Abstract
Formation of the placenta is a crucial step in mammalian pregnancy. Apart from its function in ensuring an optimal supply of nutrients and oxygen to the fetus, the placenta is also the interface at which allo-recognition of invading trophoblast cells by the maternal immune system can potentially occur. We summarise here the “state of the art” on how variability of immune system genes that code for major histocompatibility complex (MHC) molecules and natural killer receptors (NKR) may impact on human placentation. MHC and NKR are the most polymorphic human genes. Our recent reports point out that specific combinations of fetal MHC and maternal NKR genes in humans correlate with the risk of pre-eclampsia, recurrent miscarriage (RM) and fetal growth restriction (FGR). Research in this field is still at an early stage and future studies in mouse and humans will be needed before the results can be translated to clinical applications. We discuss our recent work, as well as the opportunities offered by mouse genetics, to understand the cellular and molecular mechanisms underlying immune interactions at the maternal-fetal interface.
Keywords: Uterine Natural Killer (uNK) cells, Trophoblast, Pregnancy, Major histocompatibility complex (MHC), Killer-cell immunoglobulin-like receptors (KIR), Ly49 receptors
1. Introduction
Uterine natural killer (uNK) cells are the main maternal immune cells present in decidualised endometrium prior to and during the establishment of the placenta in species with invasive haemochorial placentation (including humans and mice) [1]. NK cells are part of the lymphoid lineage and like T and B lymphocytes can be divided in several subpopulations [2]. The NK cells present in the uterus are phenotypically and functionally unlike those present in the systemic circulation [3,4]. Their exact functions in pregnancy are unknown, but available evidence points to a role in regulating the complex process of placentation. In particular, they are thought to be involved in co-ordinating access of the placental trophoblast cells to the uterine arteries. Trophoblast transformation of the arteries results in high conductance vessels, ensuring an adequate supply of oxygen and nutrients to the feto-placental unit in both human and mouse [5–8].
Many of the genes selected during evolution to regulate reproduction are invariable. However, reproduction is the only natural situation in vertebrates where two genetically different individuals coexist. Therefore, the intrinsic variability of immune system genes may well be one of the key selective pressures shaping the evolution of highly invasive haemochorial placentation. Lymphocytes have a variety of receptors capable of discerning ligands present on unhealthy cells or on those from another individual and indeed, uNK cells do have an array of receptors capable of binding fetal trophoblast ligands [9–11]. One important set of ligands for NK cells are MHC class I molecules, some of which are expressed by invasive trophoblast [12]. MHC and NKR are both highly polymorphic gene systems and indeed they are unique in their extreme variability [13,14].
We have established that certain combinations of maternal NKR and fetal MHC genotypes are risk factors for common disorders of pregnancy in humans [15–18]. Understanding how ‘good’ or ‘bad’ NKR/MHC combinations translate into different NK cell functions and subsequently affect trophoblast invasion and arterial blood flow is now a major challenge. The human maternal-fetal interface is inaccessible in early pregnancy and it is impossible to determine at this stage the pregnancies that will result in a poor outcome. To surmount these difficulties in human pregnancy research, we have begun to explore the similarities and differences between human and mice NKR and potential trophoblast ligands with the aim of developing mouse models that will elucidate how NK cell–trophoblast interactions contribute to placentation.
2. Trophoblast and decidual NK cells
The invasion of human extravillous trophoblast (EVT) into decidua with transformation of the spiral arteries is well documented [1,8]. This process is a critical determinant of reproductive outcome with defective invasion leading to major clinical problems in pregnancy, including pre-eclampsia [19], FGR [20,21] and RM [22,23]. The decidua basalis where the placenta implants is the main tissue site where cells from two individuals intermingle. Because the placental cells are semi-allogeneic, a reasonable hypothesis is that the maternal uterine immune cells regulate trophoblast behaviour. NK cells are present in great abundance at the site of placentation in both humans and mice [1] (Fig. 1). The transformation of the uterine mucosa from endometrium to decidua is characterised by the appearance of large numbers of distinctive NK cells not found elsewhere in the body [24]. Although they may influence other uterine leukocytes [25], affect glandular functions [26], modify blood flow directly by acting on spiral arteries [5] or regulate trophoblast invasion [6], their precise functions are unknown.
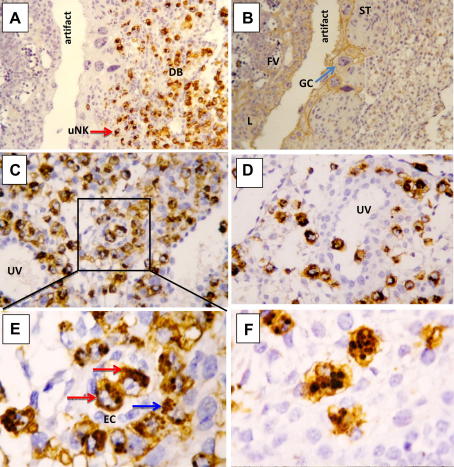
Fig. 1.
Immunohistochemistry of midsagittal sections of a mouse implantation site at gestational day 8.5. A) uNK cells, stained brown by Dolichos Biflorus Agglutinin (DBA) accumulate in the decida basalis (DB). The red arrow points to a DBA+ uNK cell; B) The adjacent section was stained with cytokeratin to identify trophoblast cells, including spongiotrophoblast (ST), invasive giant cells (GC, light blue arrow) and labirinth (L). Fetal vessels (FV) with nucleated red cells are also visible; C–D) The DBA+ uNK cells are associated with maternal uterine vessels (UV) and may participate in their remodelling; E) A higher magnification of the field in C) shows two intravascular DBA+ uNK cells (red arrows) within endothelial cells (EC) and several DBA+ uNK cells in the media (blue arrow); F) DBA+ uNK cells at this stage are large and with prominent granules.
Mice lacking uNK are fertile but display inadequate uterine vascular remodelling during pregnancy, poor decidualisation [7] and low fetal weight [27,28], highlighting the importance of NK cells in placentation. Despite the differences between mouse and humans in the anatomical details of placentation, length of gestation, time of decidualisation and location of uNK cells, there are also remarkable similarities (Table 1) [1,3,4,7,11,18,29–33]. Notably, NK cells in both species are temporally and spatially associated with trophoblast infiltration into decidua and they are particularly prominent around the spiral arteries [1,34]. In both species, there is a decline in these granulated NK cells from mid-gestation onwards so they are relatively sparse at term [35,36]. Our recent findings also show that like humans, mouse uNK cells express multiple NKR with a repertoire different from peripheral NK cells [32]. Have the NKR on these NK cells become specialised to recognise and respond to ligands on trophoblast cells?
Table 1.
Comparison of the maternal-fetal interface in humans and mice.
| Feature | Human | Mouse |
|---|---|---|
| Similarities in placenta type | Discoidal, chorio-allantoic [29] | Discoidal, chorio-allantoic [29] |
| Haemochorial placentation | Yes | Yes |
| Decidualised endometrium | Yes, during each menstrual cycle [29] | Yes, triggered by implantation [29] |
| Disproportionate feto-placental growth | Fetal growth disproportionally higher compared with placental growth in late gestation [29] | Fetal growth disproportionally higher compared with placental growth in late gestation [29] |
| Syncytial transport and barrier trophoblast | Chorionic villi with syncytiotrophoblast barrier formed by cell fusion. Diploid nuclear DNA content [30]. | Labyrinth with syncytiotrophoblast barrier formed by cell fusion. Diploid nuclear DNA content [30] |
| Invasive trophoblast | Non-proliferative, mononuclear polyploid cytotrophoblast [30]. Invasion of decidua basalis and inner third of the myometrium and differentiation into trophoblast giant cells. EVT face maternal immune system [29]. | Non-proliferative, mononuclear polyploid trophoblast with giant cells [30]. Invasion of decidua basalis. Trophoblast giant cells face maternal immune system [29] |
| Selective MHC class I expression by trophoblast | HLA-C, HLA-G and HLA-E; no HLA-A or HLA-B [12] | H2-K; no H2-D or Qa1 (in C57BL/6 mice [31]) |
| Unique uNK phenotype | CD69+ CD117+/-KLRG1high CD56superbrightCD9+CD94bright[3, 4] | CD69+ CD117+ KLRG1high NK1.1-DX5- [32]a |
| Activating NKR on uNK cells | NKG2D, NKp46, KIR2DS1 [6, 18] | NKG2D, NKp46, Ly49H, CD16 [32] |
| Inhibitory NKR on uNK cells | KLRG1, KIR2DL1/2/3, NKG2A [3, 6] | KLRG1, Ly49A, Ly49C, Ly49G2 [31, 32] |
| Vascular changes concomitant with uNK infiltration of uterus | uNK cells accumulate at site of placentation during the first trimester concentrating around spiral arteries [1] | High degree of uNK cell infiltration at gestational day 9.5 [30] around the spiral artery [7] |
| Highly polymorphic receptor/ligand systems | KIR on uNK/HLA-C on EVT [18] | Ly49s on uNK/H2-K on trophoblast giant cells [31, 32] |
| Expression of paternal MHC | Yes [18] | Yes [31] |
| Impact of paternal MHC on reproductive success | Combination of maternal KIR-AA and fetal HLA-C2 genotypes increases the risk for pre-eclampsia, FGR and RM [15,17,18] | Antigenic disparity between parental H2-linked genes affects transformation of the uterine vasculature, as well as fetal growth and placental efficiency [31] |
A different immunophenotype of decidual NK cells in C57BL/6 mice was reported by Mallidi et al., although the cells analysed in this study were mostly DBA- NK1.1+[31].
3. NK cell receptors
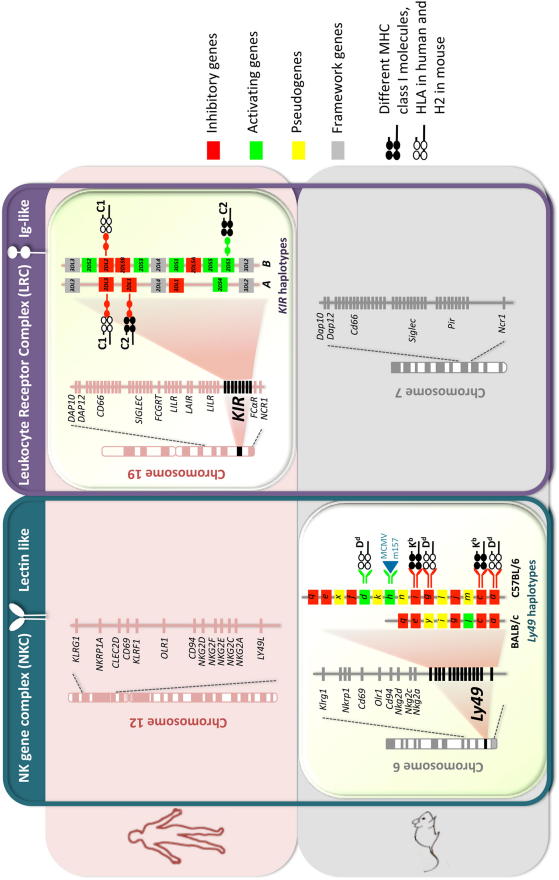
NK cell function is controlled by many activating and inhibitory receptors. Structurally, NKR are of two types: Immunoglobulin-like (Ig) receptors (human, killer immunoglobulin-like receptor, KIR) or C-type lectin-like receptors (human and mouse CD94/NKG2 and mouse Ly49). KIR genes are clustered in the leukocyte receptor complex (LRC) along with other NKR genes (e.g. LILR, LAIR, NCR1) and genes biologically relevant to pregnancy (e.g. PSG, CGB, FCRGT) on human chromosome 19. CD94/NKG2 genes together with NKG2D cluster in the NK gene complex (NKC) on human chromosome 12 and mouse chromosome 6, which also includes Ly49 genes (Fig. 2) [18,37–43].
Fig. 2.
Comparative Genomics of NKC and LRC in human and mouse. A schematic map of the NKC and the LRC encompassing genes encoding for lectin-like and Ig-like NKR and emphasizes the functional homology of human KIR[18] and mouse Ly49[38] genes that arose by convergent evolution [37]. Two typical human KIR haplotypes (A and B) and mouse Ly49 haplotypes (BALB/c and C57BL/6) are shown. The MHC class I ligands for the cognate receptors are also depicted for both human and mouse. For clarity, only one MHC class I molecules is indicated for each of the Ly49 receptors, however individual Ly49 receptors can bind multiple MHC class I molecules. For example Ly49C can bind H2-Kb and H2-Kd[39]. The figure is not drawn to scale and not all genes that map to the NKC and the LRC are indicated. Additional information can be found in references [40–43].
Contrary to the genes that code for antigen receptors on T and B lymphocytes, the genes coding for NKR do not undergo somatic recombination but are instead germ-line encoded [44]. This means they are subject to natural selection. In the case of human KIR genes, balancing selection preserves a high degree of polymorphism in the population, suggesting that no single KIR haplotype confers an absolute fitness advantage to an individual [45]. Indeed, recent comparison between human and simian KIR genes shows they are rapidly evolving [46]. KIR surface expression is stochastic on individual NK cells [47] and the variegated nature of expression results in many NK cell subsets.
Although the human KIR and mouse Ly49 families are structurally distinct, the intracellular signalling pathways of these receptors are largely conserved [44,48]. Moreover, they represent a remarkable example of convergent evolution as they mediate the same functions in the two species; they bind MHC class I molecules and regulate NK cell function [37]. Both KIR and Ly49 gene families are multigenic and polymorphic with different haplotypes having variable gene content including both inhibitory and activating receptors. In addition, both variability in KIR and Ly49 receptors and their MHC ligands determines the outcome of bone marrow transplantation (BMT) further illustrating their homologous functions [49,50]. In mice, the phenomenon of hybrid resistance has been invaluable in illuminating translational research in BMT [51]. Transplantation is, however, an artificial context and the only physiological situation in mammals where NK allo-recognition can occur is during pregnancy. Can murine models therefore unravel the secrets of natural allo-recognition occurring at the maternal-fetal interface in utero?
4. NKR on uterine NK cells and trophoblast MHC ligands
Uterine NK cells do express KIR in humans [10,11] and Ly49 in mice [32] as well as a variety of other NKR [4,52,53]. Importantly, the expression of NKR differs in human uNK cells compared to peripheral NK cells: uNK cells are defined as CD56superbright CD16− but unlike their CD56bright peripheral counterparts, they express KIR, NKG2C and NKG2E, as well as CD69, CD117, KLRG1 and CD94 [3,4]. Given the key roles of MHC class I molecules in ontogeny and function of NK cells, MHC expression on trophoblast cells is obviously important to define. In humans, the repertoire of trophoblast HLA class I expression is unique and can be summarised [12]:
-
i)
No expression of HLA-A or HLA-B molecules, which are highly polymorphic and function mainly as T cell ligands.
-
ii)
Expression of oligomorphic non-classical HLA-G and HLA-E molecules, which are ligands for LILR molecules mainly expressed by myeloid cells and the NKR, CD94/NKG2 respectively [54].
-
iii)
Expression of polymorphic classical HLA-C molecules that are the only HLA providing variability and thus may act as a fetal allogeneic ligand depending on the paternal HLA-C.
-
iv)
EVT is the predominant site of HLA-C expression in decidua and paternal HLA-C allotypes are expressed [18].
In collaboration with Myriam Hemberger, we have recently characterised the expression of trophoblast MHC in the C57BL/6 mouse and found [31]:
-
i)
Only one classical MHC class I, H2-K, but not H2-D is expressed.
-
ii)
No non-classical MHC is expressed.
-
iii)
Paternally-inherited H2-K is expressed by trophoblast invading into maternal decidua.
The H2-K molecule was expressed on the surface of trophoblast stem cells (TS) analysed by flow cytometry and by immuno-precipitation of surface proteins. This was confirmed, and intracytoplasmic staining was also seen, when implantation sites at gestation day 8.5 and TS cells were stained for co-localisation of H2-K and cytokeratin or Cdh3 in immunofluorescence microscopy [31].
The parallels, therefore, are that in both humans and mice, MHC expression is selective and differs from other somatic cells. In addition, crucially, we have found paternally inherited MHC antigens are expressed at the maternal-fetal interface in both species so that NK-mediated allo-recognition can potentially occur [18,31].
5. HLA-C and KIR
The great diversity of both NKR and MHC genes has been explained by their key roles in the effectiveness of immune responses to pathogens illustrated by a variety of human and mouse studies [55,56]. Our findings now suggest that these immune system genes also affect reproductive success [18,31].
We have a range of evidence to show that there is an important role for NKR and MHC genes in human pregnancy. Because HLA-C is the only trophoblast HLA class I molecule showing any appreciable polymorphism, we investigated expression in uNK cells of those KIR that are devoted to binding HLA-C [11]. We found that:
-
i)
Uterine NK cells express elevated levels of the KIR known to bind HLA-C (KIR2DL1/S1 and KIR2DL2/L3).
-
ii)
HLA-C tetramers bind uNK cells more than peripheral NK cells.
-
iii)
KIR2DL1 and KIR2DS1-Fc fusion proteins bind specifically to surface HLA-C molecules on normal trophoblast [18].
-
iv)
Other groups have found that uNK cell function is altered following ligation of KIR [6].
Given the difficulty in determining exactly how uNK cells might function in different pregnancies where maternal KIR and fetal HLA-C are both variable, we have used a genetic approach to show how polymorphism of KIR and HLA-C genes might affect maternal-fetal interactions [15–17]. Genetic epidemiological studies linking certain combinations of human NKR and MHC have driven the biological experiments needed to determine mechanisms of immunity to viruses and we predict a similar development in the field of reproduction. HLA-C ligands for KIR are divided into two groups based on a dimorphism at position 80 of the α1 domain of HLA-C alleles: HLA-C1 (Asn80) binds inhibitory KIR2DL2/3 and HLA-C2 (Lys80) binds inhibitory KIR2DL1 as well as activating KIR2DS1. The simplest way to analyse KIR diversity is to consider all KIR haplotypes as either A or B. KIR A haplotypes are simple with 7 genes including only one activating KIR (2DS4) that is frequently disabled. KIR B haplotypes are more variable (<12 genes); these extra genes are mostly activating [45].
We have found that in 3 different pregnancy disorders, all characterised by defective placentation (pre-eclampsia, FGR and RM), the pregnancies most at risk are those where the mother has a KIR AA genotype and there is a HLA-C2 group in the fetus [15–17]. Furthermore, when the frequency of individual KIR B haplotypes genes was compared between control and affected women, the genes most reduced in frequency in affected women were at the telomeric end of the B haplotype [18]. This is where the activating KIR for HLA-C2 allotypes, KIR2DS1, is located. An additional preliminary finding was that fetal HLA-C2 is a risk factor in KIR AA women when it is paternally rather than maternally derived [18]. This risk can be modified by the maternal C1 or C2 status in keeping with the ‘rheostat model’, according to which NK cell responsiveness is gradually modulated by inhibitory MHC-NKR interactions during education of the NK receptor repertoire [57]. This suggests that maternal NK cells are educated during their development so that they are calibrated to the levels of maternal HLA-C. Thus, uNK responses to the fetus might differ depending on the dose of fetal HLA-C2 relative to that of the mother’s HLA-C2 because of the exquisite sensitivity of NK cells to levels of self-MHC. In other words, maternal C2 genes could modify the risk conferred by fetal C2 and in keeping with this we find that the KIR AA women most at risk are those who are HLA C1/C1 haplotype carriers confronted by fetal C2 [18].
6. Mouse
The studies in humans reveal the great complexity of NKR-MHC interactions and the need to consider three variables: maternal HLA-C, fetal HLA-C and maternal KIR. Indeed, we have not yet explored the diversity further and considered the contribution of individual alleles of both HLA-C and KIR (particularly 2DL1, 2DL2, 2DL3 and 2DS4 that can all bind subsets of HLA-C allotypes [58,59]). Furthermore, the heterogenous nature of these pregnancy disorders and the lack of any easy clear clinical diagnosis of defective placentation makes further progress challenging.
We believe that mouse models can be used to dissect this complexity and determine how individual NKR/MHC combinations influence placentation. The mouse has proved crucial in unravelling how NK cells function in infections [60], BMT [50] and cancer [61]. There are also murine studies (many from Anne Croy’s pioneering work) on the role of uNK cells [62]. Most of the previous work has been conducted in syngeneic mice, however, thus excluding the impact of NKR and MHC variability on placentation.
Recently, we have used both MHC-allogeneic and -congenic mice and shown that paternal MHC affects reproductive outcome [31]. We have also developed robust outcomes of placentation and feto-placental growth, including direct measurements of trophoblast invasion and vascular remodelling. Parameters such as litter size and fetal weight directly determine reproductive success. Evaluation of spiral artery diameter, depth of EVT invasion and placental weight provides insights into indirect effects of individual NKR/MHC combinations. Furthermore, the relatively short life span of mice makes them a suitable model for studies focussing on long-term effects of impaired placentation. For example, insufficient supply of nutrients during fetal development may not have obvious direct effects for the newborn and indeed mice lacking NK cells do produce normal litter sizes. However birth weights are smaller in some NK-deficient strains, such as Irf1−/− [27] and Il15−/− mice [28] and thus insufficient nutrient supply during fetal life could prove disadvantageous later in life as it has been shown for the increased risk of hypertension and coronary heart disease in humans [63].
These findings and experimental protocols will allow future experiments where genetically modified mice, for example congenic for NKR, lacking H2-K or expressing known NK ligands as transgenes can be used. Additional questions relating to uNK cell memory and uNK cell education by maternal and fetal MHC can also be addressed in a systematic way. The phenomenon of NK cell memory [64], where NK cells respond more readily to the same insult on secondary encounter has obvious relevance to human pregnancy - the risk of pre-eclampsia is reduced after a successful first pregnancy but this effect is lost after long interbirth intervals [65].
7. Future directions
Although our initial genetic studies have revealed that particular combinations of NKR KIR and MHC HLA-C genotypes are associated with an increased risk of pregnancy disorders, much further work at both genetic and functional levels is required before these findings can be translated to the clinic. Firstly, because we have only genotyped British people of European descent to date, similar genetic studies must be performed in other populations. Because of the high incidence of pre-eclampsia in Africans, this will be a particularly informative population to study.
Furthermore, we have only performed basic KIR and HLA genotyping and not considered allelic variation. In the context of hepatitis C virus infections, where HLA-C1/C1 haplotype carriers homozygous for KIR2DL3 have a higher probability of resolution [66], certain HLA-C alleles are now emerging as particularly beneficial [67]. Those KIR that can potentially bind HLA-C2 allotypes (2DL1, 2DL2, 2DS4 and 2DS1) also show some allelic variation and this is likely to be functionally important. Future prospective studies with large numbers of well-characterised pregnancies and information on uterine artery blood flow are needed before any definitive conclusions can be reached about ‘good’ or ‘bad’ KIR/HLA-C combinations in humans. These will need to be supported by functional assays using uNK cells isolated from first trimester decidua and experiments using mouse models with defined NKR/MHC combinations. Nevertheless, the findings to date do indicate that immune system genes are likely to be important in reproductive success. Interestingly, a powerful and diverse immune system comes with a price for the individual [68] and a trade-off between its function and reproductive success has been suggested in humans [69] and described in other species such as Soay sheep [70].
Acknowledgements
We would like to thank Lucy Gardner and Katherine Sturgess for immunohistochemistry and Olympe Chazara for constructive discussions. This work was supported by The Wellcome Trust, The Medical Research Council, Wellbeing of Women, The Centre for Trophoblast Research, University of Cambridge and King's College, Cambridge.
References
- 1.Moffett A., Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 2.Colucci F., Caligiuri M.A., Di Santo J.P. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 3.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 4.Koopman L.A., Kopcow H.D., Rybalov B., Boyson J.L., Orange J.S., Schatz F. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lash G.E., Schiessl B., Kirkley M., Innes B.A., Cooper A., Searle R.F. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol. 2006;80:572–580. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- 6.Hanna J., Goldman-Wohl D., Hamani Y., Avraham I., Greenfield C., Natanson-Yaron S. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 7.Ashkar A.A., Di Santo J.P., Croy B.A. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pijnenborg R., Vercruysse L., Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Hiby S.E., King A., Sharkey A.M., Loke Y.W. Human uterine NK cells have a similar repertoire of killer inhibitory and activatory receptors to those found in blood, as demonstrated by RT-PCR and sequencing. Mol Immunol. 1997;34:419–430. doi: 10.1016/s0161-5890(97)00032-1. [DOI] [PubMed] [Google Scholar]
- 10.Verma S., King A., Loke Y.W. Expression of killer cell inhibitory receptors on human uterine natural killer cells. Eur J Immunol. 1997;27:979–983. doi: 10.1002/eji.1830270426. [DOI] [PubMed] [Google Scholar]
- 11.Sharkey A.M., Gardner L., Hiby S., Farrell L., Apps R., Masters L. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J Immunol. 2008;181:39–46. doi: 10.4049/jimmunol.181.1.39. [DOI] [PubMed] [Google Scholar]
- 12.Apps R., Murphy S.P., Fernando R., Gardner L., Ahad T., Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. doi: 10.1111/j.1365-2567.2008.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emes R.D., Goodstadt L., Winter E.E., Ponting C.P. Comparison of the genomes of human and mouse lays the foundation of genome zoology. Hum Mol Genet. 2003;12:701–709. doi: 10.1093/hmg/ddg078. [DOI] [PubMed] [Google Scholar]
- 14.Kelley J., Trowsdale J. Features of MHC and NK gene clusters. Transpl Immunol. 2005;14:129–134. doi: 10.1016/j.trim.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Hiby S.E., Regan L., Lo W., Farrell L., Carrington M., Moffett A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum Reprod. 2008;23:972–976. doi: 10.1093/humrep/den011. [DOI] [PubMed] [Google Scholar]
- 16.Parham P., Guethlein L.A. Pregnancy immunogenetics: NK cell education in the womb? J Clin Invest. 2010;120:3801–3804. doi: 10.1172/JCI44559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiby S.E., Walker J.J., O’Shaughnessy K.M., Redman C.W., Carrington M., Trowsdale J. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiby S.E., Apps R., Sharkey A.M., Farrell L.E., Gardner L., Mulder A. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steegers E.A., von Dadelszen P., Duvekot J.J., Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 20.Khong T.Y., De Wolf F., Robertson W.B., Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y.M., Bujold E., Chaiworapongsa T., Gomez R., Yoon B.H., Thaler H.T. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 22.Jauniaux E., Burton G.J. Pathophysiology of histological changes in early pregnancy loss. Placenta. 2005;26:114–123. doi: 10.1016/j.placenta.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Khong T.Y., Liddell H.S., Robertson W.B. Defective haemochorial placentation as a cause of miscarriage: a preliminary study. Br J Obstet Gynaecol. 1987;94:649–655. doi: 10.1111/j.1471-0528.1987.tb03169.x. [DOI] [PubMed] [Google Scholar]
- 24.Bulmer J.N., Morrison L., Longfellow M., Ritson A., Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991;6:791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- 25.Vacca P., Cantoni C., Vitale M., Prato C., Canegallo F., Fenoglio D. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci U S A. 2010;107:11918–11923. doi: 10.1073/pnas.1001749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hempstock J., Cindrova-Davies T., Jauniaux E., Burton G.J. Endometrial glands as a source of nutrients, growth factors and cytokines during the first trimester of human pregnancy: a morphological and immunohistochemical study. Reprod Biol Endocrinol. 2004;2:58. doi: 10.1186/1477-7827-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashkar A.A., Black G.P., Wei Q., He H., Liang L., Head J.R. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol. 2003;171:2937–2944. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- 28.Barber E.M., Pollard J.W. The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol. 2003;171:37–46. doi: 10.4049/jimmunol.171.1.37. [DOI] [PubMed] [Google Scholar]
- 29.Georgiades P., Ferguson-Smith A.C., Burton G.J. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 30.Cross J.C., Baczyk D., Dobric N., Hemberger M., Hughes M., Simmons D.G. Genes, development and evolution of the placenta. Placenta. 2003;24:123–130. doi: 10.1053/plac.2002.0887. [DOI] [PubMed] [Google Scholar]
- 31.Madeja Z., Yadi H., Apps R., Boulenouar S., Roper S.J., Gardner L. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1005342108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadi H., Burke S., Madeja Z., Hemberger M., Moffett A., Colucci F. Unique receptor repertoire in mouse uterine NK cells. J Immunol. 2008;181:6140–6147. doi: 10.4049/jimmunol.181.9.6140. [DOI] [PubMed] [Google Scholar]
- 33.Mallidi T.V., Craig L.E., Schloemann S.R., Riley J.K. Murine endometrial and decidual NK1.1+ natural killer cells display a B220+CD11c+ cell surface phenotype. Biol Reprod. 2009;81:310–318. doi: 10.1095/biolreprod.109.076448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J., Chen Z., Smith G.N., Croy B.A. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol. 2010;8:1–11. doi: 10.1038/cmi.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez-Rodriguez E.N., Nava-Salazar S., Mendoza-Rodriguez C.A., Moran C., Romero-Arauz J.F., Ortega E. Persistence of decidual NK cells and KIR genotypes in healthy pregnant and preeclamptic women: a case-control study in the third trimester of gestation. Reprod Biol Endocrinol. 2011;9:8. doi: 10.1186/1477-7827-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C., Tanaka T., Nakamura H., Umesaki N., Hirai K., Ishiko O. Granulated metrial gland cells in the murine uterus: localization, kinetics, and the functional role in angiogenesis during pregnancy. Microsc Res Tech. 2003;60:420–429. doi: 10.1002/jemt.10280. [DOI] [PubMed] [Google Scholar]
- 37.Barten R., Torkar M., Haude A., Trowsdale J., Wilson M.J. Divergent and convergent evolution of NK-cell receptors. Trends Immunol. 2001;22:52–57. doi: 10.1016/s1471-4906(00)01802-0. [DOI] [PubMed] [Google Scholar]
- 38.Carlyle J.R., Mesci A., Fine J.H., Chen P., Belanger S., Tai L.H. Evolution of the Ly49 and Nkrp1 recognition systems. Semin Immunol. 2008;20:321–330. doi: 10.1016/j.smim.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Johansson S., Salmon-Divon M., Johansson M.H., Pickman Y., Brodin P., Karre K. Probing natural killer cell education by Ly49 receptor expression analysis and computational modelling in single MHC class I mice. PLoS One. 2009;4:e6046. doi: 10.1371/journal.pone.0006046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ensembl. Mus Musculus: Chromosome 7: 1–1. http://www.ensembl.org/Mus_musculus/Location/Chromosome?r=7. 15.02.2011.
- 41.Ensembl. Mus Musculus: Chromosome 6: 1–1. http://www.ensembl.org/Mus_musculus/Location/Chromosome?r=6. 15.02.2011.
- 42.Ensembl. Homo Sapiens: Chromosome 19: 1–1. http://www.ensembl.org/Homo_sapiens/Location/Chromosome?r=19. 15.03.2011.
- 43.Ensembl. Homo Sapiens: Chromosome 12: 1–1. http://www.ensembl.org/Homo_sapiens/Location/Chromosome?r=12. 15.03.2011.
- 44.Colucci F., Di Santo J.P., Leibson P.J. Natural killer cell activation in mice and men: different triggers for similar weapons? Nat Immunol. 2002;3:807–813. doi: 10.1038/ni0902-807. [DOI] [PubMed] [Google Scholar]
- 45.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 46.Abi-Rached L., Moesta A.K., Rajalingam R., Guethlein L.A., Parham P. Human-specific evolution and adaptation led to major qualitative differences in the variable receptors of human and chimpanzee natural killer cells. PLoS Genet. 2010;6:e1001192. doi: 10.1371/journal.pgen.1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pascal V., Stulberg M.J., Anderson S.K. Regulation of class I major histocompatibility complex receptor expression in natural killer cells: one promoter is not enough! Immunol Rev. 2006;214:9–21. doi: 10.1111/j.1600-065X.2006.00452.x. [DOI] [PubMed] [Google Scholar]
- 48.Yoder J.A., Litman G.W. The phylogenetic origins of natural killer receptors and recognition: relationships, possibilities, and realities. Immunogenetics. 2011;63:123–141. doi: 10.1007/s00251-010-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasazuki T., Juji T., Morishima Y., Kinukawa N., Kashiwabara H., Inoko H. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med. 1998;339:1177–1185. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- 50.Ohlen C., Kling G., Hoglund P., Hansson M., Scangos G., Bieberich C. Prevention of allogeneic bone marrow graft rejection by H-2 transgene in donor mice. Science. 1989;246:666–668. doi: 10.1126/science.2814488. [DOI] [PubMed] [Google Scholar]
- 51.Yu Y.Y., Kumar V., Bennett M. Murine natural killer cells and marrow graft rejection. Annu Rev Immunol. 1992;10:189–213. doi: 10.1146/annurev.iy.10.040192.001201. [DOI] [PubMed] [Google Scholar]
- 52.Trowsdale J., Moffett A. NK receptor interactions with MHC class I molecules in pregnancy. Semin Immunol. 2008;20:317–320. doi: 10.1016/j.smim.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 53.El Costa H., Casemayou A., Aguerre-Girr M., Rabot M., Berrebi A., Parant O. Critical and differential roles of NKp46- and NKp30-activating receptors expressed by uterine NK cells in early pregnancy. J Immunol. 2008;181:3009–3017. doi: 10.4049/jimmunol.181.5.3009. [DOI] [PubMed] [Google Scholar]
- 54.Apps R., Gardner L., Moffett A. A critical look at HLA-G. Trends Immunol. 2008;29:313–321. doi: 10.1016/j.it.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Lanier L.L. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parham P. The genetic and evolutionary balances in human NK cell receptor diversity. Semin Immunol. 2008;20:311–316. doi: 10.1016/j.smim.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brodin P., Karre K., Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30:143–149. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Moesta A.K., Norman P.J., Yawata M., Yawata N., Gleimer M., Parham P. Synergistic polymorphism at two positions distal to the ligand-binding site makes KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J Immunol. 2008;180:3969–3979. doi: 10.4049/jimmunol.180.6.3969. [DOI] [PubMed] [Google Scholar]
- 59.Graef T., Moesta A.K., Norman P.J., Abi-Rached L., Vago L., Older Aguilar A.M. KIR2DS4 is a product of gene conversion with KIR3DL2 that introduced specificity for HLA-A∗11 while diminishing avidity for HLA-C. J Exp Med. 2009;206:2557–2572. doi: 10.1084/jem.20091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quinnan G.V., Manischewitz J.E. The role of natural killer cells and antibody-dependent cell-mediated cytotoxicity during murine cytomegalovirus infection. J Exp Med. 1979;150:1549–1554. doi: 10.1084/jem.150.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ljunggren H.G., Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bilinski M.J., Thorne J.G., Oh M.J., Leonard S., Murrant C., Tayade C. Uterine NK cells in murine pregnancy. Reprod Biomed Online. 2008;16:218–226. doi: 10.1016/s1472-6483(10)60577-9. [DOI] [PubMed] [Google Scholar]
- 63.Barker D.J., Eriksson J.G., Forsen T., Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 64.Sun J.C., Lopez-Verges S., Kim C.C., Derisi J.L., Lanier L.L. NK cells and immune "memory. J Immunol. 2011;186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skjaerven R., Wilcox A.J., Lie R.T. The interval between pregnancies and the risk of preeclampsia. New Engl J Med. 2002;346:33–38. doi: 10.1056/NEJMoa011379. [DOI] [PubMed] [Google Scholar]
- 66.Khakoo S.I., Thio C.L., Martin M.P., Brooks C.R., Gao X., Astemborski J. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 67.Knapp S., Warshow U., Hegazy D., Brackenbury L., Guha I.N., Fowell A. Consistent beneficial effects of killer cell immunoglobulin-like receptor 2DL3 and group 1 human leukocyte antigen-C following exposure to hepatitis C virus. Hepatology. 2010;51:1168–1175. doi: 10.1002/hep.23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rolff J., Siva-Jothy M.T. Invertebrate ecological immunology. Science. 2003;301:472–475. doi: 10.1126/science.1080623. [DOI] [PubMed] [Google Scholar]
- 69.Gendzekhadze K., Norman P.J., Abi-Rached L., Graef T., Moesta A.K., Layrisse Z. Co-evolution of KIR2DL3 with HLA-C in a human population retaining minimal essential diversity of KIR and HLA class I ligands. Proc Natl Acad Sci U S A. 2009;106:18692–18697. doi: 10.1073/pnas.0906051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graham A.L., Hayward A.D., Watt K.A., Pilkington J.G., Pemberton J.M., Nussey D.H. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science. 2010;330:662–665. doi: 10.1126/science.1194878. [DOI] [PubMed] [Google Scholar]