Abstract
Objectives
Adolescents with juvenile idiopathic arthritis have demonstrated substantial disagreement with their proxy’s assessment of their disability, pain, and well-being. Our objective was to describe the clinical and psychological factors associated with discordance.
Study design
This analysis included 204 proxy-adolescent (median age, 13 years) dyads that completed a Childhood Health Assessment Questionnaire for disability with 100-mm visual analogue scales for pain and well-being. Depressive symptoms in adolescents were measured by the Mood and Feelings Questionnaire and in proxies the General Health Questionnaire. Disagreement was assessed using Bland-Altman plots. Associations with discordance were identified using logistic regression analyses.
Results
There was higher agreement for disability (84%) than for pain (71%) and well-being (66%). Regression analyses found no association between age, sex, or disease duration and disagreement. However, relationships between disease activity and disagreement in outcomes were identified. Independent associations were found between increasing Mood and Feelings Questionnaire scores and disagreement in pain and well-being.
Conclusions
Proxy and adolescent reports of pain and well-being are more likely to disagree in those with severe disease. Adolescents who report depressive symptoms are also more likely to disagree with their proxy. The reasons for these are multifactorial, and considerations of both reports are important when assessing outcomes in juvenile idiopathic arthritis.
Keywords: AHAQ, Adolescent Version of the Health Assessment Questionnaire; CAPS, Childhood Arthritis Prospective Study; HRQOL, Health-related quality of life; JIA, Juvenile idiopathic arthritis; MFQ-C, Mood and Feelings Questionnaire-Child version; PGA, Physician’s Global Assessment; VAS, Visual analogue scale
For young people with juvenile idiopathic arthritis (JIA), health-related quality of life (HRQOL) and physical functioning are important outcome measures in the assessment of disease status, progression, and treatment.1 Self-report is the primary method of assessing HRQOL and physical functioning. In pediatric clinics, reporting is often replaced by proxy reports from the primary caregiver. 2 Adolescents increasingly complete the reports themselves, as they develop cognitive competencies and increasing needs for privacy. Reliance on the proxy report occurs when adolescents are unable to self-report due to illness, willingness, cognitive impairment, emotional distress, and/or verbal skills. Divergence between the proxy and adolescent reports in subjective areas such as disability, pain, and overall well-being must be identified to ensure the impact on the assessment of health in JIA is appropriately captured.
Inconsistent results have been found between child and proxy reports of pain, disability, and/or quality of life in JIA using different outcome measures across a range of age ranges (range, 8 to 18 years).3-10 Although most studies have found agreement to be moderate to good, the level of agreement can differ across the spectrum of disease severity.3,4 Detailed analysis of clinical, sociological, or psychological factors associated with disagreement is limited, but disagreement in pain scores have been reported as more likely when the child was having depressive symptoms.5 A study in cerebral palsy also demonstrated that parental stress or anxiety levels influenced parental rating of their child’s pain or quality of life.6 Demonstrating divergence between proxy and adolescent reports is important, but few studies have specifically addressed it.4
The purpose of this analysis was to describe discordance in proxy and adolescent ratings of physical disability, pain, and overall well-being in a large cohort of adolescents with JIA and to explore the factors associated with disagreement, including demographic, disease-related factors, proxy levels of anxiety, and adolescent depressive symptoms.
Methods
The subjects were registered in the larger Childhood Arthritis Prospective Study (CAPS), the details which have been described elsewhere.7 This inception cohort study recruits and follows patients younger than 16 years with new onset inflammatory arthritis from five tertiary referral centers across the United Kingdom (Liverpool, Manchester, Glasgow, Newcastle-Upon-Tyne, and London). For this analysis, patients with JIA only, defined as arthritis persisting for 6 weeks or longer in one or more joint, of no other attributable cause, were included.8
Data were collected from the rheumatologist, the hospital case notes, and directly from the child/family at their first visit to pediatric rheumatology, at 6 months and annually thereafter. These data include demographics, rheumatological examinations, and the JIA International League Against Rheumatism (ILAR) subtype.8 In addition, all the elements of the validated JIA “Core Outcome Variables”9 were collected that incorporated the total number of active joints, the total number of limited joints, a 100-mm Physician’s Global Assessment (PGA) visual analogue scale (VAS), and the Childhood Health Assessment Questionnaire validated for use in the UK population.10 This includes a 100-mm VAS for evaluation of pain and a 100-mm VAS for the evaluation of overall well-being. Each VAS score uses a range from very well (0 mm) to most unwell or severe (100 mm). All adolescents, defined as age 11 years or older at the time of completion of the questionnaire, were approached on at least one occasion to complete independently an Adolescent Version of the Health Assessment Questionnaire (AHAQ) with 100-mm VAS for pain and overall well-being.11 This version is very similar to the Childhood Health Assessment Questionnaire but uses language age appropriate to adolescents and is written in the first person. Simultaneously and independently, the adolescent’s parent or guardian completed a Childhood Health Assessment Questionnaire. Where simultaneous forms were completed at more than one time during follow-up, the earliest dyad was selected for analysis. Childhood Health Assessment Questionnaire and AHAQ scores ranged between 0 and 3 with higher scores indicating greater disability.
The proxy completed the General Health Questionnaire-30 (GHQ-30).12 This is a commonly used instrument for screening for psychiatric disorder in adults. It covers five distinct domains corresponding to anxiety, feelings of incompetence, depression, difficulty in coping, and social dysfunction. A Likert scoring method is used to score responses from strong “symptom absent” to strong “symptom present” as 0, 1, 2, and 3, with a total score ranging from 0 to 90. Studies have indicated thresholds scores of ≥40 to screen for psychiatric “caseness” in primary care populations.13,14 Additionally, adolescents age ≥11 years complete the Mood and Feelings Questionnaire-Child version (MFQ-C).15 The MFQ covers a broad range of cognitive and vegetative symptoms of depression and is commonly used to screen for core depressive symptoms in individuals between 8 and 18 years of age. The MFQ-C has 34 items and asks the responder to rate recent depressive symptoms in the last 2 weeks on a Likert scale (0 = “not true,” 1 = “sometimes true,” and 2 = “true”), with a total score ranging between 0 and 68. In addition, the MFQ-C uses simple wording for younger children as well as adolescents. Studies have shown that a cutoff score of ≥27 provides best diagnostic confidence to detect and screen for major depressive disorders (MDD).16,17
Data Analysis
The primary outcomes were agreement between the proxy-completed Childhood Health Assessment Questionnaire, pain VAS, and overall well-being VAS and the adolescent-completed AHAQ, pain VAS and overall well-being VAS. Clinical agreement was defined as ±10 mm for VAS measures and a ±0.25 for Childhood Health Assessment Questionnaire. These cutoffs were selected as they were thought to represent clinically important differences in the outcome measures.18,19 Agreement between reporters was assessed using Bland and Altman plots. This statistical analysis demonstrates the difference between the proxy and adolescent (d) reported outcome measures plotted against the mean difference between the proxy and adolescent reports (đ). The SD of the differences gives a spread of the discrepancies, and 95% of the differences are expected to lie within the 95% limits of agreement (2 SD).20,21 Kolmogorov-Smirnov tests indicated differences for each outcome measure to be approximately normally distributed.
To identify factors associated with discordance, univariate logistic regression models were developed to calculate OR of discordance with 95% CI. Covariates included (at time of AHAQ/ Childhood Health Assessment Questionnaire completion) age, sex, disease duration, physician global score, active and limited joint count, AHAQ score, adolescent pain score, adolescent well-being, MFQ, and GHQ. Variables with significance at P ≤ .05 in univariate analyses were entered into a separate multivariate model for each of the three measured outcomes to identify independent factors associated with discordance.
The study was approved by the UK Northwest Multicentre Research Ethics Committee. Parent(s)/guardians were asked to provide written consent, and children/adolescents, if considered able, were asked to provide assent.
Results
In total, 204 proxy-adolescent pairs had, respectively, completed a Childhood Health Assessment Questionnaire and AHAQ; 128 (62%) of the adolescents were female, with a median age at form completion of 13.4 years (inter quartile range [IQR], 12.1 to 14.9) (Table I). Median proxy score for the GHQ was 24 (IQR, 19 to 31); 17 (11%) proxies were found to have reached the threshold for psychiatric “caseness.” Similarly, for adolescents, the median MFQ score was 9 (IQR, 3 to 15), with 10 (7%) individuals scoring at or above the cutoff of 27.
Table I.
Baseline characteristics at time of form completion∗
| Characteristic | |
|---|---|
| n | 204 |
| Age, years | 13.4 (12.1, 14.9) |
| Female, n (%) | 128 (62) |
| Ethnicity/Caucasian, n (%) | 186 (91) |
| Disease duration, years | 0.9 (0.4, 2.3) |
| Subtype, n (%) | |
| Systemic arthritis | 13 (6) |
| Persistent oligoarthritis | 82 (40) |
| Extended oligoarthritis | 13 (6) |
| RF (−) polyarthritis | 36 (18) |
| RF (+) polyarthritis | 11 (5) |
| ERA | 27 (13) |
| PSA | 15 (7) |
| Undifferentiated | 8 (4) |
| Physician’s Global Score (0 to 100 mm) | 11 (2, 29) |
| Active joint count | 1 (0, 2) |
| Limited joint count | 0 (0, 2) |
| ESR, per mm/h | 8 (5, 18) |
| Moods and Feelings Questionnaire score (0 to 68) | 9 (3,15) |
| General Health Questionnaire score (0 to 60) | 24 (19, 31) |
All values median (interquartile range unless otherwise specified).
RF, rheumatoid factor; ERA, enthesitis-related arthritis; PSA, psoriatic arthritis; ESR, erythrocyte sedimentation rate.
Agreement in Childhood Health Assessment Questionnaire Score
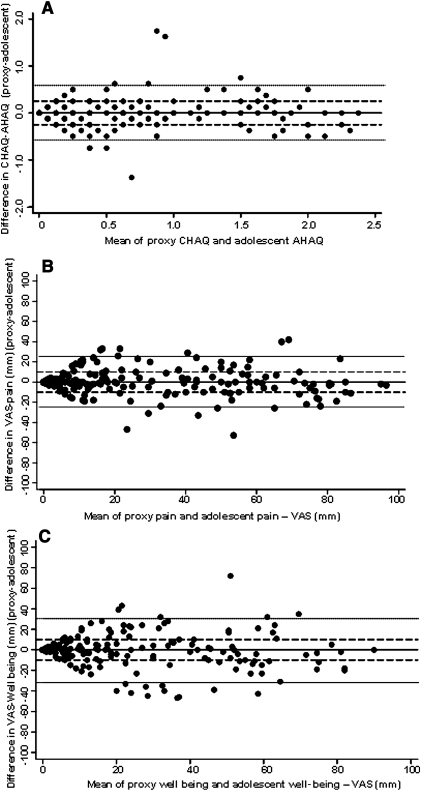
The median score (IQR) of both the Childhood Health Assessment Questionnaire and the AHAQ was 0.25 (0, 0.88). Eighty-four percent of dyads were within the agreed cutoff, with 9% proxy underestimation and 7% proxy overestimation. Across the scale, the majority of differences clustered along the line of perfect agreement, although these ranged from −1.75 to 1.38 (Figure, A). A mean of these differences −0.002 (95% CI, −0.04 to 0.04) is extremely close to zero and within the clinically defined limits of agreement (±0.25). This provides little evidence of differences between proxies and adolescents. Overall agreement was good across the scale of functional disability, with only 10 of 210 (4.8%) of points lying outside the 95% limits of agreement (−0.58, 0.59). Thus, 95% AHAQ reports are expected to lie between 0.59 and −0.58 on the Childhood Health Assessment Questionnaire /AHAQ scale. Logistic regression analyses found no association between adolescent age, sex, disease duration, active or limited joint counts, the GHQ or the MFQ, and the risk of discordance in Childhood Health Assessment Questionnaire score (Table II). In the univariate analysis only there was a weak association between increasing adolescent pain and well-being scores and disagreement.
Figure.
Bland-Altman plot of the difference between the adolescent and proxy ratings against the mean of the 2 ratings for A, CHAQ/AHAQ, B, Pain, C, Well being. Solid line, perfect agreement (no difference); Dashed Lines, clinical limit of agreement; Dotted line, 95% limit of agreement. VAS. visual analogue scale.
Table II.
Factors associated with discordance in disability, pain, and well-being scores
| Discordance in CHAQ OR (95% CI) |
Discordance in pain OR (95% CI) |
Discordance in well-being OR (95% CI) |
||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |
| Age, per year | 1.08 (0.87, 1.33) | - | 0.96 (0.81, 1.14) | - | 0.96 (0.82, 1.14) | - |
| Female | 0.75 (0.34, 1.64) | - | 1.17 (0.63, 2.16) | - | 1.59 (0.88, 2.86) | - |
| Disease duration, per year | 0.97 (0.76, 1.24) | - | 0.85 (0.69, 1.05) | - | 0.78 (0.63, 0.97) | 1.20 (0.85, 1.69) |
| AHAQ, per unit | n/a | - | 1.97 (1.26, 3.10) | 2.01 (0.89, 4.49) | 2.43 (1.54, 3.84) | 1.17 (0.49, 2.77) |
| Adolescent well-being, per 10 mm | 1.22 (1.06, 1.40) | 1.08 (0.87, 1.32) | 1.23 (1.09, 1.39) | 1.13 (0.92, 1.39) | n/a | - |
| Adolescent pain, per 10 mm | 1.22 (1.08, 1.38) | 1.16 (0.97, 1.40) | n/a | - | 1.22 (1.10, 1.35) | 1.05 (0.88, 1.26) |
| Physician’s Global Score, per 10 mm | 1.17 (0.99, 1.39) | 1.16 (1.00, 1.34) | 0.97 (0.80, 1.18) | 1.30 (1.11, 1.52) | 1.13 (0.90, 1.41) | |
| Active joint count, per joint | 1.01 (0.92, 1.11) | - | 1.04 (0.96, 1.11) | - | 1.17 (1.05, 1.30) | 1.07 (0.92, 1.23) |
| Limited joint count, per joint | 0.97 (0.86, 1.09) | - | 1.02 (0.95, 1.09) | - | 1.16 (1.04, 1.29) | - |
| ESR, per mm/h | 1.01 (0.97, 1.04) | - | 0.98 (0.94, 1.03) | - | 1.01 (0.98, 1.05) | - |
| MFQ, per unit | 1.01 (0.97, 1.05) | - | 1.04 (1.00, 1.08) | 1.05 (1.00, 1.10) | 1.05 (1.01, 1.09) | 1.06 (1.01, 1.11) |
| GHQ-30, per unit | 0.99 (0.95, 1.03) | - | 1.02 (0.99, 1.06) | - | 1.03 (1.00, 1.06) | - |
AHAQ, Adolescent Health Assessment Questionnaire; ESR, erythrocyte sedimentation rate; MFQ, Moods and Feelings Questionnaire; GHQ, General Health Questionnaire.
Agreement in Pain Score
The median proxy and adolescent pain scores were 18 (2, 44) and 15 (2, 45), respectively. Seventy-one percent of dyads agreed, with 13% proxy underestimation and 16% proxy overestimation. Analysis from the Bland-Altman plot showed that proxies, on average, scored 0.36 mm (95% CI, −1.37 mm to 2.16 mm) less than their adolescent’s report for pain (Figure, B). In terms of overall agreement, the Bland-Altman Plot indicated that 95% of the observed differences lay within 2 SD of the mean difference (SD ± 12.62 mm). These results indicate limited evidence of difference in reporting pain by proxies and adolescents. However, this spread is larger than that observed for reports of Childhood Health Assessment Questionnaire/AHAQ, and the limits of agreement (24.67 mm, 25.47 mm) exceeded the clinical level of agreement (±10 mm). At higher mean scores of pain (>65 mm) there was trend for proxies to underestimate their adolescent’s pain than overestimate it. Regression analyses found a strong trend toward an association between higher AHAQ scores and discordance in pain scores (multivariate OR, 2.01; 95% CI, 0.89, 4.49) per unit AHAQ (Table II). There was also a weak association between higher well-being scores and higher physician global scores and discordance, although these did not reach statistical significance in the multivariate analysis. In addition, there was an association between higher depressive symptoms in the child and discordance in pain scores (multivariate OR, 1.05; 1.00, 1.10 per unit MFQ). There were no associations between the directionality of discordance, underestimation or overestimation, and proxy GHQ score or the MFQ score.
Agreement in Overall Well-Being Scores
The lowest level of agreement was seen in the well-being scores, with only 66% of dyads agreeing (18% proxy underestimation and 16% proxy overestimation), although the median (IQR) scores among proxies and adolescents (11 [2, 39] and 12 [1, 39]) were very similar. The Bland-Altman plot (Figure, C) indicated that on average proxies scored −0.76 mm (95% CI, −2.95 mm, 1.43 mm) less than their adolescents. The 95% limits of agreement indicated suggest 95% of reports lied within 2 SD (15.85 mm) of the mean difference. These results provide little evidence of systematic bias in the reports of well-being, but they do indicate poorer agreement when compared with reports of physical functioning and pain. The 95% limits of agreement (−32.47 mm, 30.95 mm) exceeded clinical agreement. There was suggestion from the plots that clinical agreement was highest at lower mean ratings of well-being (<20 mm) and moderate at midrange scores. Across the midrange scale of disease severity, proxies rated well-being less severe than reports from their adolescents. In the univariate regression analysis, a number of factors were associated with discordance, including higher AHAQ, higher pain scores, and higher PGA. In addition, with each year of disease duration, proxy and adolescents were less likely to disagree in their well-being scores. In the multivariate analysis, the only factor independently associated with discordance in well-being scores were depressive symptoms in the adolescent (OR, 1.06; 95% CI, 1.01 to 1.11 per unit MFQ). There was also evidence that adolescents who rated their well-being worse than their proxy’s report (ie, VAS >10 mm higher than proxy’s rating) had higher MFQ scores (median MFQ, 12; IQR, 5, 20) than those who either agreed or reported better well-being than their proxy’s report (median MFQ, 8; IQR, 2, 14, P = .03). There was no association between proxy GHQ scores and discordance in well-being scores.
Discussion
Within one of the largest cohorts of adolescents with JIA, this study has demonstrated substantial agreement between proxy and adolescent ratings of objective, overt behaviors, and factual events, such as levels of disability. In contrast, only moderate agreement was observed for the subjective attitudes and perceptions of HRQOL, such as measures of pain and well-being, particularly when disease activity was high.
Our findings of an association between disagreement in pain and well-being scores and depressive symptoms in the adolescent confirm those from previous studies.5,22-24 Comparison with these studies is restricted by their limited focus on the adolescent with analysis in children of all ages, which are unlikely to be comparable with adolescents. One of the largest studies to date of parental and adolescent agreement in healthy children found that overall, adolescents reported poorer emotional and social health compared with their parents. These differences were exacerbated when the adolescent had additional health concerns or illness.25
In general, we observed the highest level of agreement across the recorded scores when these were most representative of a high level of well being (such as lowest symptoms, least pain). There have been reported concerns about the sensitivity of the Childhood Health Assessment Questionnaire and VAS scores to discriminate differences in patients who are well with low scores,18 and so this concordance may represent a mathematical limitation of these scores, or it may represent real concordance between proxy and adolescents when they have a high level of well-being. In contrast, we observed that agreement became more discordant with increasing disease activity, demonstrated by associated increases in pain and AHAQ/Childhood Health Assessment Questionnaire scores, PGAs, and active and limited joint counts. This was in contrast to an earlier report that found maximum discordance in those with moderate disease activity.4
We found that agreement in well-being scores was more likely with longer disease duration, confirming a previous report performed across a broader age range (9 to 18 years).26 Perceptions of the disease may vary over time in both parents and adolescents as both groups adapt and cope to the chronic illness, with benefit for those who are expressive and optimistic about their disease course in terms of clinical outcomes and psychological well-being.27
Analysis of the factors associated with different levels of concordance between the adolescent and proxy identified that depressive symptoms in adolescents resulted in a higher odds of disagreement, particularly for the subjective outcomes of pain and well-being. The relationship between these two observations may be complex, depending on factors such as the adolescent’s perception of their symptoms, the seriousness and the prognosis of JIA, their psychological response such as internalisation of symptom perceptions, and the adoption of passive or avoidance coping strategies. These could have an impact on their decision to report and communicate their symptoms to proxies, the proxy’s interpretation of behaviors in the adolescent, and the adolescent’s engagement in health-seeking behavior,28,29 thus resulting in discrepancies in reporting. Previous studies in conditions other than JIA have found that high levels of depression and anxiety in the parents have resulted in the over-reporting of their adolescent child’s symptoms.6 Our study did not demonstrate this association, which may have been a consequence of the low prevalence of anxiety symptoms among the proxies with only 17 of 204 proxies with anxiety scores above the defined cutoff for “caseness.”
The levels of agreement are consistent with a majority of papers published in this area, although this study is not without its limitations. There remains no gold standard for measuring agreement between proxy and adolescent assessments of disability, pain, and well-being. We selected scores that best represented clinically important differences in the measured outcome. Different definitions of disagreement would have altered the percentages of dyads in agreement but would not have changed the relative proportions of proxy over- or under-reporting, which was similar and equal for all three outcome measures in this study. We did not record whether the mother or father completed the questionnaire. Agreement between mothers and fathers has been reported as good,3 although agreement in ratings of current levels of pain were less concordant between children and their fathers compared with children and their mothers. Mulligan et al30 also found disagreement between mothers and fathers in their assessment of their child’s JIA, although the direction of the disagreement was not systematic. Neither of these studies provided specific analysis on adolescents limiting comparison with this study. We did not include measures of family socioeconomic status, ethnicity, or highest level of educational of the proxy.5,31,32 All of the children included in this study were English-speaking, so the results cannot necessarily be extrapolated to other languages or where questionnaires are completed with an interpreter. Finally, although the AHAQ is nearly identical to the Childhood Health Assessment Questionnaire, which has been validated for use in children greater than 7 years,22 the AHAQ has not undergone a formal validation process and therefore the possibility exists that the few observed differences we did see in disability scores may be a function of the questionnaire rather than any differences between proxy and adolescent reporting. The 100-mm VAS for pain and well-being were not changed in this study, other than asking in the first person on the adolescent questionnaire.
This cohort had a relatively low level of disease activity, which is representative of current clinical practice, and reflects assessments at different time points in their disease course. This prevented a sensitivity analysis in those with the most severe disease but equally importantly accurately reflected assessments at various time points in their disease. This is a cross-sectional study, so the results cannot be used to delineate cause and effect between risk factors and disagreement. Further follow-up of adolescents in the CAPS cohort will allow the study of changes in disagreement and disagreement with time.
Footnotes
Supported by Arthritis Research UK. The authors declare no conflicts of interest.
References
- 1.Eiser C., Jenney M. Measuring quality of life. Arch Dis Child. 2007;92:348–350. doi: 10.1136/adc.2005.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Upton P., Lawford J., Eiser C. Parent-child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res. 2008;17:895–913. doi: 10.1007/s11136-008-9350-5. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Munitis P., Bandeira M., Pistorio A., Magni-Manzoni S., Ruperto N., Schivo A. Level of agreement between children, parents, and physicians in rating pain intensity in juvenile idiopathic arthritis. Arthritis Rheum. 2006;55:177–183. doi: 10.1002/art.21840. [DOI] [PubMed] [Google Scholar]
- 4.Shaw K.L., Southwood T.R., McDonagh J.E. Growing up and moving on in rheumatology: parents as proxies of adolescents with juvenile idiopathic arthritis. Arthritis Rheum. 2006;55:189–198. doi: 10.1002/art.21834. [DOI] [PubMed] [Google Scholar]
- 5.Palermo T.M., Zebracki K., Cox S., Newman A.J., Singer N.G. Juvenile idiopathic arthritis: parent-child discrepancy on reports of pain and disability. J Rheumatol. 2004;31:1840–1846. [PubMed] [Google Scholar]
- 6.White-Koning M., Arnaud C., Dickinson H.O., Thyen U., Beckung E., Fauconnier J. Determinants of child-parent agreement in quality-of-life reports: a European study of children with cerebral palsy. Pediatrics. 2007;120:e804–e814. doi: 10.1542/peds.2006-3272. [DOI] [PubMed] [Google Scholar]
- 7.Hyrich K.L., Lal S.D., Foster H.E., Thornton J., Adib N., Baildam E. Disease activity and disability in children with juvenile idiopathic arthritis one year following presentation to paediatric rheumatology. results from the Childhood Arthritis Prospective Study. Rheumatology (Oxford) 2010;49:116–122. doi: 10.1093/rheumatology/kep352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petty R.E., Southwood T.R., Manners P., Baum J., Glass D.N., Goldenberg J. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 9.Giannini E.H., Ruperto N., Ravelli A., Lovell D.J., Felson D.T., Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40:1202–1209. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 10.Nugent J., Ruperto N., Grainger J., Machado C., Sawhney S., Baildam E. The British version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) Clin Exp Rheumatol. 2001;19:S163–S167. [PubMed] [Google Scholar]
- 11.Shaw K.L., Southwood T.R., McDonagh J.E. British Society of Paediatric and Adolescent Rheumatology. Growing up and moving on in rheumatology: a multicentre cohort of adolescents with juvenile idiopathic arthritis. Rheumatology (Oxford) 2005;44:806–812. doi: 10.1093/rheumatology/keh603. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg D. NFER Publishing; Windsor, England: 1978. Manual of the General Health Questionnaire. [Google Scholar]
- 13.Whittington J., Huppert F.A. Creating invariant subscales of the GHQ-30. Soc Sci Med. 1998;46:1429–1440. doi: 10.1016/s0277-9536(97)10133-2. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg D.P. University Press; London: 1972. The Detection of Psychiatric Illness by Questionnaire. [Google Scholar]
- 15.Messer S., Angold A., Costello E., Loeber R., Van Kammen W., Loeber M. Development of a short questionnaire for use in epidemiologic studies of depression in children and adolescents: factor composition and structure across development. Int J Methods Psychiatr Res. 1995;5:237–249. [Google Scholar]
- 16.Wood A., Kroll L., Moore A., Harrington R. Properties of the mood and feelings questionnaire in adolescent psychiatric outpatients: a research note. J Child Psychol Psychiatry. 1995;36:327–334. doi: 10.1111/j.1469-7610.1995.tb01828.x. [DOI] [PubMed] [Google Scholar]
- 17.Daviss W.B., Birmaher B., Melhem N.A., Axelson D.A., Michaels S.M., Brent D.A. Criterion validity of the Mood and Feelings Questionnaire for depressive episodes in clinic and non-clinic subjects. J Child Psychol Psychiatry. 2006;47:927–934. doi: 10.1111/j.1469-7610.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- 18.Brunner H.I., Klein-Gitelman M.S., Miller M.J., Barron A., Baldwin N., Trombley M. Minimal clinically important differences of the childhood health assessment questionnaire. J Rheumatol. 2005;32:150–161. [PubMed] [Google Scholar]
- 19.Norman G.R., Sloan J.A., Wyrwich K.W. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 20.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 21.Bland J.M., Altman D.G. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 22.Singh G., Athreya B.H., Fries J.F., Goldsmith D.P. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37:1761–1769. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- 23.Billings A.G., Moos R.H., Miller J.J., III, Gottlieb J.E. Psychosocial adaptation in juvenile rheumatic disease: a controlled evaluation. Health Psychol. 1987;6:343–359. doi: 10.1037//0278-6133.6.4.343. [DOI] [PubMed] [Google Scholar]
- 24.Doherty E., Yanni G., Conroy R.M., Bresnihan B. A comparison of child and parent ratings of disability and pain in juvenile chronic arthritis. J Rheumatol. 1993;20:1563–1566. [PubMed] [Google Scholar]
- 25.Waters E., Stewart-Brown S., Fitzpatrick R. Agreement between adolescent self-report and parent reports of health and well-being: results of an epidemiological study. Child Care Health Dev. 2003;29:501–509. doi: 10.1046/j.1365-2214.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- 26.April K.T., Feldman D.E., Platt R.W., Duffy C.M. Comparison between Children with Juvenile Idiopathic Arthritis (JIA) and their parents concerning perceived Quality of Life. Qual Life Res. 2006;15:655–661. doi: 10.1007/s11136-005-3690-1. [DOI] [PubMed] [Google Scholar]
- 27.Manne S.L., Zautra A.J. Coping with arthritis: current status and critique. Arthritis Rheum. 1992;35:1273–1280. doi: 10.1002/art.1780351106. [DOI] [PubMed] [Google Scholar]
- 28.Meyer D., Leventhal H., Gutmann M. Common-sense models of illness: the example of hypertension. Health Psychol. 1985;4:115–135. doi: 10.1037//0278-6133.4.2.115. [DOI] [PubMed] [Google Scholar]
- 29.Magnussen M.G. Characteristics of depressed and non-depressed children and their parents. Child Psychiatry Hum Dev. 1991;21:185–191. doi: 10.1007/BF00705904. [DOI] [PubMed] [Google Scholar]
- 30.Mulligan K., Etheridge A., Kassoumeri L., Wedderburn L.R., Newman S. Do mothers and fathers hold similar views about their child’s arthritis? Arthritis Rheum. 2009;61:1712–1718. doi: 10.1002/art.25008. [DOI] [PubMed] [Google Scholar]
- 31.Waters E., Stewart-Brown S., Fitzpatrick R. Agreement between adolescent self-report and parent reports of health and well-being: results of an epidemiological study. Child Care Health Dev. 2003;29:501–509. doi: 10.1046/j.1365-2214.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- 32.Ravelli A., Viola S., Migliavacca D., Pistorio A., Ruperto N., Martini A. Discordance between proxy-reported and observed assessment of functional ability of children with juvenile idiopathic arthritis. Rheumatology (Oxford) 2001;40:914–919. doi: 10.1093/rheumatology/40.8.914. [DOI] [PubMed] [Google Scholar]