Abstract
A promising strategy to accelerate joint implant integration and reduce recovery time and failure rates is to deliver a combination of certain growth factors to the integration site. There is a need to control the quantity of growth factors delivered at different times during the healing process to maximize efficacy. Polyelectrolyte multilayer (PEM) films, built using the layer-by-layer (LbL) technique, are attractive for releasing controlled amounts of potent growth factors over a sustained period. Here, we present PEM films that sequester physiological amounts of osteogenic rhBMP-2 (recombinant human bone morphogenetic protein - 2) and angiogenic rhVEGF165 (recombinant human vascular endothelial growth factor) in different ratios in a degradable [poly(β-amino ester)/polyanion/growth factor/ polyanion] LbL tetralayer repeat architecture where the biologic load scaled linearly with the number of tetralayers. No burst release of either growth factor was observed as the films degraded. The release of rhBMP-2 was sustained over a period of 2 weeks, while rhVEGF165 eluted from the film over the first 8 days. Both growth factors retained their efficacy, as quantified with relevant in vitro assays. rhBMP-2 initiated a dose dependent differentiation cascade in MC3T3-E1S4 pre-osteoblasts while rhVEGF165 upregulated HUVEC proliferation, and accelerated closure of a scratch in HUVEC cell cultures in a dose dependent manner. In vivo, the mineral density of ectopic bone formed de novo by rhBMP-2/rhVEGF165 PEM films was approximately 33% higher than when only rhBMP-2 was introduced, with a higher trabecular thickness, which would indicate a decrease in the risk of osteoporotic fracture. Bone formed throughout the scaffold when both growth factors were released, which suggests more complete remodeling due to an increased local vascular network. This study demonstrates a promising approach to delivering precise doses of multiple growth factors for a variety of implant applications where control over spatial and temporal release profile of the biologic is desired.
Keywords: Controlled drug release, BMP, VEGF, bone, hip replacement prosthesis, layer-by-layer, polyelectrolyte multilayer, dose response
1. Introduction
Approximately 10% of total hip prostheses (THP) are revised within 10 years after the procedure due to loosening of the implant [1], almost entirely because of the failure of fixation in cases of aseptic loosening [2] . In order to engineer a successful implant, it is necessary to address the bone as a highly vascularized tissue where a multistep process involving migration, proliferation, differentiation, and activation of several cell types is necessary for integrating the implant with the bone tissue [3]. Several growth factors have been implicated in this process [4], and bone morphogenetic proteins (BMP) have been shown to be some of the most potent bone tissue-forming proteins known amongst the growth factors investigated for bone tissue growth, and are the only growth factors capable of directly inducing de novo bone formation [5]. Recombinant human BMP-2 (rhBMP-2) is an osteogenic growth factor used extensively in both ectopic and orthotopic sites for bone generation; it has been demonstrated in various animal models for healing fracture sites and femur defects, such as in rats [6, 7], rabbits [8] and sheep [9], and is an approved product for use on humans in lumbar spine fusion [10] . The benefits of release of growth factors such as rhBMP-2 include rapid healing of tissue around the implant [11] and more complete bone remodeling over shorter time periods [12]. Recombinant human vascular endothelial growth factor (rhVEGF) is an angiogenic factor critical for both intramembranous and endochondral bone formation [3]. Angiogenesis is required for most tissues to develop and is a critical component of virtually all tissue-engineering strategies and the growth process and restructuring of bone tissue is greatly enhanced by increased vascularization [13] and the introduction of rhVEGF has been shown to enhance bone formation [14] through the development of the localized vascular system and increased blood flow. rhVEGF has been shown to act synergistically with rhBMP-2 to increase the growth of bone tissue [15]. Localized introduction of such growth factors has been demonstrated to greatly enhance bone tissue regeneration [16, 17]. Hence there is a therapeutic advantage to introducing growth factors from the surface of implants during the healing process.
The delivery of growth factors in vivo presents many complex challenges [18]. Growth factors, specifically rhBMP-2 and rhVEGF have been delivered therapeutically for bone regeneration applications using a variety of methods, including bolus injection [19], biodegradable polymers and hydrogels [7, 20, 21] with mixed results [22]. In cases where beneficial effects were observed, large doses of the growth factors were required (from 12 μg up to 60 mg of growth factor per gram of carrier). This raises concerns regarding the safety, cost and effective delivery of these supraphysiological amounts of recombinant proteins. Control over the amount of growth factor delivered is critical in bone tissue healing applications, where an excess of rhBMP-2 may lead to undesirable incidences of hematoma, ectopic bone formation and osteoclast activation with transiently elevated bone resorption [23]. From a delivery perspective, traditional polymer formulation techniques also have the potential to denature the protein molecules at high temperature, by exposure to unfavorable solvents or through the development of a low pH environment [24]. In addition, these systems are not easily coated onto complex geometries of implants in a conformal manner. Direct injection of growth factor to a fracture site would lead to the immediate breakdown and resorption of the protein before it could begin to exhibit desired results as the growth factor must be sustained at a minimal dose level over prolonged time periods of several weeks to be effective [25]. The ratio and timing of rhBMP-2 and rhVEGF delivered may be critical to applications involving bone tissue integration [3]; beneficial effects have been observed when rhVEGF is present in quantities of half or less than rhBMP during the first few days or weeks [26], i.e. the consolidation phase, and may even be introduced prior to the introduction of rhBMP-2 [27]. rhVEGF dosage must be tightly controlled, as excess amounts of rhVEGF from extended release can actually inhibit osteogenesis, cause severe vascular leakage and hypotension [28].
Towards addressing a lack of delivery systems where tunable control is necessary for the delivery of these potent growth factors [29], our primary goal was to develop a thin hydrolytically degradable coating that would incorporate controlled amounts of both rhVEGF and rhBMP-2, releasing them over varying periods of time with a corresponding dose dependent effect on endothelial and preosteoblast cells respectively. A suitable delivery platform would exhibit a quick release of rhVEGF and a more sustained release of rhBMP-2. Polyelectrolyte multilayer films fabricated using the layer-by-layer (LbL) assembly [30], in which a charged substrate was alternately dipped in positively and negatively charged polymer baths to build nanolayered thin films has been explored as a means to successfully release various drugs and small molecules from surfaces as well as developing LbL coatings for tissue regeneration by our group [31-35] as well as others [36-41]. The LbL assembly method is unique in its ability to sequester desired concentrations of biologic drugs for controlled local delivery using room temperature and mild aqueous conditions that preserve fragile protein activity. Orthopedic implant surfaces are a compelling application as the polyelectrolyte multilayer approach can have direct and immediate benefit in this area due to the need for sequential and controlled delivery of several therapeutic systems. Introduction of more than one growth factor in vivo would initiate the different cellular cascade necessary for robust implant integration using a powerful LbL approach to generate blends of dual growth factors that can yield idealized release behavior.
2. Materials and Methods
2.1. Materials
Poly (β-aminoester) 2, hereafter called Poly2, was synthesized as previously described [42]. Poly(acrylic acid)(PAA, 306215, Mν = 1.25MDa), 3M concentrated sodium acetate buffer (S7899, pH 5.2) and penicillin/ streptomycin solution were purchased from Sigma Aldrich (St.Louis, MO). Recombinant human BMP-2 (rhBMP-2, 4577) and recombinant human vascular endothelial growth factor C (rhVEGF-C, 165 amino acids excluding His tags at the end, 4633) was obtained from Biovision Inc. (South San Francisco, CA). rhBMP-2 ELISA kits were obtained from Peprotech Inc. (Rocky Hill, NJ). 10x Phosphate buffer Saline (PBS) was obtained from Invitrogen (Carlsbad, CA). Chondroitin sulfate sodium salt (Mn = 60000) was obtained from VWR Scientific (Edison NJ).
2.2. Preparation of polyelectrolyte solutions
Poly2 dipping solutions were 2 mg/mL in 25 mM sodium acetate buffer. Dipping solutions containing PAA and chondroitin sulfate were prepared at 2 mg/mL in 100mM sodium acetate buffer and pH-adjusted to 5.0 with 1.0M sodium hydroxide solution. rhBMP-2 and rhVEGF165 dipping solutions were 40 μg/ml in 100 mM sodium acetate buffer. Poly2, PAA and chondroitin sulfate dipping solutions were replaced every 24 hours. rhBMP-2 and rhVEGF165 dipping solutions were replenished every 24 hours by adding 40 μg/mL of growth factor to their respective pre-existing solutions. All solutions were prepared with water from a Milli-Q Plus unit (Bedford, MA) at 18.2 MΩ.
2.3. Polyelectrolyte multilayer film construction
Integra-ls® (Plainsboro, NJ) macroporous polycaprolactone/β-tricalcium phosphate (50wt% PCL/50wt% β-TCP) waffle cylinder scaffold, with diameter = 10mm and height = 2.5mm were cut in half using a razor blade and plasma etched with room air using a Harrick PDC-32G plasma cleaner on high RF power for 1 min and immediately immersed in Poly2 solution. First, rhBMP-2 nanolayered films were fabricated on this scaffold with a Carl Zeiss HMS programmable slide stainer with the following dipping protocol: 5 min in Poly2 solution, 3 washes (10, 20, 30 s), 5 min in PAA solution, 3 washes (10, 20, 30 s), 10 min in rhBMP-2 solution, 1 wash (10 s) and 5 min in PAA solution with 3 washes (10, 20, 30 s). This cycle was repeated to achieve 40, 80, 100 and 120 tetralayer films. Dipped scaffolds were allowed to dry for 48 hours at 4°C. Next, rhVEGF165 nanolayered films were then fabricated on the scaffolds using the same dipping protocol to achieve 20, 30, 40, 50 and 80 tetralayer films with the rhBMP-2 dipping solution swapped for the rhVEGF165 dipping solution and the PAA replaced with chondroitin sulfate. Concurrently, controls with single growth factor films were also fabricated. The final product was stored at 4°C until released or implanted.
2.4. Release characterization
Growth factor coated film coated scaffolds were released at 37°C into 1 mL of 1x PBS in 1.7 ml centrifuge tubes (VWR Scientific, Edison, NJ). At a series of pre-determined time points, scaffolds were transferred into new centrifuge tubes containing fresh 1ml 1x PBS. The previous centrifuge tubes along with the 1x PBS, were frozen down and stored at −20°C. Samples were analyzed using ELISA development kits according to manufacturer instructions. Release samples from consecutive time points were then combined and used in cellular assays (see below).
2.5. Cell culture
To test the efficacy of the growth factors released from the LbL films, a number of in vitro tests were performed to quantify and visualize the effect of growth factor on a cell line with a physiological response to the growth factor. rhBMP-2 initiates the differentiation of pre-osteoblast cell line MC3T3-E1 into bone. rhVEGF165 induces proliferation and migration in HUVEC’s. ). Each condition was repeat 3 times in triplicate (n=3).
2.5.1 rhBMP-2 activity assays using MC3T3-E1 pre-osteoblast cell line
MC3T3-E1 Subclone 4 cells were grown in α-MEM, supplemented with 10% FBS, and 1% of antibiotic and anti mycotic solution (containing penicillin, streptomycin and amphotericin B) in a humidified incubator (37°C; 5% CO2 in air). Culture medium was replenished every 2-3 days and cells were sub cultured when near 100% confluence with the use of 0.05% trypsin-EDTA. All cells used in these studies were less than passage number 12. MC3T3-E1 cells were seeded at 10,000 cells/well and allowed to grow to confluency in 24 well plates (visualization and quantification) in 0.5 ml of growth medium. Cells were cultured under different experimental conditions: (1) growth medium, (2) differentiation medium (growth medium supplemented with 50 mg/ml L-ascorbic acid and 10 mM b-glycerol phosphate), (3) differentiation medium supplemented with PEM released solution containing rhBMP-2 and rhVEGF165. Release samples from consecutive days were combined and the cell culture media was changed every two days until analysis for alkaline phosphatase or alizarin red as described below.
Alkaline phosphatase activity assay
Alkaline phosphatase (ALP) activity was determined on day 5 after the initiation of MC3T3-E1 osteogenic differentation by quantitation of the enzyme activity. Cells were rinsed 2x with PBS without Ca2+ and Mg2+ and fixed in 8% paraformaldehyde for 10 min at room temperature. 0.1% triton in PBS was added to the fixed cells and then frozen at 80°C for one freeze-thaw cycle. Cell lysates were transferred to an eppendorf tube, centrifuged at 15,000g for 3 min at 4°C, and the supernatant was collected. 50 μl of lysate was incubated with 150 μL of pNPP solution for 30 min at 37°C. The reaction was terminated with 0.1 M NaOH and ALP activity read on a plate reader at 405 nm. The ALP activity measurements were normalized to total protein determined by BCA assay.
Alizarin red S differentiation assays
After 14 days of exposure to different formulations of the release medium, MC3T3-E1 cells were assayed for calcium deposition using Alizarin red S (ARS). Cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min. After three rinses of 5 min in distilled water, ARS stain solution (2% ARS in distilled water pH balanced to 4.1 with 10% ammonium hydroxide) was incubated with cells for 20 min at room temperature. Cells were then washed in distilled water 4 times for 5 min each. The ARS stained cultures were imaged with phase contrast microscopy. The ARS stain was quantified using a previously published protocol [43]. The ARS stained cultures were incubated in 10% acetic acid for 30 min at room temperature and then the cell layers were disrupted by the use a pipette tip. The cell suspensions were transferred to a microcentrifuge tube, vortexed for 30 s, paraffin wrapped and heated at 85 °C for 10 min. After transferring to ice for 5 min, the tubes were centrifuged at 16,000g for 15 min and pH balanced with 10% ammonium hydroxide to pH 4.1-4.5. Triplicates with growth and differentiation medium controls were read on a 96-well plate with black sides and a clear bottom at 405 nm.
2.5.2 rhVEGF165 activity assays using Human Umbilical Vein Endothelial Cells (HUVEC) cell line
Release media was pooled and diluted 1:1 with HUVEC media (without rhVEGF). The normalized rhVEGF165 dose per milligram of scaffold was calculated, aliquoted and topped up with HUVEC media (without VEGF) for a total volume of 300 μl per well for proliferation assays and 2.5ml per well for migration assays.
HUVEC proliferation assay
1000 HUVEC’s were suspended in 300 μl of growth media and dispensed in each well of a 96 well plate (quantification) in 0.3 ml of growth medium. Cells were allowed to establish for 24 hours. HUVEC growth media was carefully removed using a pipettor without disturbing the cell layer. The combination of release and HUVEC media, was then added to the wells. Cells were allowed to proliferate for 48 hours and labeled with 10 μM bromodeoxyuridine (BrdU) kit for another 2 hours. Incorporation of BrdU into DNA was detected using an anti-BrdU peroxidase-conjugated antibody and a soluble peroxidase substrate (3,3′-5,5′-tetramethylbenzidine). The absorbance was measured at 450 nm with a 595 nm reference wavelength according to the protocol for BrdU Proliferation Assay, (Roche Diagnostics, Mannheim, Germany).
HUVEC migration assay
Confluent HUVEC monolayers in 24 well plates (Corning/Costar, Cambridge, MA) were “scratch” wounded using the tip of a universal blue pipette tip and rinsed with PBS. The HUVEC/Release media cocktail from different experimental conditions was pipetted into the plate. Cells were imaged under phase contrast microscope (Axiovert® 200 inverted microscope, Carl Zeiss, NY) immediately after adding media. HUVEC media with lacking rhVEGF was used as the control. Cells were incubated for 8 hours and the distance migrated by the cells. Cells were labeled with 1.5 μm Calcein AM Fluorescent Dye (354217, BD Biosciences, CA) for 30 min. The media and dye were aspirated and washed thrice with 1x PBS. Cells were then fixed with 8% paraformaldehyde and imaged under fluorescence (485 nm excitation 530 nm emission) at 20x magnification. The size of the scratch before and after 8 hours was measured using Axiovision® 4.5 software and the difference computed as the distance migrated by the cells.
2.5.3 In vivo evaluation of LbL films
All animal work was performed in accordance with protocols approved by the Committee on Animal Care at the Massachusetts Institute of Technology. A set of eight 100 tetralayer rhBMP-2 films (corresponding to a 6μg total scaffold load), eight 100 tetralayer rhBMP-2 films with an 80 tetralayer rhVEGF165 film on top (corresponding to a 6μg rhBMP-2 and 4μg rhVEGF165 total scaffold load) and eight 100 tetralayer Poly2/PAA bilayer films were built as implants. Sixteen 350-400 g male Sprague Dawley rats were given preoperative analgesics (1 mg/kg meloxicam and 0.05 mg/kg buprenex), and intraoperative anesthesia via 1-3% isofurane in oxygen. The right hind limb of each animal was shaved, cleaned with alcohol and povidone iodine solutions, and sterile drapes were placed around the surgical area. A 2 cm longitudinal incision, centered at the midshaft of the femur, was made laterally along the hind limb. A pocket made in the quadriceps muscle mass by blunt dissection anterior to the iliotibial band. LbL-coated PCL/βTCP scaffold was inserted into the intramuscular pocket, and wounds were closed progressively with three layers of sutures (muscle, subcutaneous, and dermal layers). Each hindlimb was implanted with either a control scaffold [P2/PAA]100, a rhBMP-2 scaffold [P2/PAA/rhBMP-2/PAA]100 or a scaffold with rhBMP-2/rhVEGF165 combination film [Poly2/PAA/rhBMP-2/PAA]100[Poly2/Chondroitin sulfate/rhVEGF165/Chondroitin sulfate]80 Postoperatively, rats were treated with buprenex and meloxicam until signs of distress dissipated. Rats were housed in separate cages with free access to food and water. At four and eight week time points, four rats from each group were sacrificed and the implants retrieved for analysis as described below.
Micro-computed tomography (MicroCT) analysis
Excised samples were immediately placed in 10% neutral buffered formalin and imaged with MicroCT (eXplore Locus, GE Medical Systems, London, Ontario) at a resolution of 27 μm with proprietary software included with the system (EVS Evolver, GE Medical Systems, Fairfield, CT). The scanning protocol was performed with a 2000 ms shutter speed, 1 x 1 binning, at X-ray tube parameters 80 kV and 450 mA. Four hundred images were taken at incremental angles, and rendered 3D images were reconstructed with the Reconstruction Utility and analyzed using MicroView (GE Healthcare, Fairfield, CT). Threshold values were chosen by visual inspection and kept constant across 1 month or 2 month data sets. Three independent regions of interest (ROIs) were chosen per sample, and the bone analysis tool was used to measure bone mineral density and stereology measurements of each sample. Three dimensional representations of bone formation and two dimensional digital slices through the samples were also taken for qualitative comparison. Each sample was measured in triplicate for each set of data.
Histological analysis
After 24 h in 10% formalin, tissues were transferred to solutions of 70% ethanol prior to decalcification. Tissues were decalcified for 5 days in a solution of 15% EDTA and 10% sodium citrate buffer, pH 7.2 at 4°C under continuous stirring. Tissues were then serially sectioned, routinely processed, and embedded in paraffin. Microscope sections (4 μm) were stained with hematoxylin and eosin (H&E), Alcian Blue, and Masson’s trichrome stains.
3. Results and Discussion
3.1 Fabricating a dual growth factor releasing layer-by-layer system
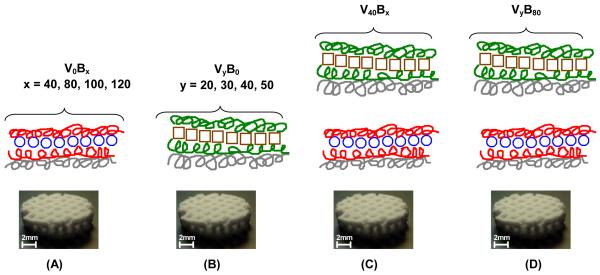
Both rhBMP-2 and rhVEGF165 are homodimeric proteins which have a similar pI of about 8.5; the non-glycosylated forms have a molecular weight of about 30 kDa and 38.2 kDa respectively. Under conditions of acidic and physiological pH, both proteins are expected to be positively charged. Hence, for the layer-by-layer film assembly, we used a tetralayer architecture with 1) Poly2, a synthetic poly(β-aminoester); 2) a biocompatible polyanion (PAA or Chondroitin sulfate); 3) a growth factor (either rhBMP-2 of rhVEGF165) and 4) a polyanion for charge reversal (Fig. 1.).
Fig. 1.
Fabrication of polyelectrolyte multilayer films using the layer-by-layer method in a tetralayer architechture with [Poly2(+)/polyanion(−)/growth factor(+)/polyanion(−)] depicted below. Four different groups were fabricated. For single growth factor films (A) rhBMP-2 PEM films with architecture [Poly2/PAA/rhBMP-2/PAA]x, with x = 40, 80, 100, 120 and (B) rhVEGF165 PEM films with architecture [Poly2/CS/rhVEGF165/CS]y, where y = 20, 30, 40, 50. For dual growth factor films, (C) y = 40 was constant and x = 40, 80, 100, 120. (D) In another formulation, x = 80 was constant and y = 20, 30, 40, 50. The substrate was always a macroporous polycaprolactone/β-tricalcium phosphate (50wt% PCL/50wt% β-TCP) waffle cylinder scaffold, with diameter = 10mm and height = 2.5mm.
In order to successfully release growth factor under physiological conditions, we chose Poly2 which is a hydrolytically degradable member of the poly (β-aminoester) (PBAE) family, which has been extensively studied in gene therapy [44] applications and used for PEM fabrication for various delivery applications from multilayer films with appropriate architectures [45]. Under neutral to acidic pH conditions of film fabrication, Poly2 is stable and the amines present along the backbone of Poly2 are protonated, yielding the positive charge necessary for electrostatic LbL systems. We anticipated that films constructed with PAA as the alternating polyanion would yield more highly ionically crosslinked films relative to chondroitin sulfate, as PAA exhibits a higher charge density. Such films should result in a more sustained release profile of rhBMP-2. An important phase of wound healing involves the secretion of glycosaminoglycans by fibroblast cells to form a hydrophilic matrix suitable for remodeling while healing. Chondroitin sulfate has been extensively investigated for wound dressing applications [46] due to its biocompatibility, non-immunogenicity and pliability. Thus it was the polyanion of choice for fabricating the rhVEGF165 PEM films. Films with tetralayer repeat units were fabricated, where the tetralayers containing rhBMP-2 were adsorbed first, and rhVEGF165 layers were adsorbed on top (Figure 1). We anticipated releasing growth factor from the surface of implants, where more rhVEGF165 would be released initially for stimulating proliferation of blood vessels in vivo, followed by more rhBMP-2, which would initiate the differentiation cascade leading to the formation of new bone.
Hydrogel based dual growth factor releasing systems have been reported [7], where components are fabricated separately and do not offer the convenience of conformal coating. Here, LbL was used to conformally coat the complex geometries of an osteoconductive macroporous polycaprolactone/β-tricalcium phosphate (50wt% PCL/50wt% β-TCP) waffle cylinder scaffold, with diameter = 10mm and height = 2.5mm. The three dimensional, porous scaffold was used for in vitro and in vivo testing as a model implant (Figure 1.). The choice of scaffold was governed by the following factors: (i) PCL is a biologically inert, biocompatible FDA approved material, (ii) βTCP is an osteoconductive material and typically both an osteoinductive agent, such as rhBMP-2, and an osteoconductive microenvironment are necessary for ectopic bone formation (iii) the total drug load scales with film surface area, allowing for a greater load per volume in a macroporous three dimensional substrate compared with a flat film [35].
3.2 In vitro kinetics of dual growth factor releasing films
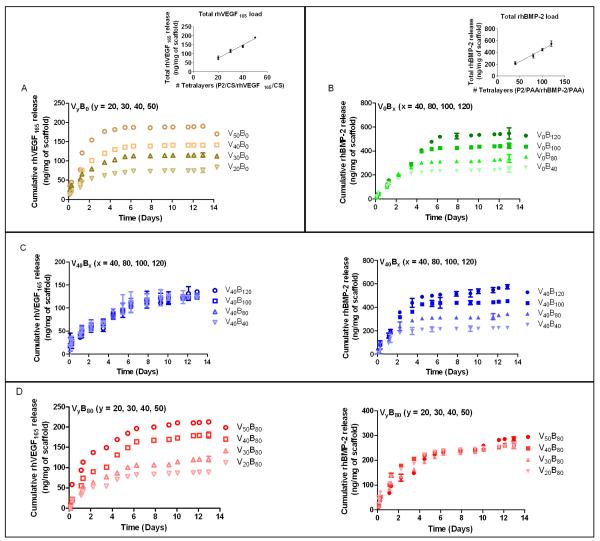
While the precise growth factor dose required would depend on the specific orthopedic application, [Poly2/PAA/rhBMP-2/PAA]x[Poly2/Chondroitin sulfate/rhVEGF165/Chondroitin sulfate]y films were constructed with the aim of demonstrating that this system could be tuned for specific applications involving bone regeneration and that the released growth factors demonstrate a dose response behavior. Release from the film was normalized to 1 milligram of scaffold weight, to provide a basis for comparison.
We tuned our system to demonstrate a control over total growth factor loading. Release of growth factors from the film occurs with a pH shift from pH 5.0 at dipping conditions to physiological pH 7.4 in 1x PBS, due to a combination of destabilization and charge imbalance in the films and from hydrolytic degradation of the Poly-2 backbone. Approximately one-third of the rhBMP-2 and two-thirds of the rhVEGF165 was released after three days. While both growth factors begin release immediately upon immersion, the release profile for rhBMP-2 indicates that it takes a longer time to elute completely from the film than rhVEGF165. The amount of growth factor released from single growth factor films (Fig. 2. A, B) and dual growth factor films (Fig. 2. C, D, E, F) is comparable, and in all cases, indicate that amounts of growth factor for each component can be independently tuned to yield a clear increase in loading with number of layers. This is an important capability for delivery film design that is not always readily attained with multilayer formulations; it indicates that the growth factor does not leach out of the film by diffusion into the dipping solution when a different growth factor is introduced. Electrostatic interactions between the growth factor and surrounding polymer chains in the film prevent it from diffusing out or being exchanged out during film assembly. Loading of rhBMP-2 and rhVEGF scaled linearly with tetralayer number with 4.50 ± 0.14 ng (R2 165 = 0.93) and 3.72 ± 0.080 ng (R2 = 0.96) of rhBMP-2 and rhVEGF165 respectively per tetralayer per milligram of scaffold (n = 3). In these films, total rhBMP-2 and total rhVEGF165 incorporation ranged from 250 and 600 ng/mg scaffold weight and 80 to 200 ng/mg scaffold weight, respectively, as a function of number of layers. A broad range of protein incorporation in films has been previously reported from 2D substrates, from 5 ng/cm2 upto 650 μg/cm2 [34, 47]. The release profiles in Figure 2 are characteristic of surface erosion behavior. The release profile in Fig. 2A indicates gradual linear release as the rhBMP-2 is completely eluted. We would expect the film would undergo bulk erosion if the water permeates into the LbL films faster than the rate of hydrolytic degradation of Poly2 and surface erosion if the rate of permeation is slower. The dominance of surface erosion is due to the prolonged half-life of Poly2 conferred by the presence of a hydrophobic region near the ester bond and a consequent decrease in water attack on the bond, hence a slower degradation rate of the polymer. Our previous work has demonstrated that a related PBAE (Poly1), with a shorter alkyl chain near the ester bonds, releases a comparable cargo much faster [34]. In case of the single and dual growth factor releasing films, rhBMP-2 release is consistent across the different groups (x = 40, 80, 100, 120) at 27.4 ± 8.38% per day averaged over the first 2 days and 5.3 ± 0.34% per day until the end of release. In single growth factor rhVEGF165 films, release is consistent across the different groups (y = 20, 30, 40, 50), at 20 ± 0.07% per day averaged over the first 5 days, after which there is no detectable release. For dual growth factor films, it is interesting to note that the amount of rhVEGF165 released drops to 15 ± 1.42% per day averaged over the first 5 days and 3.05 ± 0.96% per day until the end of release. It is likely that interdiffusion occurs with both growth factors during film assembly, leading to a mixing of BMP-2 and VEGF with in the film matrix, although a gradient of BMP-2 may still remain in the bottom layers of the film, leading to more extended release. Interdiffusion of components in polyelectrolyte multilayers has been reported for similar systems [48, 49].
Fig. 2.
Polyelectrolyte multilayer films were dipped in a Poly2/anion/growth factor/anion tetralayer repeat architecture on a macroporous polycaprolactone/β-tricalcium phosphate (50wt% PCL/50wt% β-TCP) waffle cylinder scaffold. Growth factor loading was controlled by varying the number of layers dipped on the scaffold. rhBMP-2 released over a period of about 2 weeks, whereas rhVEGF165 eluted completely within 8 days. 4 groups of films were fabricated and incubated in 1x PBS at pH 7.4 over 12 days to study growth factor release from the films. Growth factor release was quantified using ELISA and normalized per milligram of scaffold. Samples were in triplicate and the error bar is the standard deviation. Release from (A) Single growth factor rhVEGF165 release and (inset) total rhVEGF165 loading scales linearly with tetralayer number (R2 = 0.96). (B) Single growth factor rhBMP-2 release and (inset) total rhBMP-2
3.3 In vitro rhBMP-2 activity assay
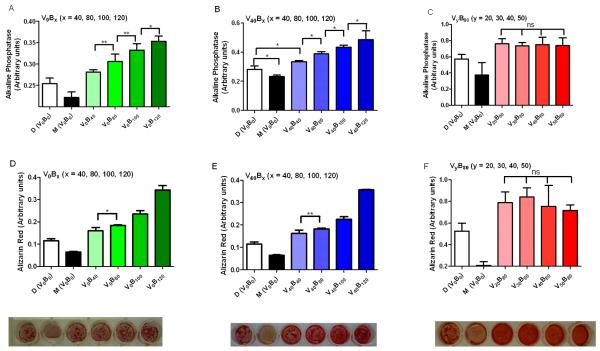
To study the rhBMP-2 dose dependent behavior of MC3T3-E1 cells in vitro, we exposed the cells to different formulations – growth media, differentiation media (growth media supplement with L-ascorbic acid and β-glycerolphosphate) and release media with the rhBMP-2 load normalized to 1 milligram of scaffold weight. Alkaline phosphatase (AP) serves as a standard early marker of induction of new bone differentiation from progenitor cells. In Figure 3A and 3B, the AP signal is dose dependent to the amount of rhBMP-2 exposed to the cells (p<0.05). In Figure 3C, AP signal is the same across the different formulations, as expected as the amount of rhBMP-2 in the formulation is the same. Alizarin red (AR) stains for calcium deposits and allows for its visualization and quantification. AR staining was performed 14 days after exposure to the different formulations. In Figures 3D, 3E, 3F, staining of Alizarin Red confirms osteoblast differentiation to a mature bone phenotype from rhBMP-2 exposure. Staining was quantified and observed to be dose dependent when the dose of rhBMP-2 was varied (Figures 3D, 3E) (p<0.05) and the intensity of the stain was the same when the rhBMP-2 dose was constant (Figure 3F). Interestingly, the staining was more diffuse when rhVEGF165 and chondroitin sulfate was part of the formulation (Fig. 3E, 3F). We have observed in our previous work [35] that the presence of chondroitin sulfate, a glycosaminoglycan, allows for a more even distribution of rhBMP-2 in culture resulting in a more diffuse stain. In all cases, the formulations significantly outperformed the differentiation induced by the growth and differentiation media.
Fig. 3.
Pre - osteoblast differentiation assay. Activity of rhBMP-2 released from the polyelectrolyte multilayer films is preserved. MC3T3-E1 cells were exposed to rhBMP-2 released from multilayer films, with the dose normalized per milligram of scaffold weight. Alkaline Phosphatase Assay demonstrates dose dependent early activation of bone differentiation cascade at Day 5. After 21 days of culture, Alizarin Red Stain confirms the presence of calcium deposits laid down during the differentiation process. Visual inspection of cultures after Alizarin red staining confirms a dose dependent presence of calcium deposits. (A, B and C) Alkaline phosphatase assay at day 3 on cells differentiated with different release formulations as depicted. (D, E and F) Alizarin red assay at day 14 on cells differentiated with different release formulation as depicted. Representative images of the alizarin red stain are below the bar graph in (D, E, F). A single factor ANOVA test allowed rejection of the null hypothesis for both assays; and a Tukey test between different groups was performed (s.d., n=9, ** p < 0.01; * p < 0.05; ns = not significant; all others p < 0.001).
3.4 In vitro rhVEGF165 activity assay
HUVEC’s have been shown to respond to rhVEGF165 concentration in a dose dependent manner at low concentrations of rhVEGF165. Complimentary to these findings, we demonstrated that the rhVEGF165 released from the LbL films was active and induced dose dependent proliferation and migration of HUVEC’s.
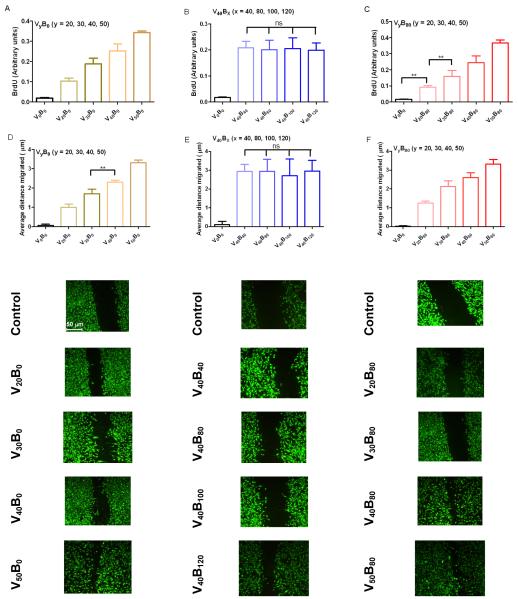
Proliferation of HUVEC’s was measured using BrdU, a synthetic nucleoside that is an analogue of thymidine and can be incorporated into the newly synthesized DNA of replicating cells substituting for thymidine during DNA replication. Cells were seeded at a low density to prevent wells from reaching confluency within the period of this study. Cells exposed to formulations were the rhVEGF165 load was changed demonstrated a dose response behavior which was statistically significant (Figure 4A, 4B) (p < 0.05). Dose response to linear increases in the number of tetralayers was also linear (R2 = 0.99) which is consistent with observations elsewhere [50]. The degree of change in proliferation was statistically insignificant when the rhVEGF165 load was kept constant (Figure 4C) (p>0.05).
Fig. 4.
Proliferation and Migration assays. Activity of rhVEGF165 released from the polyelectrolyte multilayer films is preserved. HUVEC cells exposed to rhVEGF165 dose normalized per milligram of scaffold weight exhibited increased proliferation activity, as measured by BrdU. (A) 40, 80, 100 and 120 tetralayers of rhBMP-2 followed by 40 tetralayers of rhVEGF165. (B) 40 tetralayers of rhBMP-2 followed by 20, 30, 40 and 50 tetralayers of rhVEGF165 and (C) 20, 30, 40 and 50 tetralayers of rhVEGF165. Closure of uniform “scratched” wound gap was monitored in HUVEC cell cultures over a period of 8 hours. Cells were fixed and imaged at 20x magnification (D) 40, 80, 100 and 120 tetralayers of rhBMP-2 followed by 40 tetralayers of rhVEGF165. (E) 40 tetralayers of rhBMP-2 followed by 20, 30, 40 and 50 tetralayers of rhVEGF165 and (F) 20, 30, 40 and 50 tetralayers of rhVEGF165. Calcein labeled cells demonstrate charateristics of migrating cells to close the scratch such as elongated cell bodies in all cases where released rhVEGF165 was part of the media. A single factor ANOVA test allowed rejection of the null hypothesis for both assays; and a Tukey test between different groups was performed (s.d., n=9, ** p < 0.01; * p < 0.05; ns = not significant; all others p < 0.001).
Migration of HUVEC’s was measured as the difference in the average size of the scratch made in a confluent monolayer of cells before and after incubating the cells with different formulations of release medium. Here too, a dose response behavior was observed with the scratch size decreasing linearly as a function of rhVEGF165 load (R2 = 0.99).
3.5 In vivo evaluation of LbL films using intramuscular bone formation model
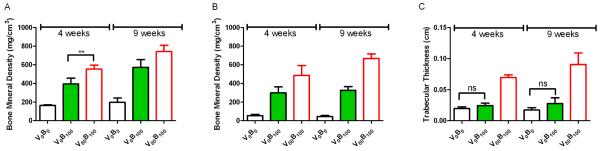
We demonstrated the efficacy of the LbL coated implants in vivo. Consistent with our previous work [35], the osteoconductive scaffold and the osteoinductive rhBMP-2 formed bone at an intramuscular ectopic site. Adding an angiogenic factor accelerated the maturation of ectopic bone. Microcomputed tomography (microCT) images at four and nine week time points showed large bone deposits created in response to rhBMP-2 LbL films. Growth factor coated implants (Figure 6) showed new plate-like bone trabeculae that progressively increased in thickness from four to nine weeks. Quantification of the bone mineral density (BMD) from microCT images demonstrated an increase of 28 ± 4.59% and 32 ± 2.73% at 4 and 9 weeks respectively (p < 0.001) for the scaffolds coated with rhBMP-2/rhVEGF165 over the scaffolds coated with just rhBMP-2. In contrast, no bone was formed in scaffolds coated with LbL films lacking growth factor.
Fig. 6.
(A) The bone mineral density (BMD) of ectopic bone formed by the rhBMP-2/rhVEGF165 combination scaffolds is 28 ± 4.59% and 32 ± 2.73% higher than bone formed by rhBMP-2 scaffold at 4 and 9 weeks respectively at the periphery of the scaffolds. (B) In the interior, the BMD for the combination growth factor scaffolds is higher than the single rhBMP-2 scaffolds indicating that more bone is present in the interior, which matures from 4 to 9 weeks as indicated by the increase in BMD. (C) The trabecular thickness of the bone formed is an important measure of bone maturation, and the trabecular thickness of the bone formed by the combination scaffolds is about 3 times and 4.5 times that of bone formed by rhBMP-2 scaffolds at 4 and 9 weeks. (s.d., n=9, ** p < 0.01; ns = not significant; all others p < 0.001)
The bone formation was restricted to the periphery of the scaffold when just rhBMP-2 was used. Over the initial 4 week time period, a greater amount of bone was present deep within scaffold when both rhBMP-2 and rhVEGF165 were released from the scaffold surface as measured by bone mineral density on the scaffold surface and the interior of the scaffold (Fig. 6A, B). Interior bone mineral density increased by 27 ± 7.1% in scaffolds with the combination growth factors, while the increase in BMP-2 eluting films was statistically insignificant. Trabecular thickness of the peripheral bone formed by the rhBMP-2/rhVEGF165 combination scaffolds, was found to be 3 fold and 4.5 fold greater than the bone formed by the BMP-2 scaffold at 4 and 9 weeks respectively (Fig. 6C).
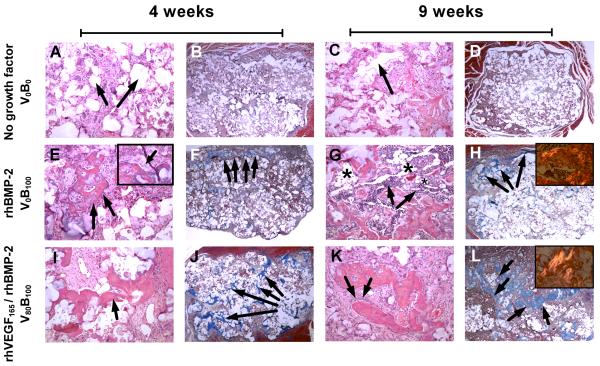
Histological sections of excised implants from the rat femur (Fig.7.) showed active bone formation and remodeling with osteocytes and cement lines where new bone is laid down by differentiating osteoblasts within scaffolds releasing either rhBMP-2 or the combination of rhVEGF165 and rhBMP-2 (Fig. 7E-7L). Bone marrow with maturing trilineage hematopoiesis was occasionally observed (Fig. 7G). Bone was deposited on the interior surface of the scaffold, and was restricted to the area of the implant (Fig 7F, 7H, 7J, 7L). This is important, as it demonstrates that the system was able to control bone formation at an ectopic site. At 4 weeks, spicules of trabecular bone were observed throughout the scaffold in combination growth factor releasing films, whereas the spicules were restricted to the periphery in single growth factor rhBMP-2 films. In both cases, mature lamellar bone was observed from 4 to 9 weeks as visualized by aligned collagen fibrils that are birefringent under polarized light. Interestingly, a large number of hematopoietic cells were observed around areas of mature lamellar bone at 9 weeks along with adipocytes, recapitulating the fatty marrow architecture and suggesting a high level of tissue maturation. Consistent with endochondral bone formation, a cartilage intermediate is visible in the bone tissue induced by growth factor PEM films (Fig. 7F, J) which is progressively replaced with bone which extends throughout the scaffold only when rhVEGF165 is present. The de novo recapitulation of a more complete bone architecture precipitated by PEM mediated release of dual growth factor rhVEGF165 and rhBMP-2 from the scaffold surface, suggests that there may be a greater initial concurrent vascularization process which mediates introduction of more cells in the interior on the scaffolds; none of the aforementioned biological processes is present in LbL scaffolds lacking BMP-2.
Fig. 7.
Histology sections from rat femur intramuscular implants. (A to D) Bone is absent in control scaffolds releasing no growth factors at 4 and 9 weeks verified by (A, C) H&E staining and (B, D) lack of brilliant blue collagen staining with Masson’s trichrome stain. (A, B) Arrows indicate pockets where the (PCL/βTCP) polymer scaffold is present at 4 weeks which degrades over (C, D) 9 weeks, without any bone growth in the vacant space. (E to H) Bone present in scaffolds releasing single growth factor rhBMP-2. (E) Presence of trabecular bone with (inset) osteoblasts laying down new bone which is (F) restricted to the periphery of the scaffold at 4 weeks. Of particular interest is (G) the abundant presence of hematopoietic cells surrounding the bone in spaces identified as fatty marrow space (asterisk), where (H) most of the bone formation is restricted to the periphery at 9 weeks and (inset) as bone matures, it goes from an unorganized collagen matrix structure to a lamellar structure with aligned collagen fibrils that are birefringent under polarized light. (I to L) Bone present in scaffolds releasing rhBMP-2 and rhVEGF165. (I) Spicules of trabecular bone which is present (J) throughout the scaffold as early as 4 weeks. (K) As osteoblasts lay down new bone, spicules mature into (L) bone that fills up the empty spaces throughout the scaffold as it degrades with (inset) a greater number of birefringent aligned collagen fibrils present than fibrils formed when single growth factor rhBMP-2 is released. Bone formed is always within the scaffold. A, C, E, G, I, K are hematoxylin and eosin (H&E) stains at 10× objective magnification. B, D, F, H, J, L are Masson’s trichrome stains at 2× objective magnification. Images in inset are taken at 20× objective magnification.
4. Conclusions
The ability to regulate the local availability of precise amounts of growth factors is a potent tool for bone tissue engineering where the differentiation of many stem cell types typically requires the action of several growth factors at distinct stages. The utility of rhBMP-2 and rhVEGF has been well documented in mediating interactions between host tissue and implant. We have presented polyelectrolyte multilayer films that are able to deliver independently tunable nanogram scale physiological amounts of active rhBMP-2 and rhVEGF over different timeframes and induce a corresponding dose response on physiologically responsive cell lines. It is important to note that the dose response behavior is restricted to micromolar scale quantities of growth factors, and we were able to demonstrate control at these levels using sustained release obtained with hydrolytic degradation. PEM films on an osteoconductive scaffold induced ectopic bone formation in the intramuscular region of a rat femur. The delivery of two growth factors - rhBMP-2 and rhVEGF - changed the microenvironment significantly compared to single growth factor rhBMP-2 such that the trabecular bone formed throughout the scaffold and was more continuous and matured faster compared to single growth factor BMP-2 delivery. The ease of fabrication of this system makes it highly flexible; it can be applied to a wide variety of tissue engineering applications, including PEM films to treat diabetic ulcers, lumbar spine fusion and fracture healing. These results are an important step toward developing a system capable of multiple biologic drug delivery in vivo, and offering successful and long lasting joint replacement wound healing and repair. Furthermore, the general demonstration of dual controlled growth factor release from a thin film coating platform is a significant advance for numerous applications from implant coatings to tissue engineering.
Fig. 5.
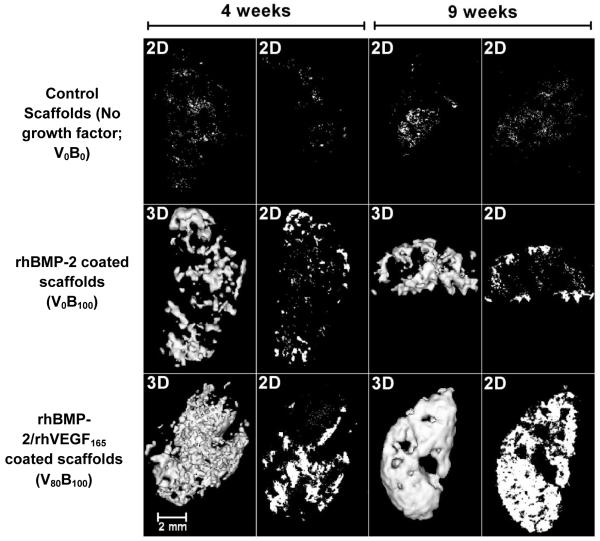
Two dimensional microCT scans (2D) and matched three dimensional reconstructions (3D) of excised PCL-βTCP half disc scaffolds, which were implanted in the intramuscular region of rats. Implants were coated with (i) no growth factor, (ii) 6 μg of single growth factor rhBMP-2 and (iii) 6 μg of single growth factor rhBMP-2 followed by 4μg of rhVEGF165. The amount of growth factor loaded was determined by fabricating triplicate companion copies along with the implanted scaffolds, releasing the growth factors in vitro and performing ELISA detection assays. (Top row) Control scaffolds without growth factors produce no detectable bone over the duration of the study. Low levels of backscatter is caused by the polymer. (Middle row) In single growth factor rhBMP-2 films lacking rhVEGF165, bone formation is restricted to the periphery of the scaffold at 4 weeks and 9 weeks. (Bottom row) As a result of increased vascularity, scaffolds releasing rhVEGF165 demonstrate a smooth, continuous profile in the ectopically formed bone which matures from 4 weeks to 9 weeks to fill the entire scaffold. In all the images, the bone formed takes the shape of the scaffold and grows inward when VEGF is present. Images are an isosurface rendering at 0.25 surface quality factor at a level threshold of 640, as defined by the proprietary Microview® software from GE Healthcare.
Acknowledgements
This work was funded by the National Institutes of Health, National Institute of Aging (5R01AG029601-04). The authors acknowledge the assistance provided by Catrina Wong and Alison Hayward, DVM (support staff, Division of Comparative Medicine, MIT) for care, handling and anesthesia of animals. The authors greatly appreciate the use of equipment available at the Institute for Soldier Nanotechnologies (ISN) and MRSEC Shared Facilities supported by the National Science Foundation (DMR-0213282) at MIT, and the support provided by the staff in the microscopy, histology and animal imaging core facilities at the Swanson Biotechnology Center in the David H. Koch Institute for Integrative Cancer Research supported by the National Institutes of Health, National Cancer Institute (2P30CA014051-39). The authors also acknowledge Integra Life Sciences (Plainsboro, NJ) and James Serdy (MIT) for providing the scaffolds used in this study.
Footnotes
5. Conflict of Interest The authors confirm that there are no known conflicts of interest associated with this publication.
References
- 1.Ince A, Rupp J, Frommelt L, Katzer A, Gille J, Lohr JF. Is “aseptic” loosening of the prosthetic cup after total hip replacement due to nonculturable bacterial pathogens in patients with low-grade infection? Clin Infect Dis. 2004;39(11):1599–1603. doi: 10.1086/425303. [DOI] [PubMed] [Google Scholar]
- 2.Harris WH, Sledge CB. Total hip and total knee replacement. N Engl J Med. 1990;323(11):725–731. doi: 10.1056/NEJM199009133231106. [DOI] [PubMed] [Google Scholar]
- 3.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr., Ferrara N, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84-A(6):1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 6.Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30(14):2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Sellers RS, Peluso D, Morris EA. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1997;79(10):1452–1463. doi: 10.2106/00004623-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Gerhart TN, Kirker-Head CA, Kriz MJ, Holtrop ME, Hennig GE, Hipp J, et al. Healing segmental femoral defects in sheep using recombinant human bone morphogenetic protein. Clin Orthop Relat Res. 1993;(293):317–326. [PubMed] [Google Scholar]
- 10.Uludag H, Gao TJ, Porter TJ, Friess W, Wozney JM. Delivery systems for BMPs: factors contributing to protein retention at an application site. J Bone Joint Surg Am. 2001;83A:S128–S135. [PubMed] [Google Scholar]
- 11.Jennissen HP. Accelerated and improved osteointegration of implants biocoated with bone morphogenetic protein 2 (BMP-2) Ann N Y Acad Sci. 2002;961:139–142. doi: 10.1111/j.1749-6632.2002.tb03067.x. [DOI] [PubMed] [Google Scholar]
- 12.Maus U, Andereya S, Gravius S, Ohnsorge JA, Niedhart C, Siebert CH. BMP-2 incorporated in a tricalcium phosphate bone substitute enhances bone remodeling in sheep. J Biomater Appl. 2008;22(6):559–576. doi: 10.1177/0885328207083311. [DOI] [PubMed] [Google Scholar]
- 13.Peng H, Usas A, Olshanski A, Ho AM, Gearhart B, Cooper GM, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20(11):2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 14.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 15.Samee M, Kasugai S, Kondo H, Ohya K, Shimokawa H, Kuroda S. Bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. J Pharmacol Sci. 2008;108(1):18–31. doi: 10.1254/jphs.08036fp. [DOI] [PubMed] [Google Scholar]
- 16.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21(24):2521–2527. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 17.Luginbuehl V, Meinel L, Merkle HP, Gander B. Localized delivery of growth factors for bone repair. Eur J Pharm Biopharm. 2004;58(2):197–208. doi: 10.1016/j.ejpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen HM, Andreassen TT, Ledet T, Oxlund H. Local injection of TGF-beta increases the strength of tibial fractures in the rat. Acta Orthop Scand. 1994;65(1):37–41. doi: 10.3109/17453679408993715. [DOI] [PubMed] [Google Scholar]
- 20.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21(5):513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 21.Saito N, Okada T, Horiuchi H, Ota H, Takahashi J, Murakami N, et al. Local bone formation by injection of recombinant human bone morphogenetic protein-2 contained in polymer carriers. Bone. 2003;32(4):381–386. doi: 10.1016/s8756-3282(03)00028-0. [DOI] [PubMed] [Google Scholar]
- 22.Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35(2):562–569. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Zara J, Siu RK, Zhang X, Shen J, Ngo R, Lee M, et al. High doses of BMP2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 2011;17(9-10) doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000;17(1):100–106. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 25.Jeon O, Song SJ, Yang HS, Bhang SH, Kang SW, Sung MA, et al. Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochem Biophys Res Commun. 2008;369(2):774–780. doi: 10.1016/j.bbrc.2008.02.099. [DOI] [PubMed] [Google Scholar]
- 26.Kokubu T, Hak DJ, Hazelwood SJ, Reddi AH. Development of an atrophic nonunion model and comparison to a closed healing fracture in rat femur. J Orthop Res. 2003;21(3):503–510. doi: 10.1016/S0736-0266(02)00209-7. [DOI] [PubMed] [Google Scholar]
- 27.Eckardt H, Bundgaard KG, Christensen KS, Lind M, Hansen ES, Hvid I. Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit tibia during distraction osteogenesis. J Orthop Res. 2003;21(2):335–340. doi: 10.1016/S0736-0266(02)00159-6. [DOI] [PubMed] [Google Scholar]
- 28.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12(6):295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Uludag H. Nanoparticulate systems for growth factor delivery. Pharm Res. 2009;26(7):1561–1580. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 30.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277:1232. [Google Scholar]
- 31.Moskowitz JS, Blaisse MR, Samuel RE, Hsu H-P, Harris MB, Martin SD, et al. The effectiveness of the controlled release of gentamicin from polyelectrolyte multilayers in the treatment of staphylococcus aureus infection in a rabbit bone model. Biomaterials. 2010;31:6019–6030. doi: 10.1016/j.biomaterials.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim BS, Smith RC, Poon Z, Hammond PT. MAD (multiagent delivery) Nanolayer: delivering multiple therapeutics from hierarchically assembled surface coatings. Langmuir. 2009;25(24):14086–14092. doi: 10.1021/la9017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shukla A, Fleming KE, Chuang HF, Chau TM, Loose CR, Stephanopoulos GN, et al. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials. 2010;31(8):2348–2357. doi: 10.1016/j.biomaterials.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald ML, Rodriguez NM, Shah NJ, Hammond PT. Characterization of tunable FGF-2 releasing polyelectrolyte multilayers. Biomacromolecules. 2010;11(8):2053–2059. doi: 10.1021/bm100413w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macdonald ML, Samuel RE, Shah NJ, Padera RF, Beben YM, Hammond PT. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials. 2011;32(5):1446–1453. doi: 10.1016/j.biomaterials.2010.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zelikin AN. Drug releasing polymer thin films: new era of surface-mediated drug delivery. ACS Nano. 2010;4(5):2494–2509. doi: 10.1021/nn100634r. [DOI] [PubMed] [Google Scholar]
- 37.Facca S, Cortez C, Mendoza-Palomares C, Messadeq N, Dierich A, Johnston APR, et al. Active multilayered capsules for in vivo bone formation. Proc Natl Acad Sci U S A. 2010;107(8):3406–3411. doi: 10.1073/pnas.0908531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dierich A, Guen E Le, Messaddeq N, Stoltz JF, Netter P, Schaaf P, et al. Bone formation mediated by synergy-acting growth factors embedded in a polyelectrolyte multilayer film. Adv Mat. 2007 Mar;19(5):693–697. [Google Scholar]
- 39.Pavlukhina S, Lu Y, Patimetha A, Libera M, Sukhishvili S. Polymer multilayers with pH-triggered release of antibacterial agents. Biomacromolecules. 2010;11(12):3448–3456. doi: 10.1021/bm100975w. [DOI] [PubMed] [Google Scholar]
- 40.Ai H, Jones SA, Lvov YM. Biomedical applications of electrostatic layer-by-layer nano-assembly of polymers, enzymes, and nanoparticles. Cell Biochem Biophys. 2003;39(1):23–43. doi: 10.1385/CBB:39:1:23. [DOI] [PubMed] [Google Scholar]
- 41.Kotov NA, Tang ZY, Wang Y, Podsiadlo P. Biomedical applications of layer-by-layer assembly: from biomimetics to tissue engineering. Adv Mat. 2006;18(24):3203–3224. [Google Scholar]
- 42.Lynn DM, Langer R. Degradable poly(beta-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. Journal of the American Chemical Society. 2000;122:10761–10768. [Google Scholar]
- 43.Gregory CA, Gunn WG, Peister A, Prockop DJ. An alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329(1):77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Chua LS, Lynn DM. Multilayered Thin Films that Sustain the Release of Functional DNA under Physiological Conditions. Langmuir. 2004;20(19):8015–8021. doi: 10.1021/la048888i. [DOI] [PubMed] [Google Scholar]
- 45.Wood KC, Chuang HF, Batten RD, Lynn DM, Hammond PT. Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer-by-layer thin films. Proc Natl Acad Sci U S A. 2006;103(27):10207–10212. doi: 10.1073/pnas.0602884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirker KR, Luo Y, Nielson JH, Shelby J, Prestwich GD. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials. 2002;23(17):3661–3671. doi: 10.1016/s0142-9612(02)00100-x. [DOI] [PubMed] [Google Scholar]
- 47.Macdonald M, Rodriguez NM, Smith R, Hammond PT. Release of a model protein from biodegradable self assembled films for surface delivery applications. J Control Release. 2008;131:228–234. doi: 10.1016/j.jconrel.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shukla A, Avadhany SN, Fang JC, Hammond PT. Tunable vancomycin releasing surfaces for biomedical applications. Small. 2010;6(21):2392–2404. doi: 10.1002/smll.201001150. [DOI] [PubMed] [Google Scholar]
- 49.Zacharia NS, Modestino M, Hammond PT. Factors influencing the interdiffusion of weak polycations in multilayers. Macromolecules. 2007;40(26):9523–9528. [Google Scholar]
- 50.Myoken Y, Kayada Y, Okamoto T, Kan M, Sato GH, Sato JD. Vascular endothelial cell growth factor (VEGF) produced by A-431 human epidermoid carcinoma cells and identification of VEGF membrane binding sites. Proc Natl Acad Sci U S A. 1991;88(13):5819–5823. doi: 10.1073/pnas.88.13.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]