SUMMARY
Excised lungs from eight marine mammal species [harp seal (Pagophilus groenlandicus), harbor seal (Phoca vitulina), gray seal (Halichoerus grypush), Atlantic white-sided dolphin (Lagenorhynchus acutus), common dolphin (Delphinus delphis), Risso's dolphin (Grampus griseus), long-finned pilot whale (Globicephala melas) and harbor porpoise (Phocoena phocoena)] were used to determine the minimum air volume of the relaxed lung (MAV, N=15), the elastic properties (pressure–volume curves, N=24) of the respiratory system and the total lung capacity (TLC). Our data indicate that mass-specific TLC (sTLC, l kg–1) does not differ between species or groups (odontocete vs phocid) and agree with that estimated (TLCest) from body mass (Mb) by applying the equation: TLCest=0.135 Mb0.92. Measured MAV was on average 7% of TLC, with a range from 0 to 16%. The pressure–volume curves were similar among species on inflation but diverged during deflation in phocids in comparison with odontocetes. These differences provide a structural basis for observed species differences in the depth at which lungs collapse and gas exchange ceases.
KEY WORDS: lung mechanics, total lung capacity, minimum air volume, excised lung, diving physiology
INTRODUCTION
In 1940, Per Scholander (Scholander, 1940) published an article that described the unusual properties of the marine mammal respiratory system. Scholander suggested that the highly compliant lung and rib cage would easily compress, the air being shunted into the rigid upper airway. This hypothesis has remained a central tenet in marine mammal diving physiology, with the assumption that this trait will reduce uptake of nitrogen (N2) and the risk of decompression sickness. Nevertheless, only a few studies have attempted to determine how pressure affects gas exchange in forced or freely diving marine mammals. These studies have assessed alveolar compression either directly by quantifying the depth-related pulmonary shunt (Kooyman and Sinnett, 1982) or indirectly by measuring N2 tension in arterial and venous blood during a dive (Falke et al., 1985; Kooyman et al., 1972) or measuring N2 removal from the muscle following a series of repeated dives (Ridgway and Howard, 1979). As the estimated depth at which alveolar collapse occurs differs considerably between studies, Bostrom and colleagues (Bostrom et al., 2008) developed a mathematical model in an attempt to understand lung compression in marine mammals. The objective was to create a theoretical framework that could be used to predict the air volumes of the various compartments of the respiratory system to the limit of collapse. The results from the model suggested that both the initial diving lung volume and the relative size and structural properties (compliance) of the upper and lower airways are important in determining the depth at which the alveoli collapse and gas exchange ceases (Bostrom et al., 2008). The model output was compared with available data for collapse depth in phocids (Falke et al., 1985; Kooyman et al., 1970; Kooyman et al., 1972; Kooyman and Sinnett, 1982) and odontocetes (Ridgway and Howard, 1979), and it was concluded that behavioral (initial diving lung volume) and structural (lung and dead-space compliance) variations among species could account for the observed differences (Bostrom et al., 2008; Fahlman et al., 2009).
Static pressure–volume (P–V) loops are commonly used to measure the physical properties (compliance) of the respiratory system. Published data exist on excised lungs for several terrestrial species, but only a few measurements have been made for marine mammals (Denison and Kooyman, 1973; Denison et al., 1971). It is therefore difficult to assess the accuracy of the model output without species-specific respiratory compliance estimates. In addition, common indices of respiratory capacity – for example, minimum air volume of the relaxed lung (MAV) and total lung capacity (TLC) – exist only for a limited number of marine mammals of different age classes (Kooyman, 1973). Therefore, this study was undertaken to increase the current knowledge of the mechanical properties of the respiratory system of marine mammals and to estimate the respiratory capacity (namely TLC and MAV) for a larger number of marine mammals. The primary objectives of the current study were, first, to compare the mechanical properties of a range of marine mammal species and, second, to estimate TLC from the P–V curves.
MATERIALS AND METHODS
Animals
Thirty-one drowned (with no sea water observed in the respiratory airways or lungs) fishery bycaught (animals that asphyxiated either in gill nets or bottom otter trawls) or stranded seals [harp seal, Pagophilus groenlandicus (Erxleben 1777); harbor seal, Phoca vitulina (Linnaeus 1758); and gray seal, Halichoerus grypus (Fabricius 1791)] or odontocetes [Atlantic white-sided dolphin, Lagenorhynchus acutus (Gray 1828); common dolphin, Delphinus delphis (Linnaeus 1758); long-finned pilot whale, Globicephala melas (Traill 1809); harbor porpoise, Phocoena phocoena (Linnaeus 1758); and Risso's dolphin, Grampus griseus (G. Cuvier 1812)] were obtained, respectively, from the NMFS Northeast Fisheries Observer Program (NEFOP) or the Marine Mammal Rescue and Research Division of the International Fund for Animal Welfare (IFAW) (Table 1). Specimens accidentally caught in the regional fisheries (bycaught) were transported by road to the Marine Research Facility (MRF) at Woods Hole Oceanographic Institution (WHOI), Woods Hole, MA, USA. Stranded animals that died or were euthanized on the beach were directly transported to WHOI. Animals were kept cold during transport and then stored at 4°C until the lungs were excised and studied less than 12h after arrival. No animal was euthanized for the purpose of this study. At the laboratory, the sex was determined, each animal weighed (±0.2 kg) and routine morphometric measurements were completed (Table 1).
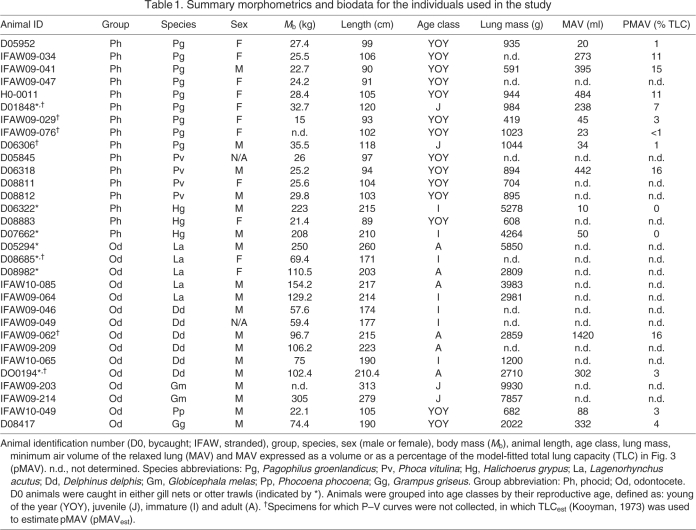
Table 1.
Summary morphometrics and biodata for the individuals used in the study
Each specimen was examined for obvious signs of scavenging, orifice discharges and bloating. Of the 31 specimens, static P–V loops and MAV were measured successfully in 24 and 15 lung samples, respectively (Table 1). In nine lung samples, both MAV and P–V data were collected (Table 1). Not all tests could be performed on all specimens owing to a variety of factors, including time-constraints and leaks during P–V experiments.
Static pressure–volume measurements
The entire respiratory tract was excised (lungs, bronchi and trachea) with the heart attached. The excised tissues were placed on a tray and the trachea intubated immediately below the pharynx using a human (Hudson RCI, Teleflex Medical, Research Triangle Drive, NC, USA) or veterinary (Equine Endotracheal Tube, Jorgensen Laboratories, Loveland, CO, USA) endotracheal tube. The endotracheal tube was attached to a manifold of three-way valves with a 3 l syringe (Series 5530, Hans-Rudolph, Kansas City, MO, USA) used to inflate the lungs in varying volume increments.
The airway opening pressure (Pao, cmH2O, 1 cmH2O=98.07 Pa) was measured using a differential pressure transducer (MPX type 339/2, Harvard Apparatus, Holliston, MA, USA) connected to an amplifier (Tam-A, Harvard Apparatus); these data were collected on a laptop computer using an A/D card (USB 1208LS, Measurement Computing, Norton, MA, USA) sampling at 2 Hz. The ambient pressure (Pamb) was used as a reference and set at 0 cmH2O.
The lungs were initially pre-conditioned by at least three inflations to a transpulmonary pressure (Ptp) of ∼30 cmH2O (Ptp=Pao–Pamb), which in mammals is often used to define the volume at TLC (Soutiere and Mitzner, 2004). The total volume used to inflate the lung to a Ptp of 30 cmH2O was divided into four or five equal increments. The P–V relationship was determined by adding or removing air in the increments determined during the pre-conditioning step. A minimum of three leak-free inflation–deflation curves was recorded. After injection or removal of an aliquot of air, the volume was held constant until the pressure had reached a plateau, usually for between 15 and 20s, before the next step-wise change in lung volume (Kooyman and Sinnett, 1979).
Minimum air volume estimates
In a subset of samples (N=15), the MAV of the relaxed (Ptp=0cmH2O) excised lungs was estimated by weighing the lung and the volume of fresh water it displaced and assuming a tissue-specific gravity of 1kgl–1 (Kooyman and Sinnett, 1979). Three estimates were obtained for each lung; if the inter-test variation was greater than 10%, tests were repeated until the variation became less than 10%.
Data processing and statistical analysis
Room temperature and ambient pressure were used to convert all volumes to standard temperature pressure, dry (STPD). We assumed intrapulmonary air to be saturated with water vapor.
We modeled the relationship between pressure and volume for the excised lung using the sigmoidal equation published by Bostrom and colleagues (Bostrom et al., 2008):
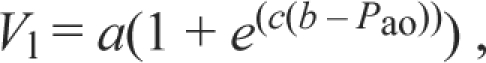 |
(1) |
where a, b and c are fitting parameters and Vl is the inflated air lung volume. The parameter a corresponds to the change in volume between the lower and upper asymptotes of the sigmoid. When inflating a relaxed excised lung to TLC, a is an estimate of the difference between TLC and MAV. The parameter b corresponds to the pressure at the inflection point of the sigmoid. The parameter c is an index of the compliance (steepness) of the curve where most of the volume change occurs (i.e. near b). Parameters were fitted using the software RGui (version 2.5.1, R Foundation for Statistical Computing, Vienna, Austria).
We used two approaches to estimate TLC. In the first, we used the MAV and a from the P–V data. For lung samples where data existed to estimate both a and MAV (N=9) (Table 1), TLC was estimated as: TLC=a+MAV. For these nine samples, the MAV was then expressed as a relative proportion of TLC as: pMAV=MAV×TLC–1. For those samples where only MAV was determined (N=7) (Table 1), pMAV was computed as: pMAVest=MAV×TLCest–1, where TLCest was computed by the equation published by Kooyman (TLCest=0.135Mb0.92) (Kooyman, 1973). The average pMAV was used to estimate TLC as: TLC=a(1–pMAV)–1. Thus, we used the average pMAV for data where both a and MAV had been computed.
In the second and more common approach, TLC was estimated as the lung volume at a Ptp=30 cmH2O (TLC30) (Kooyman and Sinnett, 1979). As the inflated volume at Ptp=30 cmH2O is an estimate of the vital capacity (VC), TLC30 was estimated as TLC30=VC(1–pMAV)–1. To compare the two estimates, TLC was plotted against TLC30. Finally, the computed TLC was plotted against animal body mass (Mb) and compared with the estimated TLCest.
The P–V relationship during inflation was analyzed by fitting the parameters a, b and c for each lung sample. TLC was estimated as described above and plotted against Mb. The scatter plot of TLC against Mb was compared with TLCest. The appearance of the deflation curve is dependent on the starting lung volume (Glaister et al., 1973). One option was to standardize the inflation volume for each sample; another option was to standardize the curves by expressing Vl as a percentage of the starting volume – that is, the maximum inflation volume. As we had data to different maximum inflation volumes for each sample, we opted for the latter to increase the statistical power. Thus, when analyzing the elastic properties of the deflation curves, Vl was expressed as a fraction of the total volume inflated. To model the biological variation within a species, and not just that of our samples, we used a nonlinear random-effects model that treated 'animal' as a random factor. To compare the curves within and between species, the 95% confidence limit was computed for each fitted model.
In this study, P-values ≤0.05 were considered as significant, whereas P≤0.1 was considered a trend. Data are presented as means ± standard error of the mean (s.e.m.), unless otherwise stated.
RESULTS
Summary morphometrics for the individuals used in the study are included in Table 1. Most odontocetes were stranded individuals (samples with an IFAW case number), whereas the seals were generally bycaught specimens (case numbers D0 and H0).
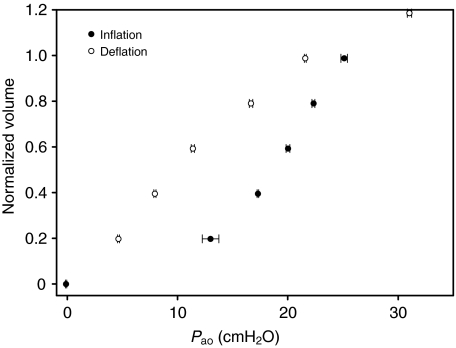
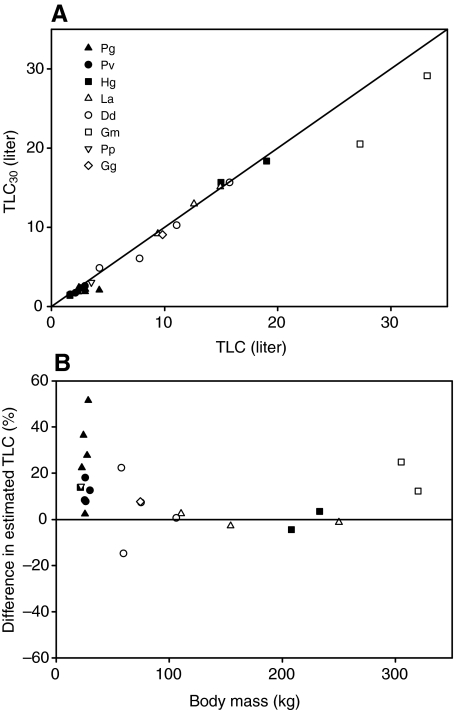
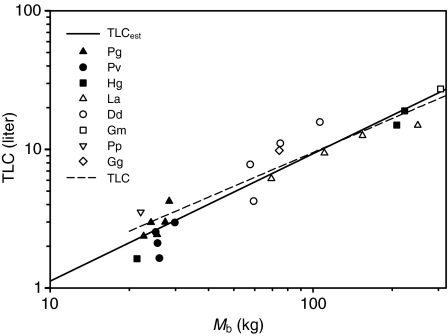
Fig. 1 shows an example of the elastic properties of an excised lung from a harbor porpoise (IFAW10-049). The experiment was repeated four times, and the error bars show the reproducibility between repeated inflation–deflation cycles. Two lung samples were difficult to inflate and were very stiff (D08685 and IFAW10-062) (Table 1); therefore, they were not included in the statistical analyses even though their data are reported in Table 1. The P–V data were used to fit the parameters in Eqn1. For lung samples where both a water-displacement measurement and inflation experiment had been performed, the average pMAV was 7% (Table 1). The pMAVest, estimated from the measured MAV and TLCest, was 5% and not different from pMAV (P>0.1, t-test). The TLC for animals where P–V data had been collected was estimated by adding the average pMAV to a – that is, TLC=a(1–0.07)–1. TLC30 was estimated as VC(1–0.07)–1. The results for the two methods of estimating TLC were plotted against each other (Fig. 2A). In addition, the relative difference between TLC and TLC30, computed as (TLC–TLC30)TLC–1×100, was plotted against Mb. TLC was on average 12% higher than TLC30, with a median of 8%. TLC was plotted against Mb (Fig.3). The estimated regression parameters from our TLC (slope: 0.81, intercept: 0.225) were not significantly different from those of the TLCest equation (slope: 0.92, intercept: 0.135) (Fig. 3). There were no apparent species-related trends, and the residuals were randomly distributed with Mb.
Fig. 1.
Mean pressure–volume data (±s.e.m., N=4) for inflation and deflation of an excised lung of a harbor porpoise (IFAW10-049). Volume includes the estimated MAV and was normalized as a proportion of the estimated total lung capacity (TLCest) (Kooyman and Sinnett, 1979), and pressure is pressure at the airway opening (Pao), which in excised lungs is the transpulmonary pressure. Error bars show the s.e.m., indicating the reproducibility between repeated inflation–deflation cycles.
Fig. 2.
(A) Total lung capacity (TLC) estimated using two methods. TLC was estimated as the volume of air in the lung between the lower and upper asymptote of the published sigmoidal function (Eqn 1) and the average minimum air volume (MAV). TLC30 was estimated as the air volume injected for a transpulmonary pressure of 30 cmH2O plus the MAV. The solid line is the line of identity. (B) Data showing the difference between TLC and TLC30 plotted against body mass (Mb, kg). Key abbreviations: Pg, Pagophilus groenlandicus; Pv, Phoca vitulina; Hg, Halichoerus grypus; La, Lagenorhynchus acutus; Dd, Delphinus delphis; Gm, Globicephala melas; Pp, Phocoena phocoena; Gg, Grampus griseus. Closed symbols represent phocids, and open symbols represent odontocetes.
Fig. 3.
Animal body mass versus model-predicted total lung capacity (TLC). TLC was estimated as TLC=a(1–0.07)–1, where a is the parameter for the difference in inflated volume between the lower and upper asymptote in Eqn 1, and 0.07 is the average estimated residual volume as determined by water-displacement experiments. The broken regression line is the best fit to the data (TLC=0.225Mb0.81), and the unbroken regression line is the equation that predicts TLC in marine mammals (TLCest=0.135Mb0.92) (Kooyman and Sinnett, 1979). Key abbreviations: Pg, Pagophilus groenlandicus; Pv, Phoca vitulina; Hg, Halichoerus grypus; La, Lagenorhynchus acutus; Dd, Delphinus delphis; Gm, Globicephala melas; Pp, Phocoena phocoena; Gg, Grampus griseus. Closed symbols represent phocids, and open symbols represent odontocetes.
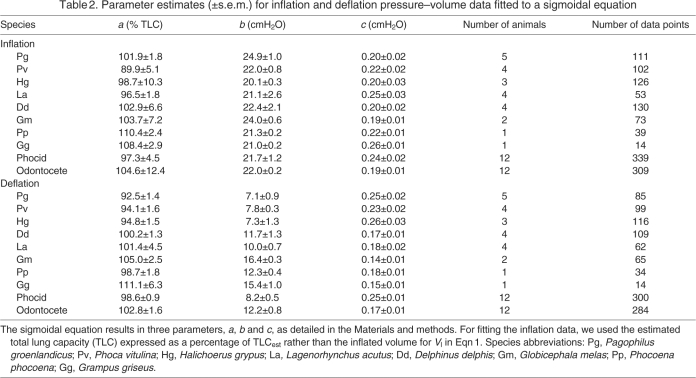
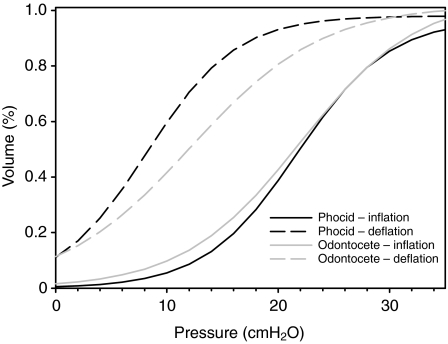
To compare the elastic properties during inflation between species, the 95% confidence limit was estimated for b and c. Data for each species were fitted to Eqn 1. For the inflation data, we used the estimated TLC [TLC=a(1–0.07)–1] expressed as a percentage of TLCest for Vl in Eqn 1. For the deflation data, Vl was adjusted as described above. The s.e.m. for each parameter was used to determine the 95% confidence limit, and differences between species were assessed by determining which samples had overlapping confidence limits. For the inflation curves, there were no differences in a, b or c between age groups, within or between species, and there were no differences between odontocetes (toothed whales) in comparison with phocids (seals) (Table 2). For the deflation curves, both b (odontocete: 12.2±0.8; phocid: 8.2±0.5) and c (odontocete: 0.17±0.01; phocid: 0.25±0.01) differed significantly between odontocetes and phocids. Again, none of the deflation parameters differed between age groups. The data for odontocetes and phocids were pooled to determine the best-fit parameters for each group, and the results are plotted in Fig. 4.
Table 2.
Parameter estimates (±s.e.m.) for inflation and deflation pressure–volume data fitted to a sigmoidal equation
Fig. 4.
The inflation and deflation curves from fitting Eqn 1 to the data for the phocids and odontocetes. The inflation volume data were expressed as a proportion of the TLCest. The deflation data were expressed as a proportion of the maximum inflation volume (including the estimated residual volume of 7%) before each deflation.
DISCUSSION
We examined the mechanical properties of lungs excised from eight different marine mammal species. Our main objectives were to estimate, first, the elastic properties and, second, the total lung capacity and minimum air volume in excised lungs from a range of marine mammals. The results from the P–V data were compared between species and groups. The parameter estimates suggest that the structural properties cause differences in behavior during deflation in seals and toothed whales. We estimated TLC, and this was plotted against Mb to assess the mass-specific TLC (sTLC, lkg–1). The mass-exponent to predict sTLC does not differ between species or groups (odontocete vs phocid) and is similar to TLCest (Kooyman, 1973).
Pressure–volume curves and the structural properties of marine mammal lungs
Two theoretical studies have suggested that the depth of alveolar collapse could be estimated using the physical properties (P–V relationship) of the different parts of the respiratory system (Bostrom et al., 2008; Fitz-Clarke, 2007). These models assume that, as the pressure increases and the gas becomes more compressed, the conducting airways remain open. This allows the air in the alveoli to move into the central airway during compression. Bostrom and colleagues (Bostrom et al., 2008) showed that their model agreed well with available data for alveolar (Falke et al., 1985; Kooyman et al., 1972; Kooyman and Sinnett, 1982; Ridgway and Howard, 1979) and tracheal volume (Kooyman et al., 1970) at different depths (Bostrom et al., 2008). However, it was also shown that varying the elastic properties of the respiratory system would significantly alter the alveolar collapse depth. As only limited information exists on the structural properties of the respiratory system in marine mammals, our aim was to compare the elastic properties in different species. Our ultimate aim is to predict the depth of alveolar collapse and improve our understanding of how gas exchange is altered during breath-hold diving. However, the data presented in this study are not suitable to make such predictions because we could not determine the small volume changes at negative transpulmonary pressures crucial to alveolar collapse. Our data do provide evidence that the elastic properties during deflation of the excised lungs differ between phocids and odontocetes. Assuming that pulmonary shunt and/or ventilation perfusion mismatch increase with decreasing alveolar volume, varying elastic properties might affect the functional gas exchange at shallow depths. Future studies need to refine the P–V curves at negative Ptp values and improve the estimates for MAV and the volume of the conducting airways. In addition, measurements are needed on live animals to test the hypothesis that the chest compliance does not affect collapse depth.
The data of the current study allow a comparison of the mechanical characteristics of the excised lungs and conducting airways in a range of marine mammals. Although it is generally believed that all marine mammals have a compliant rib cage that recoils inward to very low lung volumes and that lung collapse occurs at shallow depths, only a few studies have tested this hypothesis – and only in a limited number of species. Our data indicate that the inflation curves in excised marine mammal lungs are similar among age groups and species and between phocids and odontocetes. The inflation curves were similar to the previously published values for the California sea lion (Denison et al., 1971) and harbor porpoise (Kooyman and Sinnett, 1979). However, in the deflation curves, there was considerably more hysteresis in the P–V loop in the phocid compared with the odontocete, causing lower recoil pressures in the former. The deflation curve for the odontocete is similar to that reported for the California sea lion (Denison et al., 1971) and harbor porpoise (Kooyman and Sinnett, 1979), whereas that of the phocid is similar to that of the dog (Denison et al., 1971). When the pressure is reduced from 30 cmH2O to 10 cmH2O, the volume in the odontocete decreases by 60%, but only by 24% in the phocid. At pressures below 10 cmH2O, the deflation curve in the odontocete has greater elastance. Extending the data below MAV, the sigmoidal equation approaches 0 volume at –20cmH2O. In the California sea lion, 6% of TLC remained at Ptp=–30cmH2O, and so our extrapolation needs to be viewed carefully as we have no data at negative Ptp values. The greater pulmonary elastance in odontocetes suggests that the proportion of intrapulmonary gas remaining in the alveoli during compression might be less in odontocetes than in phocids. This agrees with data from our recent study where the lung volumes at different dive depths (pressures) were measured in post-mortem specimens, and the results indicated that collapse depths appear to be greater for cetaceans in comparison with phocids (Moore et al., 2011).
The differences in deflation curves might be due to structural or biochemical (surfactant) differences between phocids and odontocetes. In 1971, Denison and colleagues (Denison et al., 1971) showed that the reduction in alveolar volume during compression was greater in California sea lions than in dogs. It was suggested that these differences could be related to the greater abundance of elastic fibers and muscle in the supportive tissue of the lung and conducting airways in the sea lion (Denison et al., 1971; Kooyman and Sinnett, 1982); these anatomical features might contribute to the elastic differences observed in our study. In addition, preliminary results indicate that collateral ventilation – that is, gas moving laterally through the lung parenchyma – occurs in odontocetes (dolphin and pilot whale) but not in phocids (A.F., M.J.M. and C.M., unpublished), further suggesting the existence of anatomical differences. Alternatively, variation between marine and terrestrial mammals could be related to differences in alveolar surface tension. Early reports suggested that the surfactant properties of lung lavage fluid from marine mammals do not differ from those of terrestrial mammals (Denison et al., 1971; Kooyman and Sinnett, 1982). More recent work has shown that lung surfactants of pinnipeds (elephant seal, northern fur seal, California sea lion and ringed seal) have anti-adhesive properties that aid alveolar recruitment following collapse (Foot et al., 2006), but no study has reported the surfactant properties of odontocete lungs. Further work is required to determine whether the differences in hysteresis and deflation are related to differences in anatomy, biochemistry or both. In either case, the elastic properties allow us to improve predictions of the air volumes in the upper and lower airways in excised lungs at the point of collapse. Although our results illustrate the mechanical differences in excised lungs among species, studies on live animals are required in order to understand how variation in blood flow and chest compliance alters lung mechanics. For example, Leith (Leith, 1976) showed that the chest wall of a ribbon seal is highly compliant and recoils inward. Our own observations agree with this, but the chest wall in the odontocete appears to be much stiffer (A.F., M.J.M. and C.M., unpublished). The greater recoil of the odontocete chest wall might help compensate for the stiffer lung and allow for the passive exhalation reported in odontocetes (Olsen et al., 1969). Thus, future studies are needed to confirm these observations of chest compliance.
Our experimental design was similar to those used in previous studies that described the elastic properties of lungs excised from sacrificed (<19 h) harbor porpoises (Kooyman and Sinnett, 1979) and California sea lions (Denison et al., 1971). The lung samples used in this study were obtained from animals that had asphyxiated either in gill nets or bottom otter trawls (bycaught) or died during a stranding event (stranded). Therefore, the interval between death and study was often longer (>24 h) than in previous studies in the harbor porpoise (Kooyman and Sinnett, 1979) and California sea lion (Denison et al., 1971). This delay might have changed the tissue properties. In addition, bycaught animals died by drowning or asphyxiation. Asphyxiation was confirmed by the absence of water in the conducting airways. Despite these differences, the variability in the P–V relationship between lung samples within a species was reasonably low, except in two cases (D08685La and IFAW10-062Dd), and our results for the harbor porpoise are similar to those previously obtained from fresh tissues (Kooyman and Sinnett, 1979).
As for the possibility that over-inflation might have damaged the lungs, no subpleural blebs were observed in this study after inflations to Ptp<30cmH2O. Typically, these blebs develop under the visceral mesothelium after inflations to a Ptp>50 cmH2O. In addition, our results showed little variability within an individual (Fig. 1), suggesting that the lungs had not been damaged by repeated inflation–deflation cycles. In addition, no such damage was reported in lung histology of previous studies performed on fresh tissue (Denison et al., 1971; Kooyman and Sinnett, 1979), and we consider it unlikely that our results are affected by damage of the lung parenchyma.
Estimating TLC
We used two different methods to estimate TLC. In terrestrial mammals, TLC is usually defined as the air volume in the lung at a Ptp ranging between 25 and 40 cmH2O (Venegas et al., 1998). A Ptp of 30cmH2O has been used to estimate TLC in excised marine mammal lungs (Kooyman and Sinnett, 1979),. However, some species do not appear to reach maximal lung volume at Ptp values as high as 80cmH2O (Soutiere and Mitzner, 2004), and the estimated TLC will therefore vary depending on the pressure used to define TLC. For our data, most samples did not exhibit a distinct asymptote at a Ptp=30cmH2O (for example, see Fig.1), and the fitted parameter a was therefore larger than the air volume at 30cmH2O. It is possible that a standard Ptp might not be valid for all species or age classes. Alternatively, a sigmoidal equation can be used to define the upper asymptote (Glaister et al., 1973; Schroter, 1980; Venegas et al., 1998), resulting in a set of physiologically meaningful parameters that can be compared between samples from P–V curves produced under different experimental and pathological conditions. By using this approach, some estimates of TLC differed from TLCest by as much as 50%, as small differences in the shape of the P–V curves result in large differences in a. Because histological examination was not possible in all lung samples, we cannot exclude that some specimens had undergone pathological changes.
While our limited data did not reveal age differences in TLC (normalized by body weight), the lung capacity of juveniles might be different from adults. Our estimates of TLC are in general agreement with those from the equation published by Kooyman (Kooyman, 1973) in a range of species and age classes. In the present study, the average pMAV and pMAVest values were, respectively, 7 and 5% (Table 1), and measured MAV ranged between 0 and 16% of TLC. The ratio of dead space to alveolar volume is a dimensionless constant for terrestrial mammals (Leith, 1983). Our data suggest that this ratio is also constant in marine mammals, even in different age classes. In addition, the pMAV reported in this study is similar to the estimate from other species and age classes – for example, the harbor porpoise (Kooyman and Sinnett, 1979), the awake pilot whale (Olsen et al., 1969), the sei whale and fin whale [see personal communication in Kooyman and Sinnett (Kooyman and Sinnett, 1979)] – but is lower than the average in the California sea lion (Denison et al., 1971).
CONCLUSIONS
Our study confirms previous studies in the harbor porpoise (e.g. Kooyman and Sinnett, 1979) in which the residual air volume at Ptp=0 cmH2O is smaller than in terrestrial mammals. The inflation curves of excised lungs for all marine mammals studied are similar to those measured in the dog. By contrast, although the deflation curve of the phocid resembles that of the dog, that of the odontocetes is similar to the California sea lion and shows greater recoil at mid-lung volumes. These elastic differences during deflation might be due to anatomical and/or biochemical differences. Future studies are needed to confirm our findings in live animals so that the relationship between ambient pressure, alveolar size and the extent of gas exchange at depth can be assessed.
ACKNOWLEDGEMENTS
We thank the International Fund for Animal Welfare and NMFS Northeast Fisheries Observer Program and Observer Program staff and their volunteers for help throughout this study. A special thanks to all the fishermen who helped bring the bycaught animals ashore, and to Anastasia and Declan Fahlman for help with the experiments. We thank James Butler for sharing his knowledge and for the long discussions about the experimental results. Special thanks too to Dave Jones for lending his equipment throughout this study. Samples from bycaught and stranded animals were obtained with the authorization of the NOAA North East Region in Gloucester, MA, USA.
LIST OF SYMBOLS AND ABBREVIATIONS
- MAV
minimum air volume
- Mb
body mass
- Pamb
ambient pressure
- Pao
airway opening pressure
- pMAV
proportional minimum air volume
- Ptp
transpulmonary pressure
- TLC
total lung capacity
FOOTNOTES
FUNDING
This project was supported by a grant from the Office of Naval Research [award number N00014-10-1-0059]; S.H.L. was supported by HL 52586 from the National Institutes of Health.
REFERENCES
- Bostrom B. L., Fahlman A., Jones D. R. (2008). Tracheal compression delays alveolar collapse during deep diving in marine mammals. Respir. Physiol. Neurobiol. 161, 298-305 [DOI] [PubMed] [Google Scholar]
- Denison D. M., Kooyman G. L. (1973). The structure and function of the small airways in pinniped and sea otter lungs. Respir. Physiol. 17, 1-10 [DOI] [PubMed] [Google Scholar]
- Denison D. M., Warrell D. A., West J. B. (1971). Airway structure and alveolar emptying in the lungs of sea lions and dogs. Respir. Physiol. 13, 253-260 [DOI] [PubMed] [Google Scholar]
- Fahlman A., Hooker S. K., Olszowka A., Bostrom B. L., Jones D. R. (2009). Estimating the effect of lung collapse and pulmonary shunt on gas exchange during breath-hold diving: the Scholander and Kooyman legacy. Respir. Physiol. Neurobiol. 165, 28-39 [DOI] [PubMed] [Google Scholar]
- Falke K. J., Hill R. D., Qvist J., Schneider R. C., Guppy M., Liggins G. C., Hochachka P. W., Elliott R. E., Zapol W. M. (1985). Seal lungs collapse during free diving: evidence from arterial nitrogen tensions. Science 229, 556-558 [DOI] [PubMed] [Google Scholar]
- Fitz-Clarke J. R. (2007). Mechanics of airway and alveolar collapse in human breath-hold diving. Respir. Physiol. Neurobiol. 159, 202-210 [DOI] [PubMed] [Google Scholar]
- Foot N. J., Orgeig S., Daniels C. B. (2006). The evolution of a physiological system: the pulmonary surfactant system in diving mammals. Respir. Physiol. Neurobiol. 154, 118-138 [DOI] [PubMed] [Google Scholar]
- Glaister D. H., Schroter R. C., Sudlow M. F., Milic-Emili J. (1973). Bulk elastic properties of excised lungs and the effect of a transpulmonary pressure gradient. Respir. Physiol. 17, 347-364 [DOI] [PubMed] [Google Scholar]
- Kooyman G. L. (1973). Respiratory adaptations in marine mammals. Am. Zool. 13, 457-468 [Google Scholar]
- Kooyman G. L., Sinnett E. E. (1979). Mechanical properties of the harbor porpoise lung, Phocoena phocoena. Respir. Physiol. 36, 287-300 [DOI] [PubMed] [Google Scholar]
- Kooyman G. L., Sinnett E. E. (1982). Pulmonary shunts in harbor seals and sea lions during simulated dives to depth. Physiol. Zool. 55, 105-111 [Google Scholar]
- Kooyman G. L., Hammond D. D., Schroeder J. P. (1970). Bronchograms and tracheograms of seals under pressure. Science 169, 82-84 [DOI] [PubMed] [Google Scholar]
- Kooyman G. L., Schroeder J. P., Denison D. M., Hammond D. D., Wright J. J., Bergman W. P. (1972). Blood nitrogen tensions of seals during simulated deep dives. Am J. Physiol. 223, 1016-1020 [DOI] [PubMed] [Google Scholar]
- Leith D. E. (1976). Comparative mammalian respiratory mechanics. Physiologist 19, 485-510 [PubMed] [Google Scholar]
- Leith D. E. (1983). Mammalian tracheal dimensions: scaling and physiology. J. Appl. Physiol. 55, 196 [DOI] [PubMed] [Google Scholar]
- Moore M. J., Hammar T., Arruda J., Cramer S., Dennison S., Montie E., Fahlman A. (2011). Hyperbaric computer tomographic measurements of lung compression in seals and dolphins. J. Exp. Biol. 214, 2390-2397 [DOI] [PubMed] [Google Scholar]
- Olsen C. R., Hale F. C., Elsner R. (1969). Mechanics of ventilation in the pilot whale. Respir. Physiol. 7, 137-149 [DOI] [PubMed] [Google Scholar]
- Ridgway S. H., Howard R. (1979). Dolphin lung collapse and intramuscular circulation during free diving: evidence from nitrogen washout. Science 206, 1182-1183 [DOI] [PubMed] [Google Scholar]
- Scholander P. F. (1940). Experimental investigations on the respiratory function in diving mammals and birds. Hvalrådets Skr. 22, 132 [Google Scholar]
- Schroter R. C. (1980). Quantitative comparisons of mammalian lung pressure volume curves. Respir. Physiol. 42, 101-107 [DOI] [PubMed] [Google Scholar]
- Soutiere S. E., Mitzner W. (2004). On defining total lung capacity in the mouse. J. Appl. Physiol. 96, 1658-1664 [DOI] [PubMed] [Google Scholar]
- Venegas J. G., Harris R. S., Simon B. A. (1998). A comprehensive equation for the pulmonary pressure-volume curve. J. Appl. Physiol. 84, 389-395 [DOI] [PubMed] [Google Scholar]