Abstract
Background
B cell-activating factor belonging to the TNF family (BAFF) is primarily expressed by macrophages and dendritic cells, and stimulates B cell proliferation, differentiation, survival, and Ig production. In the present study, we explored the effect of activin A on BAFF expression by APCs.
Methods
To investigate the effect of activin A on BAFF expression by mouse APCs, we measured the level of BAFF expression at the transcriptional and protein levels using RT-PCR and ELISA.
Results
Activin A markedly enhanced BAFF expression in mouse macrophages and dendritic cells at both the transcriptional and protein levels. SB431542, an activin receptor-like kinase 4 (ALK4) inhibitor, completely abrogated activin A-induced BAFF transcription. Furthermore, overexpression of DN-Smad3 abolished activin-induced BAFF expression at the transcriptional and protein levels.
Conclusion
These results demonstrate that activin A can enhance BAFF expression through ALK4-Smad3 pathway.
Keywords: Activin A, BAFF, APCs, ALK4, Smad3, Ig class switch recombination
INTRODUCTION
Although Th cells have the most effect on B cell maturation and differentiation once the B cells recognize protein antigens, it is increasingly clear that antigen presenting cells (APCs) directly affect B cells. APCs enhance B cell proliferation and differentiation (1-5). B cell-activating factor belonging to the TNF family (BAFF; also known as TALL-1, THANK, BLyS, and zTNF4), derived from dendritic cells (DCs) and macrophages, plays an important role in mediating such effects (6,7). Further, it is involved in B cell survival and maturation, germinal center formation, antibody production, and class-switching recombination (CSR) (8-10).
Activation-induced deaminase (AID) is indispensable for Ig CSR (11), and it has been demonstrated that BAFF induces AID expression (12). TGF-β1, a well-known switch factor for IgA and IgG2b isotypes (13-15), stimulates mouse macrophages and DCs to express BAFF (16), suggesting that TGF-β1 can regulate Ig synthesis indirectly.
Activin A, a member of the TGF-β1 superfamily, is the dimeric protein composed of b subunits linked by a disulfide bond, that include activin A (bAbA), activin AB (bAbB), and activin B (bBbB) (17). It has overlapping biological activities with TGF-β1 (18) and signals through Smad proteins (19). We have shown that activin A stimulates mouse B cells to express IgA isotypes (20). Nevertheless, it is not known if stimulatory activity of activin A on APCs is associated with B cell activation and differentiation.
In the present study, we explored the effects of activin A on BAFF expression by APCs such as DCs and macrophages to see if activin A regulates B cell differentiation indirectly by influencing APCs. We found that activin A can potentiate APCs to express BAFF through activation of the ALK4-Smad3 pathways.
MATERIALS AND METHODS
Reagents
Activin A, TGF-β1, GM-CSF and IL-4, anti-mouse BAFF antibodies were purchased from R&D Systems (Minneapolis, MN, USA). SB431542, SB203580, PD98059, and SP600125, ABTS were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Mice
BALB/c mice were purchased from Orient Co. Ltd (Gyeonggido, Korea) and maintained on an 8:16-h light:dark cycle in an animal environmental control chamber (Daehan Biolink. Co., Korea). Animals were fed Purina Laboratory Rodent Chow 5001 ad libitum. Eight- to twelve-week-old mice were used in this study. Animal care was in accordance with the institutional guidelines of Kangwon National University.
Culture of DCs and macrophages
The mouse dendritic cell line DC2.4 and the mouse macrophage cell line RAW264.7 were cultured in DMEM (2 mM Lglutamine; 100 U/ml penicillin; 100 g/ml streptomycin) plus 10% fetal bovine serum (HyClone Labs, Logan, UT, USA) in a humidified CO2 incubator. Bone marrow stem cells were isolated from BALB/c mouse femurs and cultured with GM-CSF (10 ng/ml) and IL-4 (10 ng/ml). After 7 days, appearance of DCs was determined with mouse anti-CD11c mAb (R&D System) by FACS. This procedure resulted in approximately 80% CD11c positive cell population. For the preparation of peritoneal macrophages, mice were injected with 3 ml of 4% thioglycolate broth in PBS intraperitoneally. After 72 h, peritoneal cells were harvested by peritoneal lavage with PBS containing 2% FBS, washed twice with HBSS, resuspended in 10% FBS-DMEM, and dispensed in 100-mm culture dishes. Cells were incubated in a CO2 incubator for 2 h at 37℃, and adherent cells were used as peritoneal macrophages.
Preparation of Plasmids
A mouse BAFF promoter (pBAFF) was cloned in our laboratory (16). The DN-Smad3 plasmid was provided by Dr. M. Kato (The Cancer Institute, Tokyo, Japan).
Transfection and luciferase assays
The reporter plasmids were co-transfected with the expression plasmids and pCMVβgal (Stratagene, La Jolla, CA) into DC2.4 cells by using a MicroPoratorTM MP-100 (Digital Bio Technology, Seoul, Korea); co-transfection was performed according to the manufacturer's instructions. Briefly, 2×106 cells were mixed with the reporter plasmid and the expression plasmid and pulsed twice at 1000 V for 30 ms. Luciferase and β-galactosidase assays were performed as described (21).
RT-PCR
RNA preparation, reverse transcription, and PCR were performed as described (21). PCR primers were synthesized by Bioneer Corp. (Seoul, Korea). The primers for mouse BAFF were forward primer 5'-GCC GCC ATT CTC AAC ATG AT-3' and reverse primer 5'-TTA GGG CAC CAA AGA AGG TG-3' (product size: 468, 409 bp). All reagents for RT-PCR were purchased from Promega (Madison, WI, USA). PCR was also performed with β-actin to allow for normalization of cDNA concentrations in each set of samples. PCR products were resolved by electrophoresis on 2% agarose gels.
ELISA for mouse BAFF
ELISA was performed as described previously (16). Anti-mouse BAFF antibody (2µg/ml) was added to 96 well microplates. After incubation overnight at 4℃, the plates were washed and blocked with 1% gelatin for 1 h. Supernatant samples (50µl) or standard protein (mouse recombinant BAFF) diluted in 0.5% gelatin were added to the wells. After incubation for 1 h at 37℃, the plates were washed again, and 2µg/ml monoclonal anti-mouse BAFF antibody was added for 1 h at 37℃. The plates were then washed and incubated with goat anti-rat IgG-HRP for 1 h. After washing, 0.2 mM ABTS was added to the wells, and 10 min later, the colorimetric reaction was measured at 405 nm with a VERSAmax ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
Statistical differences between experimental groups were determined by ANO-VAs, and values of p<0.05 by unpaired two-tailed Student's t-test were considered significant.
RESULTS AND DISCUSSION
Effect of activin A on BAFF expression by mouse DCs
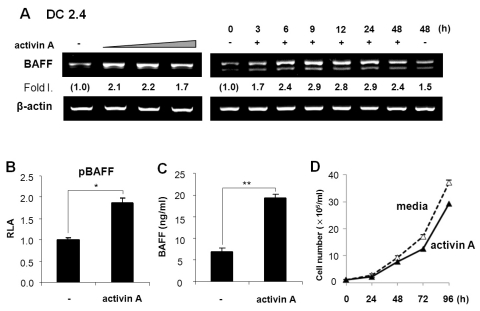
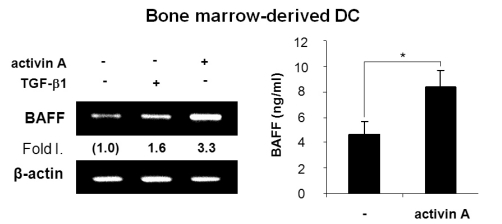
APCs are involved in B cell activation and differentiation (4,5) and BAFF is one of the important factors expressed by APCs such as macrophages and DCs (1,22,23). Since we have demonstrated that TGF-β1 stimulates mouse macrophages to express BAFF (16), it was worthwhile to investigate if activin A, a member of the TGF-β superfamily, can modulate APCs to express BAFF. To explore the possibility that activin A regulates BAFF expression in mouse DCs, we first examined the effect of activin A on the levels of endogenous BAFF transcripts. Activin A induced BAFF transcription in the mouse dendritic cell line, DC2.4. Optimal induction was observed under conditions of 10 ng/ml activin A and 9-h stimulation (Fig. 1A). Similarly, activin A increased the promoter activity of BAFF (Fig. 1B). Since activin-A increased the BAFF transcription and promoter activity, it was necessary to determine its effect at the protein level. Two types of BAFF are known to exist in a secreting and a membrane-bound form (22,7). Here, activin A increased the secretion of BAFF by DC2.4 for at least 48 hrs following stimulation (Fig. 1C). Concentration of activin A (i.e., 10 ng/ml), which significantly increased BAFF expression, little affected cell growth as shown in Fig. 1D. These results indicate that activin A actually regulates mouse DCs BAFF gene expression. Further, activin A also stimulated BAFF expression at the mRNA and protein levels in primary bone marrow-derived DCs (Fig. 2). Taken together, our results clearly show that activin A stimulates mouse DCs to express BAFF.
Figure 1.
Activin A stimulates mouse DCs to express BAFF. (A) Effect of activin A on BAFF transcriptional levels in DC2.4 cells. DC2.4 cells (a mouse DC line) were treated with activin A (5, 10, and 20 ng/ml) for 24 h (left panel). Activin A (10 ng/ml) was added to the cell cultures for the indicated times (right panel). In all experiments, BAFF mRNA levels were determined by RT-PCR. Fold increase (Fold I.) values represent relative amounts of BAFF cDNA normalized to the expression of β-actin cDNA using Kodak Molecular Imaging software. (B) Effect of activin A on BAFF promoter activity. DC2.4 cells (1×106) were transfected with the pBAFF reporter (1µg). Cells were then stimulated with activin A (10 ng/ml) and luciferase activity was determined 24 h later. Transfection efficiency was normalized by β-gal activity. Data are average luciferase activities of two independent transfections with ranges (bars). (C) Effect of activin A on BAFF secretion. DC2.4 cells were treated activin A (10 ng/ml). After 48 h of culture, supernatants were collected and the secreting form of BAFF was measured by ELISA. Data are means of triplicate samples±SEM. *p<0.05; **p<0.01. (D) Effect of activin A on the growth kinetics of DC2.4 cells. DC2.4 cells were cultured with activin A (10 ng/ml). Numbers of viable cells were enumerated by trypan blue exclusion. Data are means of triplicate samples±SEM.
Figure 2.
Effect of activin A on BAFF expression by bone marrow-derived DCs. Freshly isolated bone marrow stem cells were differentiated and incubated with activin A (10 ng/ml). After 24 h of culture, levels of BAFF transcripts were determined by RT-PCR (left panel). After 48 h of culture, BAFF secretion was measured by ELISA (right panel). Data are means of triplicate samples±SEM. *p<0.05.
Effect of activin A on BAFF expression by mouse macrophages
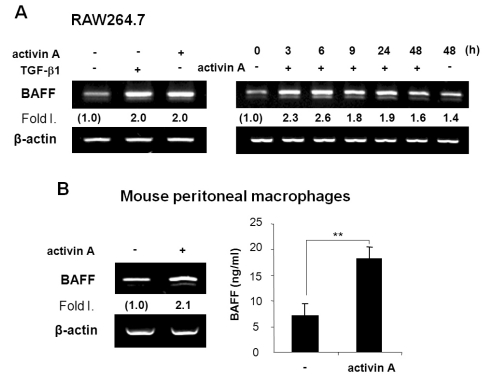
We proceeded to determine if activin A regulates BAFF expression in mouse macrophages. As shown in Fig. 3A, activin A significantly increased BAFF transcription in RAW264.7, a mouse macrophage cell line. BAFF transcript was detectable as early as 3 h after stimulation (Fig. 3A). These results are largely consistent with our early finding that TGF-β1 induces BAFF in such cell line (16). In primary peritoneal macrophages, activin A also stimulated BAFF expression at both the transcriptional and protein levels (Fig. 3B). These results reveal that, in mouse, activin A not only stimulates DCs but also macrophages to express BAFF.
Figure 3.
Activin A stimulates BAFF expression in mouse macrophage cells. (A) Effect of activin A on BAFF transcription in RAW264.7 cells. RAW264.7 cells (a mouse macrophage cell line) were treated with TGF-β (1 ng/ml) and activin A (10 ng/ml) for 24 h (left panel). Effect of activin A on BAFF transcription as a function of time (right panel). Activin A (10 ng/ml) was added to the cell cultures for the indicated times. Levels of BAFF mRNA were determined by RT-PCR. (B) Effect of activin A on BAFF expression by mouse peritoneal macrophages. Freshly isolated mouse peritoneal macrophages were incubated with activin A (10 ng/ml) for 24 h. Levels of BAFF transcripts were determined by RT-PCR (left panel). After 48 h of culture, BAFF secretion was measured by ELISA (right panel). Data are means of triplicate samples±SEM. **p<0.01.
Involvement of the Smad pathway in activin A-induced BAFF expression
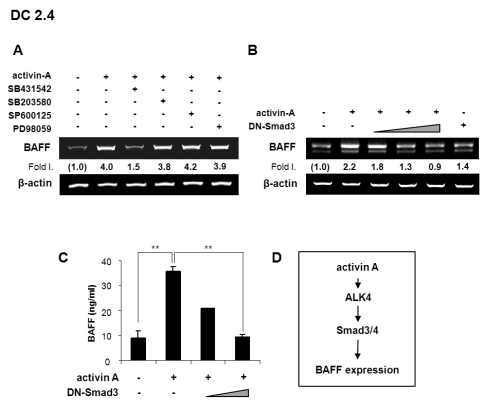
We subsequently investigated the mechanisms underlying activin A-induced BAFF expression. Since the activin A signal activates not only ALK4/Smad pathway but also mitogen-activated protein kinases (MAPKs) pathway, such as p38MAPK, JNK, and ERK (19,24-26,27), we asked the involvement of ALK4/Smad pathway and MAPK pathway in activin A-induced BAFF expression. SB431542 (an ALK4 inhibitor) completely abrogated activin A-induced BAFF transcription, whereas PD98059 (ERK inhibitor), SP600125 (JNK inhibitor), and SB203580 (p38 inhibitor) had little effect (Fig. 4A), suggesting that activin A induces BAFF expression through the ALK4 but not MAPKs pathway. Further, activin A-induced BAFF transcription was abrogated by DN-Smad3 in a dose-dependent manner (Fig. 4B). This was paralleled by changes in BAFF expression at the protein level (Fig. 4C). Taken together, these results indicate that ALK4-Smad3 pathway is mainly responsible for activin A induced BAFF expression (Fig. 4D).
Figure 4.
Activin A enhances BAFF expression through ALK4 and Smad3. (A) Activin A induces BAFF mRNA expression via ALK4. DC2.4 cells were pre-treated with 10µM SB431542, 10µM SB203580, 10µM SP600125, or 5µM PD98059 for 1 h and then stimulated with activin A (10 ng/ml) for 24 h. Levels of BAFF transcripts were determined by RT-PCR. (B) DC2.4 cells (1×106) were transfected with DN-Smad3 expression vectors (2, 4, 8µg) and stimulated with activin A (10 ng/ml) for 24 h. Levels of BAFF transcripts were determined by RT-PCR. (C) BAFF secretion by DN-Smad3-transfected DC 2.4 was measured by ELISA after 48 h incubation with activin A. Data are means of triplicate samples±SEM. **p<0.01. (D) Proposed mechanisms underlying activin A-induced BAFF expression in mouse APCs. Our results indicate that the ALK4 and Smad3/4 mediate activin A-induced BAFF expression.
In summary, we demonstrate in the present study that activin A stimulates mouse APCs to produce BAFF and that this is attributed to ALK4-Smad3 signaling. BAFF is believed to be the most important macrophage- and DC-derived B cell-activating factor (1). Among functions of BAFF, it can activate B cells to express AID gene (28,29). We have recently demonstrated that macrophage-derived BAFF induces AID expression in mouse B cells (30). Therefore, these in vitro studies imply that activin A causes APCs to produce BAFF, which may have important effects on the Ig isotype switching in vivo.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2010-0017247 and The Regional Core Research Program/Medical & Bio-Material Research Center) and by the 2nd stage of the Brain Korea 21 program. Studies were carried out in the Institute of Bioscience and Biotechnology at Kangwon National University.
Footnotes
The author have no financial conflict of interest.
References
- 1.Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell--dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464–4471. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- 2.Dubois B, Massacrier C, Vanbervliet B, Fayette J, Brière F, Banchereau J, Caux C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol. 1998;161:2223–2231. [PubMed] [Google Scholar]
- 3.Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van Kooten C, Brière F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 5.Snapper CM, Mond JJ. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J Immunol. 1996;157:2229–2233. [PubMed] [Google Scholar]
- 6.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J, Mauri D, Cavill D, Gordon TP, Mackay CR, Mackay F. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackay F, Ambrose C. The TNF family members BAFF and APRIL: the growing complexity. Cytokine Growth Factor Rev. 2003;14:311–324. doi: 10.1016/s1359-6101(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 9.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland AP, Mackay F, Mackay CR. Targeting BAFF: immunomodulation for autoimmune diseases and lymphomas. Pharmacol Ther. 2006;112:774–786. doi: 10.1016/j.pharmthera.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 12.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim PH, Kagnoff MF. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J Immunol. 1990;145:3773–3778. [PubMed] [Google Scholar]
- 14.Lebman DA, Lee FD, Coffman RL. Mechanism for transforming growth factor beta and IL-2 enhancement of IgA expression in lipopolysaccharide-stimulated B cell cultures. J Immunol. 1990;144:952–959. [PubMed] [Google Scholar]
- 15.McIntyre TM, Klinman DR, Rothman P, Lugo M, Dasch JR, Mond JJ, Snapper CM. Transforming growth factor beta 1 selectivity stimulates immunoglobulin G2b secretion by lipopolysaccharide-activated murine B cells. J Exp Med. 1993;177:1031–1037. doi: 10.1084/jem.177.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HA, Jeon SH, Seo GY, Park JB, Kim PH. TGF-beta1 and IFN-gamma stimulate mouse macrophages to express BAFF via different signaling pathways. J Leukoc Biol. 2008;83:1431–1439. doi: 10.1189/jlb.1007676. [DOI] [PubMed] [Google Scholar]
- 17.Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 18.Lebrun JJ, Chen Y, Vale W. Receptor serine kinases and signaling by activins and inhibins. In: Aono T, Sugino H, Vale WW, editors. Inhibin, Activins and Flollistatin: regulatory functions in system and cell biology. New York: Springer-Verlag New York; 1997. pp. 1–20. [Google Scholar]
- 19.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, Seo GY, Kim HA, Kim PH. Activin A stimulates IgA expression in mouse B cells. Biochem Biophys Res Commun. 2008;366:574–578. doi: 10.1016/j.bbrc.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol. 2001;31:1706–1715. doi: 10.1002/1521-4141(200106)31:6<1706::aid-immu1706>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 23.Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, Sosnovtseva S, Carrell JA, Feng P, Giri JG, Hilbert DM. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97:198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 24.Chen YG, Wang Q, Lin SL, Chang CD, Chuang J, Ying SY. Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Exp Biol Med (Maywood) 2006;231:534–544. doi: 10.1177/153537020623100507. [DOI] [PubMed] [Google Scholar]
- 25.Huang HM, Chang TW, Liu JC. Basic fibroblast growth factor antagonizes activin A-mediated growth inhibition and hemoglobin synthesis in K562 cells by activating ERK1/2 and deactivating p38 MAP kinase. Biochem Biophys Res Commun. 2004;320:1247–1252. doi: 10.1016/j.bbrc.2004.06.083. [DOI] [PubMed] [Google Scholar]
- 26.Ogihara T, Watada H, Kanno R, Ikeda F, Nomiyama T, Tanaka Y, Nakao A, German MS, Kojima I, Kawamori R. p38 MAPK is involved in activin A- and hepatocyte growth factor-mediated expression of pro-endocrine gene neurogenin 3 in AR42J-B13 cells. J Biol Chem. 2003;278:21693–21700. doi: 10.1074/jbc.M302684200. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Deng M, Parthasarathy R, Wang L, Mongan M, Molkentin JD, Zheng Y, Xia Y. MEKK1 transduces activin signals in keratinocytes to induce actin stress fiber formation and migration. Mol Cell Biol. 2005;25:60–65. doi: 10.1128/MCB.25.1.60-65.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada T, Zhang K, Yamada A, Zhu D, Saxon A. B lymphocyte stimulator activates p38 mitogen-activated protein kinase in human Ig class switch recombination. Am J Respir Cell Mol Biol. 2005;32:388–394. doi: 10.1165/rcmb.2004-0317OC. [DOI] [PubMed] [Google Scholar]
- 30.Kim HA, Seo GY, Kim PH. Macrophage-derived BAFF induces AID expression through the p38MAPK/CREB and JNK/AP-1 pathways. J Leukoc Biol. 2011;89:393–398. doi: 10.1189/jlb.1209787. [DOI] [PubMed] [Google Scholar]