Abstract
Recently, harmful algal bloom (HAB), also termed “red tide”, has been recognized as a serious problem in marine environments according to climate changes worldwide. Many novel materials or methods to prevent HAB have not yet been employed except for clay dispersion, in which can the resulting sedimentation on the seafloor can also cause alteration in marine ecology or secondary environmental pollution. In the current study, we investigated that antimicrobial peptide have a potential in controlling HAB without cytotoxicity to harmless marine organisms. Here, antimicrobial peptides are proposed as new algicidal compounds in combating HAB cells. HPA3 and HPA3NT3 peptides which exert potent antimicrobial activity via pore forming action in plasma membrane showed that HPA3NT3 reduced the motility of algal cells, disrupted their plasma membrane, and induced the efflux of intracellular components. Against raphidoflagellate such as Heterosigma akashiwo, Chattonella sp., and C. marina, it displayed a rapid lysing action in cell membranes at 1∼4 µM within 2 min. Comparatively, its lysing effects occurred at 8 µM within 1 h in dinoflagellate such as Cochlodium polykrikoides, Prorocentrum micans, and P. minimum. Moreover, its lysing action induced the lysis of chloroplasts and loss of chlorophyll a. In the contrary, this peptide was not effective against Skeletonema costatum, harmless algal cell, even at 256 µM, moreover, it killed only H. akashiwo or C. marina in co-cultivation with S. costatum, indicating to its selective algicidal activity between harmful and harmless algal cells. The peptide was non-hemolytic against red blood cells of Sebastes schlegeli, the black rockfish, at 120 µM. HAB cells were quickly and selectively lysed following treatment of antimicrobial peptides without cytotoxicity to harmless marine organisms. Thus, the antibiotic peptides examined in our study appear to have much potential in effectively controlling HAB with minimal impact on marine ecology.
Introduction
Harmful algal bloom (HAB), commonly called “red tide” and caused by massive and exceptional overgrowth of microalgae and cyanobacteria around the coasts, has increased globally with serious implications for the aquaculture industry and human health [1]. HAB not only leads to huge economic loss but also contributes to pollution of the coastal areas. This phenomenon often causes damage such as mass mortalities of fish, marine mammals, shellfish, and other oceanic life due to toxins and depletion of oxygen [2]. Furthermore, ingestion of seafood that has accumulated toxins produced by HAB results in illness and death in both humans and animals [2], [3]. Previous studies conducted in the Florida gulf coast have also found that exposure to aerosolized toxins from contaminated water leads to respiratory problems in humans [4], [5].
Many efforts have been made to prevent and mitigate the impact of HAB. Several reports suggest suppressing or killing harmful algal species using mechanical, biological, and chemical methods [6]–[10]. Despite several trials, a general method for controlling drastically increased blooming is limited due to differences in marine ecologies throughout the world. At times, secondary pollution of the marine environment is caused by imprudent treatments for preventing HABs. For example, clay or yellow loess was used to remove HAB organisms by sedimentation in Korea, Japan, and other countries; however, this method occasionally creates secondary effects on the bottom-dwelling organisms or ecological and environmental problems through dispersal of a large amount of clay to sea [11]–[13]. Likewise, although chemical compounds with algicidal activity have been isolated from various sources, it is possible to cause ecological changes as these reagents are not easily biodegraded and can accumulate in marine organisms over long periods similar to agricultural chemicals. Presently, alternative methods or materials are needed to minimize such problems.
It is well-known that several species of dinophyceae belonging to the genus Prorocentrum and Cochlodinium and raphidophyceae belonging to the genus Chattonella and Heterosigma, HAB cells used in present study, acutely produce toxins that poison shellfish and other marine organisms [14]–[16]. On the other hand, HPA3 (19-mer) and HPA3NT3 (15-mer) peptides, that are suggested to prevent HAB cells in here, are the modified antimicrobial peptides (AMPs) of the HP(2–20) peptide secreted from Helicobacter pylori [17]. They act against pathogenic bacteria and fungi through a pore-forming mechanism in plasma membranes [17].
AMPs have a potential for effectively managing HABs because they exert a selective algicidal action against toxic, rather than non-toxic, algal species. Moreover, compared to other chemical materials or microbiological treatments, HABs eliminate toxic algal organisms more quickly by bursting the cells. The aims of this study were (1) to determine algicidal activity of AMPs, (2) to prove the process of algal cell lysis after expose to an AMP under light microscopy, (3) to investigate selective targeting of toxic algal cells, and (4) to document the mode of algicidal action. Herein, we demonstrate the potent algicidal activity of an antimicrobial peptide (AMP) and its potential role as a novel material that selectively acts against these toxic algal species.
Methods
Materials
SYTOX Green was obtained from Molecular Probes (Eugene, OR) and 5(6)-carboxytetramethylrhodamine N-succinimidyl ester (TAMRA) were purchased from Sigma-Aldrich (St. Louis, MO). 9-fluorenylmethoxycarbonyl (Fmoc) amino acids were from CEM Co. (Matthews, NC). All other reagents were of analytical grade.
Peptide synthesis
Peptides were synthesized by Fmoc solid-phase methods on Rink amide 4-methyl benzhydrylamine resin (Novabiochem; 0.55 mmol/g) by using a Liberty microwave peptide synthesizer (CEM Co.). The protocols for peptide cleavage and purification used for this study were previously described [17].
Algal cultures
Prorocentrum minimum (D-120), Prorocentrum micans (D-077), Heterosigma akashiwo (RA-020, RA-018), Chattonella sp. (RA-005), and Skelectonema costatum (B-796) were provided by the Korea Marine Microalgae Culture Center, Busan, Republic of Korea. Cochlodinium polykrikoides, H. akashiwo, and Chattonella marina were collected for this study from the coastal area of the South Sea in Korea. All but one species were grown and maintained in an f/2 medium [18] at 20°C with 14/10 h light/dark illumination cycles under cool white fluorescent light (120 µmol photons m−2s−1). S. costatum was grown under the same conditions except that this organism was cultured with a light density of 30 µmol photons m−2s−1.
Algicidal activity
Algal cultures grown at mid-exponential phase were introduced to a 24-well tissue culture plate at a concentration of 2∼4×104 cells/mL, and two-fold serial dilutions of each peptide with f/2 medium were added in a range from 0.25 to 256 µM. After a 1 h (for raphidophyceae), 4 h (for dinophyceae), or 72 h (for S. costatum) incubation, the numbers of surviving cells were counted in a hemacytometer with a Sedgwick–Rafter counting chamber under an inverted microscopy. Inhibitory concentrations (ICs) of peptides were defined by counting the number of burst cells in which cell envelopes or membranes were completely disrupted, but not non-swimming cells. Under the same conditions, peptides were added at IC90 to C. marina cultures and the time-dependent algicidal action of the peptides was recorded under inverted microscopy with a DP71 camera (Olympus, Tokyo, Japan).
Pre-cultured H. akashiwo and P. minimum cells were adjusted to a concentration of 2.1×104 cells/mL and S. costatum cell cultures were adjusted to 4.2×104 cells/mL. After mixing cultures of H. akashiwo with S. costatum or P. minimum with S. costatum, 2 or 8 µM of HPA3NT3 were added and cells were counted at indicated times in a hemacytometer with a Sedgwick–Rafter counting chamber.
Confocal laser scanning microscopy (CLSM)
The cellular distribution of peptides was examined by using TAMRA-labeled peptides and CLSM. Algal cells were incubated with TAMRA-HPA3 or -HPA3NT3 at IC50 for 5 min (H akashiwo and C. marina) or 1 h (P. minimum), after which the cells were washed three times with f/2 medium by centrifugation (×1000 g, 5 min) and fixed with 2% (v/v) glutaraldehyde. Localization of TAMRA-labeled peptides was then observed using an inverted LSM510 laser-scanning microscope (Carl Zeiss, Gőttingen, Germany). To detect TAMRA-labeled peptides, 543-nm light from a helium neon laser was directed at a DIC/543 beam splitter. Images were then recorded digitally in a 512×512 pixel format in serial sections from the top to bottom of the algal cells.
Assay for chlorophyll a
To measure chlorophyll a concentrations, H. akashiwo cells incubated in the absence or presence of the peptides were harvested by centrifugation (at 3,000×g for 10 min) at the indicated times. The cell pellets were resuspended and extracted in 90% acetone for 24 h at 4°C. The samples were then centrifuged at 10,000×g for 10 min to remove cell debris and chlorophyll a concentrations were determined as described by in Jeffrey and Humphrey [18].
SYTOX green uptake
Algal cells were grown to mid-exponential phase in the above culture condition and adjusted to 4×104 cell/mL in f/2 media, after which they were incubated with 0.5 µM SYTOX Green for 15 min in the dark. After addition of the peptides at the indicated concentrations, an increase in fluorescence from the binding of the cationic dye to intracellular DNA was monitored over time. The excitation and emission wavelengths were 485 nm and 520 nm, respectively. All fluorescence values were plotted by base fluorescence that was obtained in algal cells without peptide.
Hemolysis
Fresh fish blood was collected from Sebastes schlegeli and immediately injected into heparin blood collection tubes (BD Vacutainer, Franklin Lakes, NJ). After gently mixing, red blood cells (RBCs) were centrifuged at 800×g and washed with PBS until the supernatant was clear. The RBCs (8% (v/v) of the final concentration) were added to 2-fold serially dilutions of peptide with PBS. After incubation with mild agitation for 1 h at 37°C, the samples were then centrifuged at 800×g for 10 min and absorbance of the supernatant was then measured at 414 nm. Complete (100%) or no hemolysis was defined as the absorbance of the RBCs containing 1% Triton X-100 or PBS alone, respectively. Each measurement was made in triplicate, and percentage hemolysis was calculated using Equation 1:
| (1) |
Time-dependent elimination of red tide by peptide
H. akashiwo cells grown at mid-exponential phase were transferred to a hexahedron cell (12×12×45 mm) at a density of 1×108 cells/m: and then incubated with 40 µg/mL of HPA3NT3 peptide. Immediately after adding the peptide, the reduction in viable cell numbers was continuously recorded for 20 min by using a digital video recorder. The individual images were extracted at 1 min intervals by video imaging software.
Results
AMPs have potent algicidal activity against harmful algal species
As shown in Table S1, they had a higher hydrophobicity and hydrophobic moment than parent peptide, HP (2–20), indicating that they favor interaction with cellular membranes. The anti-algal activity of HPA3 and HPA3NT3 peptides was evaluated by the observation of decreased motility and bursting of harmful algal species, Cochlodinium polykrikoides, Prorocentrum minimum and micans (dinophyceae or dinoflagellate), Chattonella marina and sp., and Heterosigma akashiwo (raphidophyceae or raphidoflagellate) under light microscopy (Table 1). HP (2–20) led to weak growth-inhibition in almost algal cells, while HPA3 and HPA3NT3, having additional cationicity and hydrophobicity, were more potent. HPA3NT3 had better algicidal action against all harmful algal species compared to the other peptides, at concentrations ranging from 1 to 8 µM. This peptide was particularly more active against raphidoflagellate than dinoflagellate. Generally, swimming movements of the algal cells were inhibited by treatments with the peptides at 1/4 concentrations of IC90, and lysis of occurred at concentrations over IC50.
Table 1. The algicidal activity of the peptides against various marine algal strains.
| Strains | Inhibitory concentration (µM) | |||||
| HP(2–20) | HPA3 | HPA3NT3 | ||||
| IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | |
| Dinophyceae | ||||||
| Cochlodinium polykrikoides * | 64 | 128 | 4 | 8 | 4 | 8 |
| Prorocentrum minimum D-120 | 128 | >256 | 16 | 32 | 4 | 8 |
| Prorocentrum micans D-077 | 128 | >256 | 16 | 32 | 4 | 8 |
| Raphidophyceae | ||||||
| Heterosigma akashiwo RA-020 | 32 | 128 | 2 | 2–4 | 0.5 | 1 |
| Heterosigma akashiwo RA-018 | 64 | 128 | 2 | 2–4 | 0.5 | 1 |
| Heterosigma akashiwo * | 64 | 128 | 2 | 4 | 0.5 | 1 |
| Chattonella sp. RA-005 | 64 | 128 | 2 | 4 | 1 | 2 |
| Chattonella mirina * | 32 | 64 | 4 | 8 | 2 | 4 |
| Bacillariophyceae | ||||||
| Skeletonema costatum B-796 | 256 | >256 | 128 | >256 | >256 | >256 |
*Algal strains for this study were isolated from the South Sea of Korea.
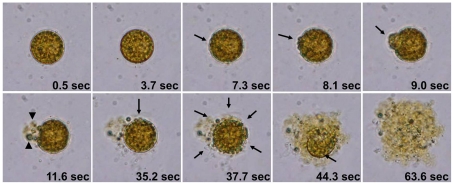
Upon exposure to HPA3 and HPA3NT3, C. marina and H. akashiwo cells swelled, their plasma membranes were disrupted, and cytoplasmic organelles and materials were released. Ultimately, cell lysis was complete within 2 min (Fig. 1 and Movie S1). This short period required for cell destruction suggested that electrostatic force of peptides led to rapid binding on algal cell membranes although membrane lipid composition is diverse according to algal species. Fig. 2 and Movie S2 show the time-dependent lysis of C. marina cell in the presence of HPA3NT2. With the addition of peptide, motility of the cell stopped, cells became rounded (0.5 sec) by inducing permeability in cell membrane, all within a very short period. At 7.3 sec, the integrity of plasma membrane was changed. Continuously, a small swelling on the cells expanded (arrow at 8.1 and 9.0 sec), burst, and intracellular organelles were released (arrowheads at 11.6 sec) through one part of the membrane that was attacked by many peptides. Interestingly, lysis of chloroplast membranes resulted in loss of chloroplast coloring. Images taken at 37.7 sec showed that the lysis of the plasma membrane and release of chloroplast membranes were ongoing in several parts of the cell. A layer of cell membrane disappeared and chloroplasts were almost destroyed at 63.6 sec.
Figure 1. Morphological changes of rapidoflagellates in the presence of peptides.
C. marina (A) and H. akashiwo RA-018 (B) cells incubated without (1) or with HP(2–20) (2), HPA3 (3), and HPA3NT3 (4) peptides each at IC90 for 2 min. Bar: 100 µm for all panels.
Figure 2. Time-dependent changes of C. marina cells in the presence of HPA3NT3.
After 4 µM of peptide of was introduced to algal cells, cell morphology was digitally recorded by video. The images were extracted from video files at the indicated times. Arrows and arrowheads indicate the damaged regions of the plasma membrane and the released chloroplasts, respectively.
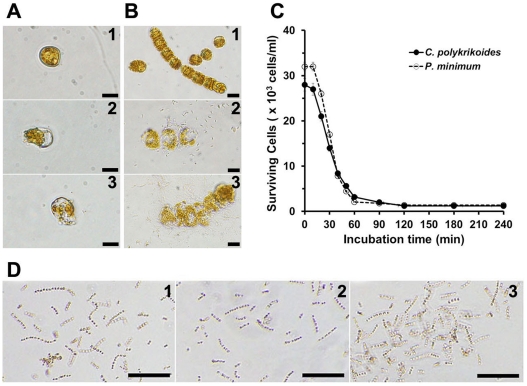
As shown in Fig. 3A and B, the mode of action of HPA3 and HPA3NT3 on C. polykrikoides and P. minimum was similar to that on raphidoflagellate, but complete lysis of dinoflagellate required long-term exposure to the peptides (1 h) compared to raphidoflagellate (2 min). Long chains of C. polykrikoides were broken and motility of P. minimum was significantly decreased during the first 10 min after introducing of peptides, but changes in morphology of individual cells was remarkable. Subsequently, non-motile cells expanded, cell envelopes and membranes were destroyed, and efflux of intracellular materials followed within 1 h (Fig. 3A–C). Differences in the kinetics of initial cell interaction and complete lysis between dinoflagellate and raphidoflagellate were dependent on the absence or presence of cell envelope and the number of outer membranes of the chloroplasts.
Figure 3. Algicidal effects of peptides against dinoflagellates and diatoms.
(A and B) P. minimum (A) and C. polykrikoides (B) cells without (1) or with HPA3 (2) and HPA3NT3 (3) each at each IC90. (C) Density of surviving cells at the indicated times. (D) S. costaum cells incubated without (1) or with 128 µM HPA3 (2) and 256 µM HPA3NT3 (3) for 3 days at 20°C. Bars: 50 µm for panels A and B and 100 µm for panel D.
On the other hand, although the growth of S. costatum, a harmless diatom, was inhibited at 256 µM of HPA3, half of cells were survival (Fig. 3D–2). However, a long-term exposure to 256 µM HPA3NT3 did not lead to any changes in morphology or survival of S. costatum cells (Fig. 3D–3).
Selective algicidal action of HPA3NT3
To investigate selective algicidal action of HPA3NT3, harmful (H. akashiwo or P. minimum) and harmless (S. costatum) algal cells were co-cultured in the presence of the peptide. After pre-culturing both types of algal cells, S. costatum cultures (4.2×104 cells/mL) were mixed with H. akashiwo or P. minimum cultures (2.1×104 cells/mL) in different cell densities because S. costatum can be preyed upon by HAB [19], [20]. HPA3NT3 was introduced to combinations of co-cultured cells at concentrations of 2 µM or 8 µM (IC90 against H. akashiwo and P. minimum, respectively). Survival of the algal cells was monitored over time for 48 hr (Fig. 4A and C) and the cells were counted. In control experiment without peptide, the growth patterns of the two co-cultures in the absence of peptide were shown that growth of S. costatum with P. minimum was little inhibited compared to co-culture of S. costatum and H. akashiwo (Fig. 4A and C) because S. costatum was purposely fed to P. minimum. While addition of HPA3NT3 resulted in H. akashiwo or P. minimum cells lysis and aggregation within 10 min (Fig. 4B–2- arrow) or 1 h (Fig. 4D–2- arrow), respectively, cell density of S. costatum was significantly increased for 24 h although its growth rate was slightly inhibited for 2 (Fig. 3A) or 6 h (Fig. 3C) following peptide exposure. In addition, presence of peptide led to enhanced S. costatum growth, indicating that selective killing and rapid lysis action of HPA3NT3 against only harmful algal cells played an important role in the propagation of S. costatum.
Figure 4. The selective algicidal action of HPA3NT3 in co-cultivations of harmful and harmless algal cells.
(A) The changes of cell density in co-cultures of S. costatum (square) and H. akashiwo (circle) in the absence (filled and 1) or presence (open and 2) of 2 µM HPA3NT3. (B) Images of surviving cells after 6 h of incubation with and without 2 µM HPA3NT2. (C) The changes of cell density in co-cultures of S. costatum (square) and P. minimum (triangle) in the absence (filled and 1) or presence (open and 2) of 8 µM HPA3NT3. (D) Images of surviving cells after 24 h of incubation with and without 8 µM HPA3NT2. Bar: 50 µm for panels B and D.
Cellular targets of HPA3 and HPA3NT3 algicidal action
To identify cellular regions of peptide action in harmful algal cells, the N-termini of HPA3 and HPA3NT3 were labeled with carboxytetramethylrhodamine (TAMRA) to produce the peptides TAMRA-HPA3 and TAMRA-HPA3NT3. Prior to this experiment, we examined whether labeling with this dye has any effect on the peptide algicidal activity, and found that the peptides' biological functions and abilities were not limited. Algal cells incubated with TAMRA-labeled peptides at IC50 were observed under confocal laser scanning microscopy (CLSM). In order to minimize a misjudgement of planes due to the large size and dimension of algal cells, images were serially recorded on 12 planes from the top to bottom of the cells. The localization of TAMRA-HPA3 in the cell surfaces appeared as an intensive red pigmentation on the plasma membrane and chloroplasts of H. akashiwo and C. marina (Fig. S1). Likewise, P. minimum with thick envelopes of cellulose was also targeted (Fig. S1), although how the peptides passed across these envelopes was unclear. As shown in Fig. 5, chloroplast membranes were a main target of TAMRA-HPA3NT3. In order to investigate other intracellular targets, C. marina cells in which lysis was ongoing were examined (Fig. 5B). Fluorescence was more concentrated in chloroplasts than in the plasma membrane and released cytoplasmic materials. This indicated that HPA3NT3 possesses significant affinity for the chloroplast envelope and thylakoid membranes composed of phosphatidylglycerol and sulfoquinovosyldiacylglycerol [21], [22]. Cationic peptide binds to negatively charged lipids through electrostatic interactions.
Figure 5. Localization of TAMRA-HPA3NT3 in serial sections.
(A) C. marina cell treated with 2 µM of TAMRA-HPA3NT3 for 2 min. (B) P. minimum cell treated with 4 µM of TAMRA-HPA3NT3 for 30 min. The images were automatically recorded on 12 planes from the top to bottom of the cell. Numbers in left top of each panel represent the distance of focus from the top of the cell.
Mode of action of HPA3NT3
To determine how HPA3NT3 kills HAB cells, we investigated the peptide-induced damage in two regions, the plasma membrane and chloroplast. First, uptake of SYTOX green, a vital dye which does not penetrate the membrane of live cells by itself, was used to analyse H. akashiwo and S. costatum cells treated with HPA3NT3. After pre-incubating algal cells with this dye, HPA3NT3 was added at 1/2 and 1×IC50 that resulted in only loss of cell motility (Fig. 6A). In this assay, the reason of peptide treatment at not IC90 but IC50 value was that nucleic acids released by complete lysis of algal cells at IC90 can bind with non-penetrating or free dyes. In H. akashiwo cells, fluorescence emitted upon dye binding to cytoplasmic nucleic acids was increased in a concentration- and time-dependent manner, indicating that HPA3NT3 directly acts on plasma membranes leading to an influx of dye. Additionally, maximal fluorescence intensity was recorded at 10 min in the presence of 0.5 µM HPA3NT3. On the contrary, HPA3NT3 did not induce permeability of the plasma membrane in S. costatum, a harmless diatom, as evidenced by no change in fluorescence over 30 min in the presence of the peptide; further incubation with the peptide for 2 h did not induce any change in fluorescence (data not shown).
Figure 6. Mode of action of HPA3NT3 in algal cells.
(A) Uptake of SYTOX green in H. akashiwo and S. costatum cells treated with HPA3NT3. (B) Concentrations of chlorophyll a in cell treated with HPA3NT3.
We next investigated the effect of HPA3NT3 peptide on chloroplasts because these organelles leaked out from H. akashiwo cells upon damage to the plasma membranes. Chlorophyll a concentrations in the control cells not exposed to the peptide were maintained for almost 60 min, but the concentrations in cells treated with 0.5 and 1 µM HPA3NT3 were significantly reduced in a time-dependent manner (Fig. 6B). These data indicate that the peptide disrupts both the envelope and thylakoid membranes of chloroplasts, corresponding to the rapid loss of green coloring observed in the cells.
Cytotoxicity of peptides against fish red blood cells (fRBCs)
We collected RBCs from rockfish, Sebastes schlegeli, in order to examine cytotoxic effects of the peptides on fish cell. Melittin, a cytotoxic peptide which was used as a negative control, caused 100% hemolysis at 8 µM, but HPA3 and HPA3NT3 induced 75.6% and 1.0% hemolysis, respectively, at 64 µM (Fig. 7). Treatment with 128 µM HPA3NT3, 128 times IC90 for H. akashiwo cells, resulted in 5.3% hemolysis. These data suggested that HPA3NT3, but not HPA3, may be used as an algicidal agent without being cytotoxic to other marine organisms.
Figure 7. Hemolytic effects of peptides.
Peptides with indicated concentrations incubated for 1 h with red blood cells collected from Sebastes schlegeli, and the released hemoglobin was then calculated.
In vitro clearance of red tide by addition of HPA3NT3
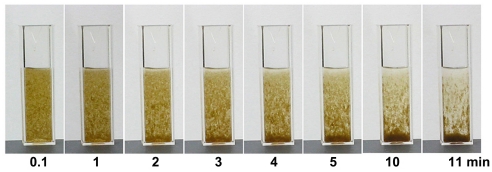
To further investigate the effectiveness of HPA3NT3 in controlling HAB and the associated high densities of harmful algal cells, we scaled up an algicidal assay for this peptide. As shown in Fig. 8, 1×108 cells/mL of H. akashiwo were incubated with 40 µg/mL of HPA3NT3, and the reduction of the algal bloom was continuously recorded for 20 min. The algal bloom immediately reacted to the addition of peptide, and debris from lysed cells began to precipitate at 1 min. Continuous sedimentation was almost completed at 11 min and the extremely turbid media was clear (Fig. 8). This rapid clearance corresponded to the rapid peptide-induced plasma and chloroplast membrane lysis in harmful algal cells.
Figure 8. In vitro clearance of red tide.
40 µg/mL of HPA3NT3 was mixed with H. akashiwo of 1×108 cells/mL, and sedimentation of algal cells or clearance of turbid seawater continuously recorded.
Discussion
During the recent decades, problems with HABs have significantly increased due to factors such as extensive costal eutrophication and global climate change [23]–[25]. Suggested methods for controlling HABs are limited to a few strategies such as the use of algicidal viruses [26], [27], predators of toxic algal species [19], [28], algicidal agents produced from bacteria [8], [9], [29], UV irradiation [30], [31], and clay sedimentation [6], [12]. However, the only used method is flocculation of harmful algae through clay dispersal [6], [7], [12], but this method is associated with secondary contamination and changes in marine environments and ecologies when the clay was highly dispersed [11], [13]. Presently, new materials or methods that are able to substitute for the above management strategies are urgently required.
The study on the algicidal activity of antibiotic peptides against harmful algal species is rare, although one peptide with two standard and three unusual amino acids containing hydroxyl groups has been reported [32]. In our study, we demonstrated that antimicrobial peptides may be useful in the prevention of HABs due to several characteristics: (1) selective algicidal action, (2) membranolytic action, (3) non- or low cytotoxic, and (4) standard amino acid composition.
Potent and selective algicidal activity of HPA3 and HPA3NT3 antimicrobial peptides
We hypothesized that membranolytic action of HPA3 and HPA3NT3 would lead to algicidal activity because eukaryotic phytoplankton cells are usually surrounded by phospholipid and glycolipid bilayers although the lipid composition varies according to species. In this study, we showed that both HPA3 and HPA3NT3 have a potent algicidal activity against two types of harmful algal cells through lysing cell membranes and chloroplasts. Other studies reported that some fatty acids with anti-algal activity cause a leakage of intracellular K+ resulting from damage to the plasma membrane in phytoplankton and cyanobacteria, and dissociated phycobilins from thylakoids [33]–[35]. Sokolov et al. [36] suggested that fatty acids are able to cause membrane permeability through the formation of ion channels in the phospholipid bilayers, thereby altering the membrane structure.
The present study revealed that algicidal action of both peptides were more rapid and effective against raphidoflagellate such as H. akashiwo, C. marina, and C. sp. than dinoflagellate like C. polykrikoides, P. minimum, and P. micans. Generally, cell envelopes of raphidoflagellate are mainly composed of glycocalyx and plasma membranes, but dinoflagellate used in this study possesses an “armored” type of cell wall in which the plasma membrane is typically covered by thick plates of cellulose [37]–[40]. It was known that morphologies of raphidoflagellate are easily changed by chemical and physical treatment [39], therefore it was not surprising to observe that the efflux of intracellular components from algal cells was quickly induced by the peptides. Generally AMPs with cationicity act to membrane and have membrane permeating activity, therefore, they can be easily bind to plasma membrane of raphidoflagellate and can disrupt through electrostatic interaction and amphipathic character. This effect required more time in dinoflagellate because the peptides are difficult to reach the plasma membrane across cellulose layers of their cell wall and the breakdown of this layer was not easy. Moreover, as shown in Fig. 2 and Fig. 6, the peptides interacted with and lysed the chloroplasts and other cell organelles in harmful algal cells. Raphidoflagellate chloroplasts are composed of two membrane layers while dinoflagellate contains two of chloroplast envelope plus postulated plasmalemma [41]–[43]. This may explain the differences in the kinetics of peptide action against raphidoflagellate and dinoflagellate.
S. costatum is a ubiquitous and relatively harmless diatom (bacillariophyceae) which provides to coastal fisheries as a food source due to fast growing rate and plays a role of marine re-mineralization [44], [45] although it is often forms spring blooms in China [46]. The population and growth of S. costatum was not affected by the HPA3NT3 peptide, even at high concentration. In addition, co-culture experiments with harmful and harmless algal cells showed that only harmful algal cells were killed by the peptide without any impact on the harmless algal cell (Fig. 4). Generally, an organic casing of the diatom cell wall is coated by siliceous frustule which is forms a joint or gasket through callose, a structural β-1,3-linked glucan [47], [48]. Perhaps, this siliceous coat will interrupt the peptides' attack by providing a protective barrier that prevents the peptide from reaching the plasma membrane. This may contribute to the selective algicidal activity of the HPA3NT3 peptide.
Plausible mode of action of HPA3 and HPA3NT3
As shown in the microscopic observation, both peptides disrupted the plasma and chloroplast membranes of harmful algal cells. TAMRA-HPA3NT3 localized to the chloroplast membranes more than the plasma membranes of H. akashiwo and P. minimum (Fig. 5A). In particular, C. marina cells undergoing lysis of the plasma membrane showed concentrated fluorescence in the chloroplasts (Fig. 5B). Results from experiments measuring the influx of SYTOX Green dye, which flowed into the cytoplasm and led to increased fluorescence upon binding to nucleic acids when the plasma membrane was damaged, provided insight into the membrane-permeable action of the peptides. HPA3NT3 induced a significant increase in fluorescence in H. akashiwo cells for 10 min at concentrations below MIC90, meaning that the peptide damaged the plasma membrane. In addition, concentrations of chlorophyll a were decreased by treatment with HPA3NT3. Since both peptides are amphipathic compounds containing hydrophobic and hydrophilic moieties, they can easily adhere on the plasma membrane and insert themselves into the lipid bilayer. Therefore, we suggest that the entrance of peptides through the damaged plasma membrane allows targeting of the chloroplast membranes. Moreover, chloroplast membranes contain sulfoquinovosyldiacylglycerol and phosphatidylglycerol [41], negatively charged lipids, to which cationic peptides are able to bind through electrostatic interactions. On the other hand, plasma membranes of dinoflagellate used in this study are covered by cellulose plates; however, the peptides were able to kill these cells within 1–2 hr. Although the binding affinity of the peptides for cellulose was weak [17], they slowly pass through the polysaccharide layer and disrupt plasma and chloroplast membranes. In contrast to raphidoflagellate, S. costatum did not effectively uptake SYTOX Green in the presence of 512 µM of HPA3NT3. We suggest that the interaction of peptide with the interior plasma membrane may be protected by the siliceous cover.
Cytotoxicity of peptides and in vitro clearing of red tide
The cytotoxicity of anti-algal compounds must be considered before using these reagents in marine environments. The present study showed that 128 µM of HPA3 and HPA3NT3 caused 97.3% and 5.3% hemolysis, respectively, in fresh red blood cells (RBCs) collected from Sebastes schlegeli, a black rockfish, indicating that HPA3NT3 peptide is less cytotoxic. Moreover, it was previously reported that HPA3NT3 possesses low hemolytic and cytotoxic activity against human RBCs and HaCaT cell [17]. The membranolytic action of HPA3NT3 prefers negatively charged phospholipids (such as PG, cardiolipin, and phosphatidylserine) over zwitterionic phospholipids (such as phosphatidylethanolamine and phosphatidylcholine) [17]. This strong action for negatively charged phospholipids particularly contributes to the selective lysis of harmful algal cells. Although HPA3NT3 cytotoxicity should be studied in other organisms, we propose that this peptide is a safe algicidal compound at least in fish. In vitro clearance experiments showed that dispersed HPA3NT3 removed algal bloom from top to bottom with 11 min through rapid sedimentation of lysed algal cells (Fig. 8). This experiment performed in high density of algal cells showed that the peptide can quickly control algal-blooming in limited areas.
As the peptides used in this study were composed of common amino acids, there are a number of reasonable advantages for using these for controlling HABs. First, these peptides can be naturally biodegraded in marine environments. Biodegradable property is one of important factors selecting algicidal materials for use in the ocean because algicidal chemicals are frequently noxious to marine organisms or human, and like agricultural chemicals, can accumulate in humans through the food chain. Therefore, these peptides, which are easily biodegraded in marine ecosystems, are comparatively safe. Another potential of them is that their sequences are easily able to be converted into genetic resource and to produce in a great quantity because they are ribosomally synthesized. Recent studies proposed that HABs can be controlled by parasites of harmful algal species [49] or single-stranded RNA viruses [26], [27]. Furthermore, more active and safe peptides could be developed by substitution of amino acids based upon the amino acid sequence of HPA3NT3 .
In practical terms, use of antimicrobial peptides in preventing HABs will be limited due to the high cost of chemical synthesis. This problem could be solved by processes such as mass production in microbes or plants, and enhancement of algicidal by gene-transfer into specific parasites or RNA viruses for harmful algal cells as these peptides are composed entirely of standard amino acids. Although we are only beginning to realize the potential of antimicrobial peptides in this field, further investigation will result in the discovery of practical applications for these peptides.
Supporting Information
Localization of TAMRA-HPA3 in algal cells. (A) H. akashiwo cell treated with 2 µM of TAMRA-HPA3 for 2 min. (B) C. marina cell treated with 4 µM of TAMRA-HPA3 for 2 min. (C) P. minimum cell treated with 16 µM of TAMRA-HPA3 for 30 min. All images were recorded with a intermediated focus between the top and bottom of cells.
(TIF)
Sequence, molecular mass, mean hydrophobicity ( H ), and mean relative hydrophobic moment ( µ H) of the peptides used in this study.
(PDF)
The morphological changes of C. marina with 4 µM of HPA3 were digitally recorded by video under microscopy.
(MPG)
After 4 µM of HPA3NT3 of was introduced to C. marina , cell morphology was digitally recorded by video under microscopy. Snapshots of Figure 2 were extracted from this movie file.
(MPG)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Pioneer Research Center Program through the National Research Program of Korea (http://www.nrf.re.kr/html/kr) funded by the Ministry of Education, Science and Technology (Grant No. 2008-2000122). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Edvardsen B, Imai I. The ecology of harmful flagellates within Prymnesiophyceae and Rapidophyceae. In: Granéli E, Turner JT, editors. Ecology of Harmful Algae. Berlin Springer; 2006. pp. 67–79. [Google Scholar]
- 2.Nishibori N, Niitsu M, Fujihara S, Sagara T, Nishio S, et al. Occurrence of the polyamines caldopentamine and homocaldopentamine in axenic cultures of the red tide flagellates Chattonella antiqua and Heterosigma akashiwo (Raphidophyceae). FEMS Microbiol Lett. 2009;298:74–78. doi: 10.1111/j.1574-6968.2009.01701.x. [DOI] [PubMed] [Google Scholar]
- 3.Watkins SM, Reich A, Fleming LE, Hammond R. Neurotoxic shellfish poisoning. Mar Drugs. 2008;6:431–455. doi: 10.3390/md20080021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoagland P, Jin D, Polansky LY, Kirkpatrick B, Kirkpatrick G, et al. The costs of respiratory illnesses arising from Florida gulf coast Karenia brevis blooms. Environ Health Perspect. 2009;117:1239–1243. doi: 10.1289/ehp.0900645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, et al. Aerosolized red-tide toxins (brevetoxins) and asthma. Chest. 2007;131:187–194. doi: 10.1378/chest.06-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengco MR, Anderson DM. Controlling harmful algal blooms through clay flocculation. J Eukaryot Microbiol. 2004;51:169–172. doi: 10.1111/j.1550-7408.2004.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DM. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast Manag. 2009;52:342. doi: 10.1016/j.ocecoaman.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayali X, Azam F. Agicidal bacteria in the sea and their impact on algal blooms. J Eukaryot Microbiol. 2004;51:139–144. doi: 10.1111/j.1550-7408.2004.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Kim JF, Yim JH, Kwon SK, Lee CH, et al. Red to red - the marine bacterium Hahella chejuensis and its product prodigiosin for mitigation of harmful algal blooms. J Microbiol Biotechnol. 2008;18:1621–1629. [PubMed] [Google Scholar]
- 10.Pearson LA, Moffitt MC, Ginn HP, Neilan BA. The molecular genetics and regulation of cyanobacterial peptide hepatotoxin biosynthesis. Crit Rev Toxicol. 2008;38:847–856. doi: 10.1080/10408440802291513. [DOI] [PubMed] [Google Scholar]
- 11.Bricelj VM, Malouf RE. Influence of algal suspended sediment concentrations on the feeding physiology of the hard clam Mercenaria mercenaria. Mar Biol. 1984;84:155–165. [Google Scholar]
- 12.Sun XX, Lee YJ, Choi JK, Kim EK. Synergistic effect of sophorolipid and loess combination in harmful algal blooms mitigation. Mar Pollut Bull. 2004;48:863–872. doi: 10.1016/j.marpolbul.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Park YT, Lee WJ. Changes of bacterial population during the decomposition process of red tide dinoflagellate, Cochlodinium polykrikoides in the marine sediment addition of yellow loess. Korean J Fish Aqua Sci. 1998;31:920–926. [Google Scholar]
- 14.Dominguez HJ, Paz B, Daranas AH, Norte M, Franco JM, et al. Dinoflagellate polyether within the yessotoxin, pectenotoxin and okadaic acid toxin groups: characterization, analysis and human health implications. Toxicon. 2010;56:191–217. doi: 10.1016/j.toxicon.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Watkins SM, Reich A, Fleming LE, Hammond R. Neurotoxic shellfish poisoning. Mar Drugs. 2008;6:431–455. doi: 10.3390/md20080021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haque SM, Onoue Y. Variation in toxin compositions of two harmful raphidophytes, Chattonella antiqua and Chattonella marina, at different salinities. Environ Toxicol. 2002;17:113–118. doi: 10.1002/tox.10039. [DOI] [PubMed] [Google Scholar]
- 17.Park SC, Kim MH, Hossain MA, Shin SY, Kim Y, et al. Amphipathic alpha-helical peptide, HP (2–20), and its analogues derived from Helicobacter pylori: pore formation mechanism in various lipid compositions. Biochim Biophys Acta. 2008;1784:783–788. doi: 10.1016/j.bbamem.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Jeffrey SW, Humphrey GF. New spectrophotometric equations for determining chlorophyll a,b,c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanzen. 1975;167:191–194. [Google Scholar]
- 19.Yoo YD, Jeong HJ, Kim MS, Kang NS, Song JY, et al. Feeding by phototrophic red-tide dinoflagellates on the ubiquitous marine diatom Skeletonema costatum. J Eukaryot Microbiol. 2009;56:413–420. doi: 10.1111/j.1550-7408.2009.00421.x. [DOI] [PubMed] [Google Scholar]
- 20.Yoo YD, Jeong HJ, Kang NS, Song JY, Kim KY, et al. Feeding by the newly described mixotrophic dinoflagellate Paragymnodinium shiwhaense: feeding mechanism, prey species, and effect of prey concentration. J Eukaryot Microbiol. 2010;57:145–158. doi: 10.1111/j.1550-7408.2009.00448.x. [DOI] [PubMed] [Google Scholar]
- 21.Wada H, Murata N. The essential role of phosphatidylglycerol in photosynthesis. Photosynth Res. 2007;92:205–215. doi: 10.1007/s11120-007-9203-z. [DOI] [PubMed] [Google Scholar]
- 22.Sato N, Hagio M, Wada H, Tsuzuki M. Environmental effects on acidic lipids of thylakoid membranes. Biochem Soc Trans. 2000;28:912–914. [PubMed] [Google Scholar]
- 23.Chambouvet A, Morin P, Marie D, Guillou L. Control of toxic marine dinoflagellate blooms by serial parasitic killers. Science. 2008;322:1254–1257. doi: 10.1126/science.1164387. [DOI] [PubMed] [Google Scholar]
- 24.Anderson DM, Burkholder JM, Cochlan WP, Glibert PM, Gobler CJ, et al. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae. 2008;8:39–53. doi: 10.1016/j.hal.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sellner KG, Doucette GJ, Kirkpatrick GJ. Harmful algal blooms: causes, impacts and detection. J Ind Microbiol Biotechnol. 2003;30:383–406. doi: 10.1007/s10295-003-0074-9. [DOI] [PubMed] [Google Scholar]
- 26.Mizumoto H, Tomaru Y, Takao Y, Shirai Y, Nagasaki K. Diverse responses of the bivalve-killing dinoflagellate Heterocapsa circularisquama to infection by a single-stranded RNA virus. Appl Environ Microbiol. 2008;74:3105–3111. doi: 10.1128/AEM.02190-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagasaki K. Dinoflagellates, diatoms, and their viruses. J Microbiol. 2008;46:235–243. doi: 10.1007/s12275-008-0098-y. [DOI] [PubMed] [Google Scholar]
- 28.Jeong HJ, Kim JS, Yoo YD, Kim ST, Kim TH, et al. Feeding by the heterotrophic dinoflagellate Oxyrrhis marina on the red-tide raphidophyte Heterosigma akashiwo: a potential biological method to control red tides using mass-cultured grazers. J Eukaryot Microbiol. 2003;50:274–282. doi: 10.1111/j.1550-7408.2003.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima T, Miyazaki Y, Matsuyama Y, Muraoka W, Yamaguchi K, et al. Producing mechanism of an algicidal compound against red tide phytoplankton in a marine bacterium gamma-proteobacterium. Appl Microbiol Biotechnol. 2006;73:684–690. doi: 10.1007/s00253-006-0507-2. [DOI] [PubMed] [Google Scholar]
- 30.Sakai H, Oguma K, Katayama H, Ohgaki S. Effects of low or medium-pressure UV irradiation on the release of intracellular microcystin. Water Res. 2007;41:3458–3464. doi: 10.1016/j.watres.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Gao K, Guan W, Helbling EW. Effects of solar ultraviolet radiation on photosynthesis of the marine red tide alga Heterosigma akashiwo (Raphidophyceae). J Photochem Photobiol B. 2007;86:140–148. doi: 10.1016/j.jphotobiol.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Ishida K, Murakami M. Kasumigamide, an antialgal peptide from the cyanobacterium Microcystis aeruginosa. J Org Chem. 2000;65:5898–5900. doi: 10.1021/jo991918f. [DOI] [PubMed] [Google Scholar]
- 33.Wu JT, Chiang YR, Huang WY, Jane WN. Cytotoxic effects of free fatty acids on phytoplankton algae and cyanobacteria. Aquat Toxicol. 2006;80:338–345. doi: 10.1016/j.aquatox.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Alamsjah MA, Hirao S, Ishibashi F, Fujita Y. Isolation and structure determination of algicidal compounds from Ulva fasciata. Biosci Biotechnol Biochem. 2005;69:2186–2192. doi: 10.1271/bbb.69.2186. [DOI] [PubMed] [Google Scholar]
- 35.Alamsjah MA, Ishibe K, Kim D, Yamaguchi K, Ishibashi F, et al. Selective toxic effects of polyunsaturated fatty acids derived from Ulva fasciata on red tide phyotoplankter species. Biosci Biotechnol Biochem. 2007;71:265–268. doi: 10.1271/bbb.60475. [DOI] [PubMed] [Google Scholar]
- 36.Sokolov Y, Mirzabekov T, Martin DW, Lehrer RI, Kagan BL. Membrane channel formation by antimicrobial protegrins. Biochim Biophys Acta. 1999;1420:23–29. doi: 10.1016/s0005-2736(99)00086-3. [DOI] [PubMed] [Google Scholar]
- 37.Parrish CC. Dissolved and particulate marine lipid classes: a review. Mar Chem. 1988;23:17–40. [Google Scholar]
- 38.Marshall JA, Nichols PD, Hallegraeff GM. Chemotaxonomic survey of sterols and fatty acids in six marine raphidophyte algae. J Appl Phycol. 2002;14:255–265. [Google Scholar]
- 39.Kim D, Okamoto T, Oda T, Tachibana K, Lee KS, et al. Possible involvement of the glycocalyx in the ichthyotoxicity of Chattonella marina (Raphidophyceae): immunological approach using antiserum against cell surface structures of the flagellate. Mar Biol. 2001;139:624–632. [Google Scholar]
- 40.Wada M, Hara Y, Kato M, Yamada M, Fujii T. Diurnal appearance, fine structure, and chemical composition of fatty particles in Heterosigma akashiwo (Raphidophyceae). Protoplasma. 1987;137:134–139. [Google Scholar]
- 41.Gibbs S. The chloroplasts of some algal groups may have evolved from endosymbiotic eukaryotic algae. Ann N Y Acad Sci. 1981;361:193–207. doi: 10.1111/j.1749-6632.1981.tb46519.x. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs MA, Connell L, Cattolico RA. A conserved His-Asp signal response regulator-like gene in Heterosigma akashiwo chloroplasts. Plant Mol Biol. 1999;41:645–655. doi: 10.1023/a:1006394925182. [DOI] [PubMed] [Google Scholar]
- 43.Iwataki M, Hansen G, Moestrup Ø, Matsuoka K. Ultrastructure of the harmful unarmored dinoflagellate Cochlodinium polykrikoides (Dinophyceae) with reference to the apical groove and Flagellar apparatus. J Eukaryot Microbiol. 2010;57:308–321. doi: 10.1111/j.1550-7408.2010.00491.x. [DOI] [PubMed] [Google Scholar]
- 44.Ianora A, Miralto A, Poulet SA, Carotenuto Y, Buttino I, et al. Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature. 2004;429:403–407. doi: 10.1038/nature02526. [DOI] [PubMed] [Google Scholar]
- 45.Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, et al. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang D, Di B, Wei G, Ni I, Oh IS, et al. Spatial, seasonal and species variations of harmful algal blooms in the South Yellow Sea and East China Sea. Hydrobiologia. 2006;568:245–253. [Google Scholar]
- 47.Volcani BE. Cell wall formation in diatoms: morphogenesis and biochemistry. In: Simpson TL, Volcani BE, editors. Silicon and Siliceous Structures in Biological Systems. New York Springer; 1981. pp. 157–200. [Google Scholar]
- 48.Hecky RE, Mopper K, Kilham P, Degens ET. The amino acid and sugar composition of diatom cell-walls. Mar Biol. 1973;19:323–331. [Google Scholar]
- 49.Chambouvet A, Morin P, Marie D, Guillou L. Control of toxic marine dinoflagellate blooms by serial parasitic killers. Science. 2008;322:1254–1257. doi: 10.1126/science.1164387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Localization of TAMRA-HPA3 in algal cells. (A) H. akashiwo cell treated with 2 µM of TAMRA-HPA3 for 2 min. (B) C. marina cell treated with 4 µM of TAMRA-HPA3 for 2 min. (C) P. minimum cell treated with 16 µM of TAMRA-HPA3 for 30 min. All images were recorded with a intermediated focus between the top and bottom of cells.
(TIF)
Sequence, molecular mass, mean hydrophobicity ( H ), and mean relative hydrophobic moment ( µ H) of the peptides used in this study.
(PDF)
The morphological changes of C. marina with 4 µM of HPA3 were digitally recorded by video under microscopy.
(MPG)
After 4 µM of HPA3NT3 of was introduced to C. marina , cell morphology was digitally recorded by video under microscopy. Snapshots of Figure 2 were extracted from this movie file.
(MPG)