Abstract
Mechanisms to explain tumor cell resistance to drugs that target the microtubule cytoskeleton have relied on the assumption that the drugs act either to suppress microtubule dynamics or to perturb the balance between assembled and nonassembled tubulin. Recently, however, it was found that these drugs also alter the stability of microtubule attachment to centrosomes, and do so at the same concentrations that are needed to inhibit cell division. Based on this new information, a new model is presented that explains resistance resulting from a variety of molecular changes that have been reported in the literature. The improved understanding of drug action and resistance has important implications for chemotherapy with these agents.
Keywords: drug resistance, tubulin, mitosis, cell division, paclitaxel, colchicine, vinblastine, chemotherapy, cancer, microtubule detachment, microtubule dynamics, cell motility
Drugs that target the microtubule cytoskeleton play a major role in the treatment of cancer. Vinca alkaloids like vinblastine and vincristine have been used since the 1960’s and remain major drugs for the treatment of leukemia, lymphoma, and a variety of other diseases. More recently, taxanes such as paclitaxel and docetaxel have shown strong activity in treating tumors in breast, ovaries, lung, and other tissues. Like most chemotherapeutic agents, these compounds are administered at high concentrations that correspond to their maximum tolerated doses and therefore they inflict considerable morbidity in patients. At these high doses, the drugs cause mitotic spindles to malfunction, chromosomes to misalign, and the mitotic checkpoint to become activated [1]. Prolonged activation of the checkpoint ultimately leads to cell death through apoptosis, or to slippage past the checkpoint and re-entry into G1 without cell division. The resultant multiploid cells eventually die by other mechanisms [2]. Because of these actions that affect mitosis, microtubule drugs are often called antimitotic agents or spindle poisons.
In addition to their effects on cell division, microtubule drugs have also been found to inhibit angiogenesis [3]. An important area of cancer research is to find drugs that are able to inhibit the growth of blood vessels into emerging tumors and thereby limit the size of tumors so that they don’t disrupt normal organ function [4]. Microtubule drugs appear to be especially potent agents for this purpose and may present an opportunity to devise new therapies that are able to utilize these inhibitors in a way that limits their toxicity. The effective use of these agents requires a detailed understanding of how they act and how cells become resistant to their actions.
1. Mechanism of drug action
The cellular microtubule network is created by nucleated assembly at the centrosome, an organelle located near the interphase nucleus that replicates and divides to form the spindle poles during mitosis [5]. Tubulin αβ heterodimers are added in a sequential and polarized manner to form 25 nm hollow tubes whose plus-ends grow out in all directions to the plasma membrane as their minus-ends remain embedded at the centrosome or spindle poles. At steady state, unassembled tubulin heterodimers rapidly exchange with the polymer that comprises the cytoskeleton. This turnover occurs predominantly at the microtubule plus-ends that have been shown to undergo frequent transitions between growth and shortening interspersed with periods of pause, a behavior that has been named “dynamic instability” [6]. A transition from growth or pause to shortening is called “catastrophe;” whereas a transition from shortening to growth or pause is referred to as “rescue.” In addition, a parameter called “dynamicity” that measures the total change in length (growth plus shortening) per unit time is often used as an indicator of dynamic activity [7]. These episodes of growth and shortening are stochastic events that result in a “search and capture” behavior allowing the microtubule cytoskeleton to rapidly remodel in response to changes in cell shape or to cellular and environmental cues [8]. This behavior is believed to be essential for microtubule mediated functions such as mitosis [9]. The role of dynamic instability in determining overall microtubule polymer levels, however, is not clear; and widely differing levels of dynamicity have been reported for various cell lines, yet they all divide normally [10–13]. Thus, the ability of microtubule dynamics to influence microtubule content or mitotic progression remains to be established.
Drugs that affect microtubule formation have been known for many decades. Generally, they fall into two classes: some like colchicine and vinblastine inhibit assembly, whereas others like paclitaxel and epothilones promote microtubule assembly and stabilize the polymer. Despite these opposing actions, both drug classes inhibit microtubule mediated processes and block cells in mitosis. A potential explanation for this observation has come from extensive studies showing that virtually all drugs that interfere with microtubule function suppress microtubule dynamics and do so at concentrations that block mitosis without affecting microtubule polymer levels [14]. The effects on microtubule polymer levels presumably occur at higher concentrations and are believed to represent distinct actions of the drugs [14]. Although these observations would seem to argue that the drugs inhibit mitosis by suppressing microtubule dynamics, recent experiments discussed below cast doubt on this conclusion.
2. Resistance to microtubule inhibitors
2.1. Tubulin mutations
The selection of mammalian cells resistant to the effects of microtubule inhibitors has been reviewed elsewhere [15–17]. Although a few of these mutants appear to have an alteration in drug binding, they were isolated from multistep procedures and likely contained multiple genetic changes including functional haploidization of the gene encoding the major expressed β-tubulin isoform [18–20]. The preponderance of mutants that have been isolated were shown to have alterations in microtubule assembly that could counteract the presence of the selecting drug [21–31]. Single step selections in CHO cells, for example, showed that alterations in α- or β-tubulin could confer resistance to any given microtubule inhibitor and resulted in cell lines with a common set of characteristics [32]. These common phenotypes included relatively low 3–5 fold resistance and predictable cross-resistance patterns; i.e., cells resistant to a microtubule destabilizing drug such as vinblastine were cross-resistant to other destabilizing drugs regardless of their binding sites, but were more sensitive to drugs that stabilize microtubules. Conversely, cells selected for resistance to paclitaxel were cross-resistant to other stabilizing drugs but were more sensitive to agents that inhibit assembly. Moreover, it was possible to isolate cell lines that in addition to being drug resistant, were also dependent on the drug for proliferation, a phenotype that is clearly at odds with a mechanism involving diminished drug binding [29, 33].
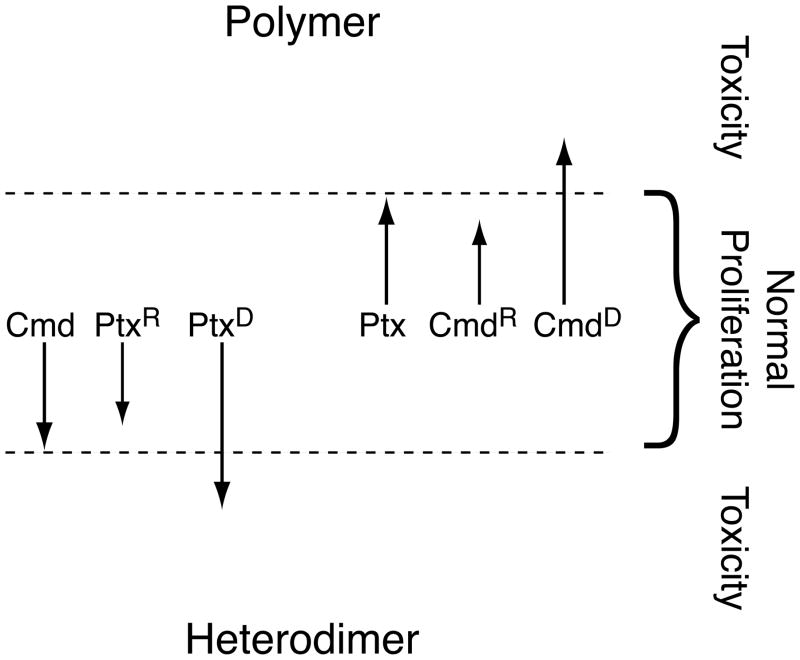
The properties of the mutant cell lines are consistent with a model in which the balance between unassembled tubulin and microtubule polymer can be altered by drug treatment or by mutations in tubulin (Fig. 1). Cells can tolerate small changes in this balance, but perturbations outside of the normal limits, caused by toxic drug concentrations or by tubulin mutations, prevent cell proliferation by impeding the ability of the cells to successfully complete mitosis. Colcemid resistance mutations increase the amount of polymer; thus, the mutant cells are more sensitive to paclitaxel and other drugs that also increase the amount of polymer; but they are resistant to all the drugs that decrease the amount of polymer. In contrast, paclitaxel resistance mutations decrease the amount of polymer; thus the mutant cells are more sensitive to colcemid and other drugs that decrease polymer; but they are resistant to all the drugs that increase microtubule assembly. When mutations move the balance between assembled and unassembled tubulin outside of normal limits, a dependence phenotype is created. Thus, paclitaxel dependent mutants have too little polymer for normal microtubule function and can’t survive unless paclitaxel or another stabilizing drug is present at concentrations sufficient to restore the normal balance [32]. Similarly, colcemid dependent mutants have too much polymer and require colchicine or another destabilizing drug to rescue their proliferation [34]. Confirmation of this model came from direct measurements showing that colcemid resistant mutants have more polymer, paclitaxel resistant cells have less polymer, and paclitaxel dependent cells have much less polymer than wild-type cells [27, 35].
Fig. 1.
Relationship between microtubule polymer levels and drug resistance. The model assumes that cells remain viable within a small range of polymer levels denoted by the dashed lines, but they experience toxicity (failure to divide) outside of that range. Drugs like colcemid (Cmd) become toxic at concentrations that lower polymer levels beneath the lower limit; drugs like paclitaxel (Ptx) become toxic at concentrations that raise polymer levels beyond the upper limit. Drug resistance mutations move the polymer levels in a direction that opposes the selecting drug (paclitaxel resistance (PtxR) to lower levels; colcemid resistance (CmdR) to higher levels). When the mutations are strong enough to move the polymer levels into the toxic zones, drug dependence (PtxD; CmdD) occurs.
Despite their prevalence in drug resistant cell lines, tubulin mutations have not been found at an appreciable frequency in tumors from patients with various forms of cancer [36–42]. These negative results have led to a general perception that tubulin mutations are not responsible for clinical resistance to microtubule drugs [43]. It should be pointed out, however, that most studies examined tumors from patients who had not been treated with microtubule drugs. Given that tubulin is a highly conserved protein, it is not surprising that spontaneous non-synonymous mutations would be rare. Only four such mutations or polymorphisms have been reported in untreated cancer patients, and they were not shown to be associated with clinical response to therapy [38, 44, 45]. However, subsequent transfection experiments showed that three of the mutations / polymorphisms were able to confer resistance to paclitaxel [46]. The results suggest that in rare cases, spontaneous tubulin mutations and polymorphisms may be capable of altering the inherent response of patients to therapy, but they do not address the frequency of tubulin mutations in acquired drug resistance. To obtain this information, studies are needed to assess the tubulin status in new and recurrent tumors from individual patients before and after drug treatment. One promising study in this direction reported that vincristine resistance in tumor cells from children treated for acute lymphocytic leukemia correlated with elevated levels of microtubule polymer; and xenografts selected for vincristine resistance also had elevated polymer and were more sensitive to paclitaxel as predicted by the model in Fig. 1 [47]. Unfortunately, the cells were not analyzed for the existence of mutations in tubulin.
2.2. Overexpression of tubulin isotypes
In addition to tubulin mutations, altered tubulin expression is a potential mechanism of resistance. Mammalian tubulin is encoded by a multigene family that produces at least 7-8 distinct isotypes for each of the α- and β-tubulin proteins [48–50]. The α-tubulins are very homologous and differ from one another at fewer than 10 amino acid residues, but the β-tubulins are more heterogeneous and can differ in as many as 20% of their roughly 445 residues. Most studies examining the effects of tubulin overproduction on drug resistance have focused on the β-subunit because it is more heterogeneous and isotype specific antibodies are readily available. However, the application of new approaches such as mass spectroscopy may allow future studies into the potential role of α-tubulin isotypes in cellular drug responses as well [51]. Initial studies using multistep selections to generate paclitaxel resistant cell lines implicated β1, β2, β3, and β4a in drug resistance [52–55]; but the conclusions relied on correlations that were compromised by the likelihood that the multistep selection had introduced multiple genetic changes and by the difficulties in accurately determining changes in tubulin protein composition. A more direct approach to assess the effects of tubulin isotype overproduction on drug resistance used tetracycline regulated expression of specific β-tubulin cDNAs in transfected CHO cells. These experiments showed that overexpressing β1, β2, or β4b had no effect on microtubule assembly or drug resistance [56]. However, very high overexpression of β3, a brain specific isotype, was able to lower the amount of microtubule polymer and confer weak (1.5 fold) resistance to paclitaxel [57]. Overexpression of β4a, another brain specific isotype, increased the cell sensitivity to paclitaxel because of a presumptive increase in drug binding affinity [58]. Very dramatic changes were produced by the overexpression of β5, a minor but widely expressed isotype. The microtubules appeared highly disrupted, the amount of polymer was greatly reduced, and the cells became paclitaxel resistant and dependent as β5 expression increased [59]. Very recent studies examining the effects of overexpressing β6, a platelet specific tubulin isotype, showed dramatic disruption of the microtubule cytoskeleton and inhibition of cell division, but indicated that there was no resistance to paclitaxel [60].
It thus appears that overexpressing the repertoire of β-tubulin isotypes identified only two that were capable of conferring paclitaxel resistance: β3, which conferred weak resistance, and β5, which conferred robust resistance and dependence. The transfection studies also provided insights into the mechanism by which tubulin overexpression is able to confer drug resistance. The two isotypes that were capable of conferring resistance to paclitaxel reduced the level of microtubule polymer and conferred increased sensitivity to drugs that inhibit microtubule assembly. Cells overexpressing these isotypes therefore resemble the cells with tubulin mutations described earlier. This point is especially relevant in the case of β5 overexpression where it was shown that drug resistance could be traced to serine 239 [61]. Mutation of this amino acid to cysteine, the residue found in the β1, β2, and β4 isotypes that fail to confer drug resistance, eliminated the ability of β5 to disrupt the cytoskeleton or confer resistance to paclitaxel. These studies demonstrated that tubulin isotypes act like tubulin mutants and confer drug resistance through a mechanism that is similar to the one described in Fig. 1.
2.3. Overexpression of microtubule interacting proteins
Although tubulin is the main constituent of microtubules, a large number of additional proteins interact with microtubules and it is possible that some of them could be involved in conferring drug resistance. The first microtubule associated proteins (MAPs) that were identified are the so-called structural MAPs that include MAP2 and tau from brain tissue as well as the more ubiquitously expressed MAP4 [62]. These proteins copurify with microtubules during in vitro assembly and were found to enhance assembly when added to purified tubulin [63]. Whether the proteins have the same activity in vivo, however, is an open question. It was shown, for example, that overexpressing tau or MAP4 had no effect on microtubule assembly or resistance to paclitaxel in CHO cells, preventing MAP4 binding to microtubules produced no phenotype in human fibroblasts, and overexpressing or silencing tau had no effect on the sensitivity of breast cancer cells to paclitaxel [64–66]. Based on these observations, it appears unlikely that changes in structural MAPs play a significant role in cellular drug resistance, though some like tau can affect microtubule organization and they all can potentially affect microtubule function in complex tissues.
More recently, other proteins have been identified that are more likely to play some role in microtubule assembly and drug resistance [67, 68]. One such protein is stathmin, an oncoprotein that sequesters tubulin or promotes microtubule catastrophe in vitro depending on the pH of the assay [69]. Given that these actions should lower microtubule polymer levels, overexpression of stathmin would be expected to confer resistance to paclitaxel (see Fig. 1). Support for this prediction came from A549 cells selected for resistance to paclitaxel that had increased expression of stathmin [70]. The results were complicated, however, because the mutant cells came from a multistep procedure and they had an alteration in α-tubulin in addition to the change in stathmin. It is therefore not clear what role, if any, the increased stathmin played in drug resistance. More direct experiments involving transfection have given conflicting results. Consistent with Fig. 1, stathmin depletion caused increased sensitivity to paclitaxel and increased resistance to vinblastine [71]. However, a second laboratory reported that overexpression of stathmin reduced microtubule assembly as expected, but the increased stathmin conferred resistance to both paclitaxel and vinblastine in contradiction to Fig. 1 [72]. Thus, while there is evidence that stathmin can affect the sensitivity of cells to microtubule drugs, further work is needed to understand how it acts and what drugs are affected.
Another microtubule interacting protein with a potential for affecting drug sensitivity is mitotic centromere associated kinesin (MCAK). MCAK and related proteins of the kin 13 gene family have been shown to catalyze microtubule depolymerization in vitro [73, 74]. Based on this action, Fig. 1 predicts that MCAK overexpression should confer resistance to paclitaxel, and recent experiments confirm this prediction. Tetracycline regulated overexpression of MCAK in CHO cells reduced microtubule polymer, conferred resistance to paclitaxel and epothilone A, and increased sensitivity to colcemid [75]. The results indicate that altered MCAK expression affects sensitivity to microtubule drugs by a mechanism that is similar to the one that mediates the effects of tubulin mutations as well as overexpression of particular tubulin isotypes. It will be of interest to determine whether other microtubule interacting proteins also play a role in drug resistance.
3. Limitations in current models of drug resistance
Two major models have been proposed to explain how microtubule drugs inhibit mitosis and how cells acquire resistance to their action. Model 1 posits that drugs inhibit mitosis by suppressing microtubule dynamics and that tubulin mutations confer resistance by increasing dynamics [14]. As evidence for this mechanism, a paclitaxel resistant (and dependent) human lung carcinoma cell line was found to have significantly increased microtubule dynamics compared to the parental cells [76]. From this it was surmised that the mutant cells could tolerate more paclitaxel-induced suppression of dynamics. Model 2, on the other hand, relates drug resistance to changes in the level of microtubule polymer and has already been described in some detail (Fig. 1).
There are problems with both models. The idea that microtubule dynamics are the most critical target for drug action and that mutant cells gain resistance by altering their dynamics seems to be contradicted by the observation that microtubule dynamics can differ widely among various cell lines without obvious effects on their ability to divide [10–13]. Moreover, the paclitaxel dependent lung carcinoma cell line mentioned above is rescued by the addition of paclitaxel, but the authors have not shown that it is also rescued by vinblastine, colcemid, or any of the other microtubule inhibitory drugs that are known to also suppress dynamics. Finally, the model cannot explain the opposing effects of drugs on microtubule assembly, or the drug cross resistance/increased sensitivity patterns of hundreds of mutants in CHO and other cell lines [15]. This is particularly true for paclitaxel dependent mutants that are rescued by drugs that promote microtubule assembly but not by drugs that inhibit assembly, and for colcemid dependent cells that can be rescued by drugs that inhibit assembly but not by drugs that promote assembly.
Model 2, involving microtubule polymer levels, is consistent with the known effects of the drugs on microtubule assembly. It is also consistent with changes in microtubule assembly and drug sensitivity seen in cells altered by expression of mutant tubulin, tubulin isotypes, or microtubule interacting proteins. The model does not, however, explain how a change in microtubule polymer is able to affect microtubule function. In an attempt to bridge the two models, microtubule dynamics were recently examined in mutant cell lines with the expectation that changes in specific parameters might underlie the differences in polymer content and response to drugs. Two paclitaxel dependent mutants were initially chosen for those studies: one with a mutation in α-tubulin and one with a mutation in β-tubulin. Both mutants had less than half the normal amount of microtubule polymer, and were unable to progress normally through mitosis, but they could re-enter G1 without dividing to form large multinucleated cells [29, 33, 35]. Changes in dynamic parameters were unable to explain these phenotypes [77]. When incubated without paclitaxel, both cell lines had slightly suppressed dynamics, including growth and shortening rates, that couldn’t explain the decrease in microtubule content. Moreover, paclitaxel addition maximally suppressed the dynamics at concentrations that were 5–10 fold lower than those needed to rescue cell division. Similar low drug concentrations also maximally suppressed microtubule dynamics in wild-type cells and had no effect on cell division. Thus, in contradiction to Model 1, suppression of dynamics by paclitaxel appeared to play little, if any, role in the ability of either wild-type or mutant cell lines to proliferate.
4. Novel aspects of microtubule drug action
The surprising inability of paclitaxel-induced suppression of microtubule dynamics to inhibit cell division in wild-type cells or rescue cell division in mutant cells led to a search for new mechanisms by which microtubule drugs might act. In the studies with paclitaxel dependent CHO cells it was observed that the large multinucleated cells resulting from paclitaxel withdrawal had abundant microtubule fragments scattered throughout the cytoplasm. Live cell imaging showed that those fragments were generated by microtubule detachment from centrosomes, a process that occurs at low frequency in wild-type cells but at much higher frequencies in the mutant cell lines [77]. In addition, paclitaxel was able to reduce the frequency of detachment at the same concentrations that rescued cell division. The results indicated that paclitaxel rescued cell division in these mutants by suppressing microtubule detachment from centrosomes, and further suggested that microtubule destabilizing drugs such as colcemid and vinblastine might act by increasing the frequency of detachment. In confirmation of this prediction, it was shown that mitotic inhibitory concentrations of all tested microtubule destabilizing drugs were able to increase the detachment frequency and produce microtubule fragments in a variety of cell lines [78]. The microtubule fragments produced by detachment shortened, but never grew, from their minus-ends, thus potentially helping to explain the lower microtubule content in the paclitaxel dependent cell lines as well as in wild-type cells treated with microtubule destabilizing drugs. These newly reported drug actions on microtubule attachment to centrosomes are consistent with the long recognized opposing actions of microtubule stabilizing versus destabilizing drugs on microtubule assembly, the changes in polymer content in mutant cell lines, and the drug cross resistance/increased sensitivity patterns of mutant cells.
Altered microtubule detachment has been associated with the different molecular changes that produce drug resistance. For example, a paclitaxel resistant cell line with a mutation in tubulin required less vinblastine than wild-type cells to accumulate microtubule fragments whereas a colcemid resistant tubulin mutant required more vinblastine [78]. In cells with changes in β-tubulin isotype composition, it was reported that β5 overexpression disrupted microtubule assembly and conferred resistance and dependence to paclitaxel [59]. The overexpression of β5 was shown to affect several of the dynamic instability parameters, including the microtubule shortening rate, but the parameters were normalized by paclitaxel concentrations that were well below those needed to rescue cell division [79]. The cells also exhibited an elevated frequency of microtubule detachment that was only reversed by the higher paclitaxel concentrations capable of rescuing proliferation. Thus, microtubule detachment explained not only the effects of tubulin mutations, but also changes that resulted from overexpression of a specific tubulin isotype. Similarly, it was recently reported that MCAK overexpression is able to confer paclitaxel resistance. The transfected cells displayed microtubule fragments that were generated by detachment from centrosomes and could be reversed by paclitaxel treatment [75]. These studies not only demonstrated that overexpression of a microtubule interacting protein could produce drug resistance by a similar mechanism as tubulin mutations, they also implicated MCAK in the mechanism by which microtubules detach, a process about which little is currently known.
It is not yet clear how changes in microtubule-centrosome attachment affect mitosis. The currently accepted view of the mitotic spindle apparatus is that it consists of continuous microtubules that link the spindle poles (centrosomes) to kinetochores on the condensed chromosomes (kinetochore fibers) and to the plasma membrane (astral fibers), or that interdigitate near the center of the cell (interpolar fibers) [80]. Several publications, however, point to an alternative structure. For example, electron microscopic reconstruction from serial sections of mammalian spindles has advanced the idea that as many as 50% of interpolar microtubules lack attachment to the spindle poles [81]. Another study using live cell imaging has noted the presence of peripheral microtubule fragments that become incorporated into the central spindles of LLCPK1α cells [82]. The requirement of microtubule fragments for spindle formation is consistent with a report that the frequency of microtubule detachment increases when cells enter mitosis, indicating that fragment creation is a cell cycle regulated process [78]. Experiments using speckle microscopy have discovered large numbers of microtubule fragments in meiotic spindles, leading to a model in which the spindle can be viewed as a “tiled array” of overlapping microtubule fragments held together by microtubule motor proteins [83, 84]. Although evidence indicates that microtubule fragments can form away from the spindle poles [85], they can also be generated by detachment and it is not yet known how much each process contributes to their formation.
Genetic evidence supporting a requirement for microtubule fragments has come from C. elegans where it was shown that mei-1, a gene that encodes a katanin-like protein known to sever microtubules, is required for assembly and function of the meiotic spindle [86]. However, inhibition of katanin activity does not appear to prevent cell division in mammalian cell lines [87]. This discrepancy could be explained if mammals use a different mechanism, perhaps involving MCAK, to generate microtubule fragments for mitotic spindle assembly. As discussed earlier, elevated MCAK production has been shown to increase the frequency of microtubule detachment and generate fragments, and it is located at the centrosome where fragments for spindle assembly would need to be produced. Moreover, MCAK is degraded at the metaphase to anaphase transition and so its levels are low during interphase when the detachment frequency is low, but the levels increase and reach a maximum in mitosis, the time when microtubule detachment is highest [88]. In addition to changes in protein level, MCAK is also regulated by phosphorylation during mitosis, and this may further control its microtubule detachment activity [89]. Thus, MCAK is properly situated and regulated to be a key player in microtubule detachment and spindle assembly. Its ability to stimulate microtubule disassembly by binding and stabilizing curved profilaments at microtubule minus ends [74] is consistent with the fact that dispersed tubulin mutations, overexpression of specific tubulin isotypes, or drug binding to tubulin can all affect microtubule detachment. The variety of changes that alter the detachment frequency argues that it is the overall stability of the lattice rather than perturbation of a specific site that affects microtubule attachment, and MCAK induced disassembly fits this paradigm well.
5. A new model for drug resistance
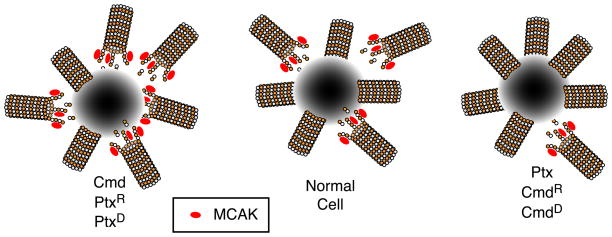
A new model to explain the mechanism by which drug resistance emerges is presented in Fig. 2. The model shows microtubules attached to only one of the two spindle poles. In normally dividing cells, microtubule detachment provides the proper balance of continuous and fragmented microtubules to maintain spindle assembly and function. The binding of microtubule destabilizing drugs, paclitaxel resistance mutations, overexpression of isotypes like β5, or overexpression of MCAK causes an increase in microtubule detachment such that there are too few anchored microtubules for normal spindle assembly or function. Conversely, the binding of microtubule stabilizing agents or colcemid resistance mutations inhibit microtubule detachment and leave the cells with too few microtubule fragments to construct a normal spindle apparatus. The model predicts that any change able to stimulate detachment will confer resistance to paclitaxel and other microtubule stabilizing drugs; whereas any change that inhibits detachment will confer resistance to colcemid, vinblastine, and other microtubule destabilizing drugs. This simple model can account for the properties of mutant cell lines, including their drug cross resistance patterns and their changes in microtubule polymer levels. The model previously described in Fig. 1 linking polymer levels to drug resistance is predictive and consistent with these new ideas but is not mechanistic. Rather, it represents one of the consequences of microtubule detachment.
Fig. 2.
Microtubule detachment and drug resistance. This model assumes that a normal balance of attached and non-attached microtubules is needed for normal spindle function and successful cell division. Drugs like colcemid (Cmd), or tubulin mutations that produce paclitaxel resistance (PtxR) or dependence (PtxD), increase the frequency of detachment and disrupt spindle assembly and function. Drugs like paclitaxel (Ptx), or mutations that produce colcemid resistance (CmdR) or dependence (CmdD), decrease the frequency of detachment and thereby also disrupt spindle assembly and function. There is evidence to indicate that MCAK is involved in microtubule detachment, thus explaining why overproduction of the protein confers resistance to drugs like paclitaxel that inhibit detachment. The diagram depicts microtubules bound to and detaching from one of the two spindle poles.
Given that an increased frequency of microtubule detachment produces fragments that are easily monitored by immunofluorescence, it would seem that the presence of these fragments should be diagnostic for whether the cells are likely to be paclitaxel resistant. This prediction has turned out to be mostly true. Microtubule fragments are common in paclitaxel dependent cell lines with mutations in tubulin, cells overexpressing β5-tubulin, and cells overexpressing MCAK. One exception to this general rule, however, is found in cells that overexpress β6-tubulin, a platelet specific isotype [60]. Cells transfected with β6 produce very abundant microtubule fragments that accumulate and bundle near the cell periphery in a manner that resembles marginal band formation in developing platelets [90]. However, the cells do not appear to be paclitaxel resistant and the formation of fragments is not reversed by drug treatment as is seen in paclitaxel dependent cell lines with mutations in tubulin. The reason for this discrepancy appears to reside in the observation that the cells do not have increased microtubule detachment from centrosomes. Instead, the fragments are produced by an unknown cell cycle dependent process during mitosis and the fragments persist throughout subsequent cell cycles because β6 expression severely depresses dynamics thereby making the fragments extraordinarily stable [60]. These observations demonstrate that it is the effects on microtubule detachment that cause changes in drug sensitivity, and they indicate that microtubule fragments are frequently, but not always, a diagnostic marker for detachment.
6. Role of microtubule dynamics in cell motility
The observation that drugs can suppress microtubule dynamics at sublethal concentrations indicates that dynamics play less of a role in mitosis than is currently thought [77, 78]. On the other hand, a number of publications have reported that sublethal concentrations of microtubule drugs can inhibit cell motility and angiogenesis (reviewed in [3]). Although some of these authors speculated that the low drug concentrations were acting to suppress microtubule dynamics, direct measurements were not carried out. More recently, it was shown that vascular endothelial cell migration could be inhibited with very low concentrations of paclitaxel or vinblastine, but the inhibition correlated with a drug-induced increase rather than suppression of dynamics [91, 92]. In contrast, a clear correlation between suppression of microtubule dynamics and inhibition of cell motility came from experiments showing that colcemid and vinblastine suppress microtubule dynamics and inhibit CHO cell migration in parallel at concentrations that are about 10-fold lower than their IC50 for inhibition of cell division [78]. Similar results were obtained using human cell lines and additional microtubule drugs.
A role for microtubule dynamics in regulating the ability of cells to migrate was further solidified by experiments with cells that express β3-tubulin. Expression of this isotype is normally restricted to brain and Sertoli cells [48–50], but β3 has also been found in a number of tumors where its presence appears to correlate with tumor aggressiveness and poor clinical outcomes [93, 94]. The deleterious effects of β3 expression could at least partially be attributed to its demonstrated role in conferring resistance to paclitaxel [54, 55, 57], but recent work indicates that it plays a much bigger role in cell migration. Microtubules in a CHO cell line with tetracycline regulated expression of β3 were previously shown to resist the ability of paclitaxel to suppress dynamics when β3 was expressed [95]. More recently, it was shown that very low concentrations of paclitaxel can suppress dynamics and inhibit cell migration when β3 was absent, but 10-fold higher drug concentrations were needed to suppress dynamics and inhibit cell migration when β3 was present [96]. The parallel shift in the dose responses provides strong evidence that paclitaxel inhibits cell migration via its effects on microtubule dynamics. The same authors further showed that several common human tumor cell lines including HeLa and MCF7 are heterogeneous for β3 expression on a cell-to-cell basis, and that low paclitaxel concentrations selectively inhibited the migration of the β3 negative cells. The results suggest that the ability of β3 to counteract paclitaxel inhibition of cell motility could underlie the observation that tumors with β3 expression are more aggressive. It should also be pointed out that the ability of β3 to shift the dose response for suppression of microtubule dynamics into the range of concentrations that normally inhibit cell division, may explain why prior investigators using human tumor cell lines found a misleading correlation between suppression of microtubule dynamics and inhibition of mitosis.
7. Microtubule drugs and cancer treatment
The prior discussion indicates that there is still much to be learned about how microtubule drugs act and how best to utilize them to treat patients with cancer. Current therapies use these agents at high concentrations in an effort to directly kill tumor cells by inhibiting mitosis. At these high concentrations, however, patients experience considerable morbidity and it is necessary to temporarily suspend treatment to allow bone marrow recovery. During this recovery, however, the tumor cells also resume growth and thereby enhance their ability to acquire additional mutations that could increase their aggressiveness and resistance to therapy. Although this high dose approach has been successful in many cases, there are still far too many patients that do not respond to therapy or who relapse during or after cessation of treatment. The ability of drugs like vincristine to produce microtubule fragments at the doses that are effective for inhibiting cell division suggests the possibility of personalizing and improving therapy by looking for these fragments in easily accessible tumor cells such as in patients undergoing treatment for leukemia. Similarly, it may be possible to look for fragments in tumor cells from relapsed patients previously treated with paclitaxel as an indication that some of the cells may have acquired drug resistance.
The observation that low drug concentrations alter microtubule dynamics without affecting cell division suggests the possibility that these much less toxic drug concentrations may be able to provide a therapeutic benefit by inhibiting processes such as angiogenesis and tumor metastasis that are dependent upon cell migration. Ideally, such therapy would be given continuously on an outpatient basis and cause little or no toxicity. The goal would not be to eradicate tumors, but rather to limit their size and ability to spread to distant tissues, thus providing the patient with an extended normal life even while harboring small tumors in the body. This chronic administration of low drug concentrations is often referred to as metronomic therapy, and there have been some studies indicating its potential usefulness (reviewed in [97]). Considerably more work needs to be done in this area to establish the effectiveness of metronomic therapy relative to standard therapy, and to find the optimum drug concentrations that limit tumor size yet minimize side effects to the patient. Further cell culture studies are needed to find drug combinations that may act synergistically and to define the mechanisms by which tumor cells can gain resistance to this approach. The finding that β3 expression can overcome paclitaxel inhibition of cell migration is one example of resistance and there are likely to be others [96]. In addition, it will be important to find agents that can selectively target β3 expressing cells and there have already been some promising advances in this direction [98–100].
Acknowledgments
Work in our laboratory is supported by grant CA85935 from the National Institutes of Health (to FC).
Abbreviations
- CHO
Chinese hamster ovary
- Cmd
colcemid
- MAPs
microtubule associated proteins
- MCAK
mitotic centromere associated kinesin
- Ptx
paclitaxel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 2.Blagosklonny MV. Mitotic arrest and cell fate: why and how mitotic inhibition of transcription drives mutually exclusive events. Cell Cycle. 2007;6:70–74. doi: 10.4161/cc.6.1.3682. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz EL. Antivascular actions of microtubule-binding drugs. Clin Cancer Res. 2009;15:2594–2601. doi: 10.1158/1078-0432.CCR-08-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdollahi A, Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Updat. 2010;13:16–28. doi: 10.1016/j.drup.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Wiese C, Zheng Y. Microtubule nucleation: gamma-tubulin and beyond. J Cell Sci. 2006;119:4143–4153. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]
- 6.Mitchison T, Kirschner MW. Dynamic instability of microtubules. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 7.Jordan MA, Wilson L. Use of drugs to study role of microtubule assembly dynamics in living cells. Methods Enzymol. 1998;298:252–276. doi: 10.1016/s0076-6879(98)98024-7. [DOI] [PubMed] [Google Scholar]
- 8.Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 9.Wollman R, Cytrynbaum EN, Jones JT, Meyer T, Scholey JM, Mogilner A. Efficient chromosome capture requires a bias in the 'search-and-capture' process during mitotic-spindle assembly. Curr Biol. 2005;15:828–832. doi: 10.1016/j.cub.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Cassimeris L, Pryer NK, Salmon ED. Real-time observation of microtubule dynamic instability in living cells. J Cell Biol. 1988;107:2223–2231. doi: 10.1083/jcb.107.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honore S, Kamath K, Braguer D, Horwitz SB, Wilson L, Briand C, Jordan MA. Synergistic suppression of microtubule dynamics by discodermolide and paclitaxel in non-small cell lung carcinoma cells. Cancer Res. 2004;64:4957–4964. doi: 10.1158/0008-5472.CAN-04-0693. [DOI] [PubMed] [Google Scholar]
- 12.Panda D, DeLuca K, Williams D, Jordan MA, Wilson L. Antiproliferative mechanism of action of cryptophycin-52: kinetic stabilization of microtubule dynamics by high-affinity binding to microtubule ends. Proc Natl Acad Sci U S A. 1998;95:9313–9318. doi: 10.1073/pnas.95.16.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999;10:947–959. doi: 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature Reviews. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 15.Cabral F. Mechanisms of resistance to drugs that interfere with microtubule assembly. In: Fojo AT, editor. Cancer Drug Discovery and Development: The Role of Microtubules in Cell Biology, Neurobiology, and Oncology. Humana Press; Totowa, NJ: 2008. pp. 337–356. [Google Scholar]
- 16.Cabral F. Factors determining cellular mechanisms of resistance to antimitotic drugs. Drug Resistance Updates. 2001;3:1–6. doi: 10.1054/drup.2000.0172. [DOI] [PubMed] [Google Scholar]
- 17.Orr GA, Verdier-Pinard P, McDavid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–7295. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannakakou P, Gussio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC, Fojo T. A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. Proc Natl Acad Sci USA. 2000;97:2904–2909. doi: 10.1073/pnas.040546297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannakakou P, Sackett DL, Kang Y-K, Zhan Z, Buters JTM, Fojo T, Poruchynsky MS. Paclitaxel-resistant human ovarian cancer cells have mutant β-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272:17118–17125. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, O'Brate A, Zhou W, Giannakakou P. Resistance to microtubule-stabilizing drugs involves two events: beta-tubulin mutation in one allele followed by loss of the second allele. Cell Cycle. 2005;4:1847–1853. doi: 10.4161/cc.4.12.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Garay ML, Chang L, Blade K, Menick DR, Cabral F. A β-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J Biol Chem. 1999;274:23875–23882. doi: 10.1074/jbc.274.34.23875. [DOI] [PubMed] [Google Scholar]
- 22.Hari M, Loganzo F, Annable T, Tan X, Musto S, Morilla DB, Nettles JH, Snyder JP, Greenberger LM. Paclitaxel-resistant cells have a mutation in the paclitaxel-binding region of beta-tubulin (Asp26Glu) and less stable microtubules. Mol Cancer Ther. 2006;5:270–278. doi: 10.1158/1535-7163.MCT-05-0190. [DOI] [PubMed] [Google Scholar]
- 23.Hari M, Wang Y, Veeraraghavan S, Cabral F. Mutations in α- and β-tubulin that stabilize microtubules and confer resistance to colcemid and vinblastine. Mol Cancer Ther. 2003;2:597–605. [PubMed] [Google Scholar]
- 24.He L, Yang CH, Horwitz SB. Mutations in β-tubulin map to domains involved in regulation of microtubule stability in epothilone-resistant cell lines. Mol Cancer Ther. 2001;1:3–10. [PubMed] [Google Scholar]
- 25.Kavallaris M, Tait AS, Walsh BJ, He L, Horwitz SB, Norris MD, Haber M. Multiple microtubule alterations are associated with Vinca alkaloid resistance in human leukemia cells. Cancer Res. 2001;61:5803–5809. [PubMed] [Google Scholar]
- 26.Liaw TY, Salam NK, McKay MJ, Cunningham AM, Hibbs DE, Kavallaris M. Class I beta-tubulin mutations in 2-methoxyestradiol-resistant acute lymphoblastic leukemia cells: implications for drug-target interactions. Mol Cancer Ther. 2008;7:3150–3159. doi: 10.1158/1535-7163.MCT-08-0442. [DOI] [PubMed] [Google Scholar]
- 27.Minotti AM, Barlow SB, Cabral F. Resistance to antimitotic drugs in Chinese hamster ovary cells correlates with changes in the level of polymerized tubulin. J Biol Chem. 1991;266:3987–3994. [PubMed] [Google Scholar]
- 28.Poruchynsky MS, Kim JH, Nogales E, Annable T, Loganzo F, Greenberger LM, Sackett DL, Fojo T. Tumor cells resistant to a microtubule-depolymerizing hemiasterlin analogue, HTI-286, have mutations in alpha- or beta-tubulin and increased microtubule stability. Biochemistry. 2004;43:13944–13954. doi: 10.1021/bi049300+. [DOI] [PubMed] [Google Scholar]
- 29.Schibler M, Cabral F. Taxol-dependent mutants of Chinese hamster ovary cells with alterations in α- and β-tubulin. J Cell Biol. 1986;102:1522–1531. doi: 10.1083/jcb.102.4.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verrills NM, Flemming CL, Liu M, Ivery MT, Cobon GS, Norris MD, Haber M, Kavallaris M. Microtubule alterations and mutations induced by desoxyepothilone B: implications for drug-target interactions. Chem Biol. 2003;10:597–607. doi: 10.1016/s1074-5521(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Veeraraghavan S, Cabral F. Intra-allelic suppression of a mutation that stabilizes microtubules and confers resistance to colcemid. Biochemistry. 2004;43:8965–8973. doi: 10.1021/bi049637b. [DOI] [PubMed] [Google Scholar]
- 32.Cabral F, Brady RC, Schibler MJ. A mechanism of cellular resistance to drugs that interfere with microtubule assembly. Ann NY Acad Sci. 1986;466:745–756. doi: 10.1111/j.1749-6632.1986.tb38456.x. [DOI] [PubMed] [Google Scholar]
- 33.Cabral F. Isolation of Chinese hamster ovary cell mutants requiring the continuous presence of taxol for cell division. J Cell Biol. 1983;97:22–29. doi: 10.1083/jcb.97.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitfield C, Abraham I, Ascherman D, Gottesman MM. Transfer and amplification of a mutant β-tubulin gene results in colcemid dependance: use of the transformant to demonstrate regulation of β-tubulin subunit levels by protein degradation. Mol Cell Biol. 1986;6:1422–1429. doi: 10.1128/mcb.6.5.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barlow SB, Gonzalez-Garay ML, Cabral F. Paclitaxel-dependent mutants have severely reduced microtubule assembly and reduced tubulin synthesis. J Cell Sci. 2002;115:3469–3478. doi: 10.1242/jcs.115.17.3469. [DOI] [PubMed] [Google Scholar]
- 36.Achiwa H, Sato S, Shimizu S, Maeda H, Niimi T, Takahashi T, Ueda R, Mitsudomi T. Analysis of beta-tubulin gene alteration in human lung cancer cell lines. Cancer Lett. 2003;201:211–216. doi: 10.1016/s0304-3835(03)00473-7. [DOI] [PubMed] [Google Scholar]
- 37.de Castro J, Belda-Iniesta C, Cejas P, Casado E, Fresno Vara JA, Hardisson D, Sanchez JJ, Feliu J, Ordonez A, Nistal M, Gonzalez-Baron M. New insights in beta-tubulin sequence analysis in non-small cell lung cancer. Lung Cancer. 2003;41:41–48. doi: 10.1016/s0169-5002(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa S, Miyoshi Y, Egawa C, Ishitobi M, Tamaki Y, Monden M, Noguchi S. Mutational analysis of the class I beta-tubulin gene in human breast cancer. Int J Cancer. 2002;101:46–51. doi: 10.1002/ijc.10575. [DOI] [PubMed] [Google Scholar]
- 39.Maeno K, Ito K, Hama Y, Shingu K, Kimura M, Sano M, Nakagomi H, Tsuchiya S, Fujimori M. Mutation of the class I beta-tubulin gene does not predict response to paclitaxel for breast cancer. Cancer Lett. 2003;198:89–97. doi: 10.1016/s0304-3835(03)00279-9. [DOI] [PubMed] [Google Scholar]
- 40.Sale S, Sung R, Shen P, Yu K, Wang Y, Duran GE, Kim JH, Fojo T, Oefner PJ, Sikic BI. Conservation of the class I beta-tubulin gene in human populations and lack of mutations in lung cancers and paclitaxel-resistant ovarian cancers. Mol Cancer Ther. 2002;1:215–225. [PubMed] [Google Scholar]
- 41.Tsurutani J, Komiya T, Uejima H, Tada H, Syunichi N, Oka M, Kohno G, Fukuoka M, Nakagawa K. Mutational analysis of the beta-tubulin gene in lung cancer. Lung Cancer. 2002;35:11–16. doi: 10.1016/s0169-5002(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 42.Urano N, Fujiwara I, Hasegawa S, Miyoshi Y, Noguchi S, Takiguchi S, Yasuda T, Yano M, Monden M. Absence of beta-tubulin gene mutation in gastric carcinoma. Gastric Cancer. 2003;6:108–112. doi: 10.1007/s10120-003-0235-6. [DOI] [PubMed] [Google Scholar]
- 43.Berrieman HK, Lind MJ, Cawkwell L. Do beta-tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol. 2004;5:158–164. doi: 10.1016/S1470-2045(04)01411-1. [DOI] [PubMed] [Google Scholar]
- 44.McDaid HM, Mani S, Shen HJ, Muggia F, Sonnichsen D, Horwitz SB. Validation of the pharmacodynamics of BMS-247550, an analogue of epothilone B, during a phase I clinical study. Clin Cancer Res. 2002;8:2035–2043. [PubMed] [Google Scholar]
- 45.Yee KW, Hagey A, Verstovsek S, Cortes J, Garcia-Manero G, O'Brien SM, Faderl S, Thomas D, Wierda W, Kornblau S, Ferrajoli A, Albitar M, McKeegan E, Grimm DR, Mueller T, Holley-Shanks RR, Sahelijo L, Gordon GB, Kantarjian HM, Giles FJ. Phase 1 study of ABT-751, a novel microtubule inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2005;11:6615–6624. doi: 10.1158/1078-0432.CCR-05-0650. [DOI] [PubMed] [Google Scholar]
- 46.Yin S, Bhattacharya R, Cabral F. Human mutations that confer paclitaxel resistance. Mol Cancer Ther. 2010;9:327–335. doi: 10.1158/1535-7163.MCT-09-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ong V, Liem NL, Schmid MA, Verrills NM, Papa RA, Marshall GM, Mackenzie KL, Kavallaris M, Lock RB. A role for altered microtubule polymer levels in vincristine resistance of childhood acute lymphoblastic leukemia xenografts. J Pharmacol Exp Ther. 2008;324:434–442. doi: 10.1124/jpet.107.128926. [DOI] [PubMed] [Google Scholar]
- 48.Luduena RF. Multiple forms of tubulin: different gene products and covalent modifications. Internatl Rev Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan KF. Structure and utilization of tubulin isotypes. Ann Rev Cell Biol. 1988;4:687–716. doi: 10.1146/annurev.cb.04.110188.003351. [DOI] [PubMed] [Google Scholar]
- 50.Leandro-Garcia LJ, Leskela S, Landa I, Montero-Conde C, Lopez-Jimenez E, Leton R, Cascon A, Robledo M, Rodriguez-Antona C. Tumoral and tissue-specific expression of the major human beta-tubulin isotypes. Cytoskeleton (Hoboken) 2010;67:214–223. doi: 10.1002/cm.20436. [DOI] [PubMed] [Google Scholar]
- 51.Miller LM, Xiao H, Burd B, Horwitz SB, Angeletti RH, Verdier-Pinard P. Methods in tubulin proteomics. Methods Cell Biol. 2010;95:105–126. doi: 10.1016/S0091-679X(10)95007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haber M, Burkhart CA, Regl DL, Madafiglio J, Norris MD, Horwitz SB. Altered expression of M beta 2, the class II beta-tubulin isotype, in a murine J774.2 cell line with a high level of taxol resistance. J Biol Chem. 1995;270:31269–31275. doi: 10.1074/jbc.270.52.31269. [DOI] [PubMed] [Google Scholar]
- 53.Jaffrezou JP, Dumontet C, Derry WB, Duran G, Chen G, Tsuchiya E, Wilson L, Jordan MA, Sikic BI. Novel mechanism of resistance to paclitaxel (Taxol) in human K562 leukemia cells by combined selection with PSC 833. Oncology Res. 1995;7:517–527. [PubMed] [Google Scholar]
- 54.Kavallaris M, Kuo DYS, Burkhart CA, Regl DL, Norris MD, Haber M, Horwitz SB. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100:1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ranganathan S, Benetatos CA, Colarusso PJ, Dexter DW, Hudes GR. Altered beta-tubulin isotype expression in paclitaxel-resistant human prostate carcinoma cells. Br J Cancer. 1998;77:562–566. doi: 10.1038/bjc.1998.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blade K, Menick DR, Cabral F. Overexpression of class I, II, or IVb β-tubulin isotypes in CHO cells is insufficient to confer resistance to paclitaxel. J Cell Sci. 1999;112:2213–2221. doi: 10.1242/jcs.112.13.2213. [DOI] [PubMed] [Google Scholar]
- 57.Hari M, Yang H, Zeng C, Canizales M, Cabral F. Expression of class III β-tubulin reduces microtubule assembly and confers resistance to paclitaxel. Cell Motil Cytoskeleton. 2003;56:45–56. doi: 10.1002/cm.10132. [DOI] [PubMed] [Google Scholar]
- 58.Yang H, Cabral F. Heightened sensitivity to paclitaxel in class IVa β-tubulin transfected cells is lost as expression increases. J Biol Chem. 2007;282:27058–27066. doi: 10.1074/jbc.M704101200. [DOI] [PubMed] [Google Scholar]
- 59.Bhattacharya R, Cabral F. A ubiquitous β-tubulin disrupts microtubule assembly and inhibits cell proliferation. Mol Biol Cell. 2004;15:3123–3131. doi: 10.1091/mbc.E04-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang H, Ganguly A, Yin S, Cabral F. Megakaryocyte lineage-specific class VI β-tubulin suppresses microtubule dynamics, fragments microtubules, and blocks cell division. Cytoskeleton (Hoboken) 2011;68:175–187. doi: 10.1002/cm.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhattacharya R, Cabral F. Molecular basis for class V β-tubulin effects on microtubule assembly and paclitaxel resistance. J Biol Chem. 2009;284:13023–13032. doi: 10.1074/jbc.M900167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olmsted JB. Non-motor microtubule-associated proteins. Curr Opin Cell Biol. 1991;3:52–58. doi: 10.1016/0955-0674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 63.Sloboda RD, Dentler WL, Rosenbaum JL. Microtubule-associated proteins and the stimulation of tubulin assembly in vitro. Biochemistry. 1976;15:4497–4505. doi: 10.1021/bi00665a026. [DOI] [PubMed] [Google Scholar]
- 64.Barlow SB, Gonzalez-Garay ML, West RR, Olmsted JB, Cabral F. Stable expression of heterologous microtubule associated proteins in Chinese hamster ovary cells: evidence for differing roles of MAPs in microtubule organization. J Cell Biol. 1994;126:1017–1029. doi: 10.1083/jcb.126.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spicakova T, O'Brien MM, Duran GE, Sweet-Cordero A, Sikic BI. Expression and silencing of the microtubule-associated protein Tau in breast cancer cells. Mol Cancer Ther. 2010;9:2970–2981. doi: 10.1158/1535-7163.MCT-10-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang XM, Peloquin JG, Zhai Y, Bulinski JC, Borisy GG. Removal of MAP4 from microtubules in vivo produces no observable phenotype at the cellular level. J Cell Biol. 1996;132:345–357. doi: 10.1083/jcb.132.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cassimeris L, Spittle C. Regulation of microtubule-associated proteins. Int Rev Cytol. 2001;210:163–226. doi: 10.1016/s0074-7696(01)10006-9. [DOI] [PubMed] [Google Scholar]
- 68.Howard J, Hyman AA. Microtubule polymerases and depolymerases. Curr Opin Cell Biol. 2007;19:31–35. doi: 10.1016/j.ceb.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Howell B, Larsson N, Gullberg M, Cassimeris L. Dissociation of the tubulin-sequestering and microtubule catastrophe-promoting activities of oncoprotein 18/stathmin. Mol Biol Cell. 1999;10:105–118. doi: 10.1091/mbc.10.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martello LA, Verdier-Pinard P, Shen H-J, He L, Torres K, Orr GA, Horwitz SB. Elevated levels of microtubule destabilizing factors in a taxol-resistant/dependent A549 cell line with an a-tubulin mutation. Cancer Res. 2003;63:1207–1213. [PubMed] [Google Scholar]
- 71.Iancu C, Mistry SJ, Arkin S, Wallenstein S, Atweh GF. Effects of stathmin inhibition on the mitotic spindle. J Cell Sci. 2001;114:909–916. doi: 10.1242/jcs.114.5.909. [DOI] [PubMed] [Google Scholar]
- 72.Alli E, Bash-Babula J, Yang J-M, Hait WN. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864–6869. [PubMed] [Google Scholar]
- 73.Moore A, Wordeman L. The mechanism, function and regulation of depolymerizing kinesins during mitosis. Trends Cell Biol. 2004;14:537–546. doi: 10.1016/j.tcb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 75.Ganguly A, Yang H, Cabral F. Overexpression of mitotic centromere-associated Kinesin stimulates microtubule detachment and confers resistance to Paclitaxel. Mol Cancer Ther. 2011;10:929–937. doi: 10.1158/1535-7163.MCT-10-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goncalves A, Braguer D, Kamath K, Martello L, Briand C, Horwitz S, Wilson L, Jordan MA. Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc Natl Acad Sci USA. 2001;98:11737–11742. doi: 10.1073/pnas.191388598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ganguly A, Yang H, Cabral F. Paclitaxel dependent cell lines reveal a novel drug activity. Mol Cancer Ther. 2010;9:2914–2923. doi: 10.1158/1535-7163.MCT-10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang H, Ganguly A, Cabral F. Inhibition of cell migration and cell division correlates with distinct effects of microtubule inhibiting drugs. J Biol Chem. 2010;285:32242–32250. doi: 10.1074/jbc.M110.160820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhattacharya R, Yang H, Cabral F. Class V β-tubulin alters dynamic instability and stimulates microtubule detachment from centrosomes. Mol Biol Cell. 2011;22:1025–1034. doi: 10.1091/mbc.E10-10-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Civelekoglu-Scholey G, Scholey JM. Mitotic force generators and chromosome segregation. Cell Mol Life Sci. 2010;67:2231–2250. doi: 10.1007/s00018-010-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mastronarde DN, McDonald KL, Ding R, McIntosh JR. Interpolar spindle microtubules in PTK cells. J Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tulu US, Rusan NM, Wadsworth P. Peripheral, non-centrosome-associated microtubules contribute to spindle formation in centrosome-containing cells. Curr Biol. 2003;13:1894–1899. doi: 10.1016/j.cub.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 83.Burbank KS, Groen AC, Perlman ZE, Fisher DS, Mitchison TJ. A new method reveals microtubule minus ends throughout the meiotic spindle. J Cell Biol. 2006;175:369–375. doi: 10.1083/jcb.200511112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang G, Houghtaling BR, Gaetz J, Liu JZ, Danuser G, Kapoor TM. Architectural dynamics of the meiotic spindle revealed by single-fluorophore imaging. Nat Cell Biol. 2007;9:1233–1242. doi: 10.1038/ncb1643. [DOI] [PubMed] [Google Scholar]
- 85.Goshima G, Kimura A. New look inside the spindle: microtubule-dependent microtubule generation within the spindle. Curr Opin Cell Biol. 2010;22:44–49. doi: 10.1016/j.ceb.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 86.Srayko M, O'toole ET, Hyman AA, Muller-Reichert T. Katanin disrupts the microtubule lattice and increases polymer number in C. elegans meiosis. Curr Biol. 2006;16:1944–1949. doi: 10.1016/j.cub.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 87.Buster D, McNally K, McNally FJ. Katanin inhibition prevents the redistribution of gamma-tubulin at mitosis. J Cell Sci. 2002;115:1083–1092. doi: 10.1242/jcs.115.5.1083. [DOI] [PubMed] [Google Scholar]
- 88.Ganguly A, Bhattacharya R, Cabral F. Cell cycle dependent degradation of MCAK: evidence against a role in anaphase chromosome movement. Cell Cycle. 2008;7:3187–3193. doi: 10.4161/cc.7.20.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ems-McClung SC, Walczak CE. Kinesin-13s in mitosis: Key players in the spatial and temporal organization of spindle microtubules. Semin Cell Dev Biol. 2010;21:276–282. doi: 10.1016/j.semcdb.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hartwig J, Italiano JJ. The birth of the platelet. J Thromb Haemost. 2003;1:1580–1586. doi: 10.1046/j.1538-7836.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- 91.Pasquier E, Honore S, Pourroy B, Jordan MA, Lehmann M, Briand C, Braguer D. Antiangiogenic concentrations of paclitaxel induce an increase in microtubule dynamics in endothelial cells but not in cancer cells. Cancer Res. 2005;65:2433–2440. doi: 10.1158/0008-5472.CAN-04-2624. [DOI] [PubMed] [Google Scholar]
- 92.Pourroy B, Honore S, Pasquier E, Bourgarel-Rey V, Kruczynski A, Briand C, Braguer D. Antiangiogenic concentrations of vinflunine increase the interphase microtubule dynamics and decrease the motility of endothelial cells. Cancer Res. 2006;66:3256–3263. doi: 10.1158/0008-5472.CAN-05-3885. [DOI] [PubMed] [Google Scholar]
- 93.Ploussard G, Terry S, Maille P, Allory Y, Sirab N, Kheuang L, Soyeux P, Nicolaiew N, Coppolani E, Paule B, Salomon L, Culine S, Buttyan R, Vacherot F, de la Taille A. Class III beta-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy. Cancer Res. 2010;70:9253–9264. doi: 10.1158/0008-5472.CAN-10-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seve P, Dumontet C. Is class III beta-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol. 2008;9:168–175. doi: 10.1016/S1470-2045(08)70029-9. [DOI] [PubMed] [Google Scholar]
- 95.Kamath K, Wilson L, Cabral F, Jordan MA. βIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem. 2005;280:12902–12907. doi: 10.1074/jbc.M414477200. [DOI] [PubMed] [Google Scholar]
- 96.Ganguly A, Yang H, Cabral F. Class III β-tubulin counteracts the ability of paclitaxel to inhibit cell migration. Oncotarget. 2011;2:368–377. doi: 10.18632/oncotarget.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 98.Dumontet C, Jordan MA, Lee FF. Ixabepilone: targeting betaIII-tubulin expression in taxane-resistant malignancies. Mol Cancer Ther. 2009;8:17–25. doi: 10.1158/1535-7163.MCT-08-0986. [DOI] [PubMed] [Google Scholar]
- 99.Ferlini C, Raspaglio G, Mozzetti S, Cicchillitti L, Filippetti F, Gallo D, Fattorusso C, Campiani G, Scambia G. The seco-taxane IDN5390 is able to target class III beta-tubulin and to overcome paclitaxel resistance. Cancer Res. 2005;65:2397–2405. doi: 10.1158/0008-5472.CAN-04-3065. [DOI] [PubMed] [Google Scholar]
- 100.Ferlini C, Raspaglio G, Cicchillitti L, Mozzetti S, Prislei S, Bartollino S, Scambia G. Looking at drug resistance mechanisms for microtubule interacting drugs: does TUBB3 work? Curr Cancer Drug Targets. 2007;7:704–712. doi: 10.2174/156800907783220453. [DOI] [PubMed] [Google Scholar]