Abstract
This study investigated two mining lakes located in the north of Lower Austria. These lakes arose 45 years ago when open cast lignite mining ceased. The lakes are separated by a 7-m wide dam. Due to the oxidation of pyrite, both lakes have been acidified and exhibit iron, sulphate, and heavy metal concentrations several orders of magnitude higher than in circumneutral lakes. The water column of both lakes is divided into two layers by a pronounced chemocline. The smaller mining lake (AML), with pH close to of 2.6, is the most acidic lake in Austria, whereas flooding with stream water and by drainage from the surrounding fields neutralized the adjacent larger pit lake. The goal of our study was to investigate the effect of flooding on its physical, chemical and biological properties, in comparison to the pristine AML. Even relative to other extremely acidic lakes, the flora and fauna in the AML was reduced and composed of only two flagellate, one ciliate, and one rotifer species. The simplified pelagic food web in the mixolimnion consisted of heterotrophic bacteria, the mixotrophic flagellates Chlamydomonas acidophila and Ochromonas sp., the ciliate Oxytricha sp., and the rotifer Cephalodella sp. The latter two are as yet undescribed new species. The heliozoan Actinophrys sp. that may act as top predator occurred only in low abundance. The euglenid Lepocinclis buetschlii formed a stable deep chlorophyll maximum (DCM) at 7 m depth. Highest cell numbers of L. buetschlii in the DCM exceeded 108 L−1. The neutralized mining lake harboured higher plankton diversity similar to that of natural circumneutral lakes. A peak of at least 16 different phytoplankton taxa was observed during summer. The zooplankton consisted of several copepod species, daphnids and other cladocerans, and at least six different rotifer species. Several fish species occurred in the neutralized lake. Although the effect of non-permanent flooding was largely sustainable, interannual fluctuations of the pH affected the plankton community and reduced its species diversity.
Keywords: Acid mining lake, Neutralization, Meromixis, Deep chlorophyll maximum, Phytoplankton, Zooplankton, Biodiversity, Langau
Introduction
Very acidic (pH 2.8–3.5) and extremely acidic lakes (pH < 2.8, Nixdorf et al., 2005) are common in areas where coal was mined. Due to the oxidation of pyrite, which is formed in brown and black coal deposits, many of these pit or mining lakes (ML) have been acidified and are characterized by high conductivity and concentrations of iron, sulphate, and heavy metals several orders of magnitude higher than in circumneutral lakes (summarized by Geller et al., 1998). As a consequence of the harsh environmental conditions, biodiversity in these habitats is reduced to a few species. Fish, at the top, and autotrophic picoplankton at the bottom of the pelagic food chain are completely lacking in acid mining lakes. Coccal cyanobacteria can only be found at pH >4.5 (Steinberg et al., 1999). Abundances of heterotrophic bacteria are about one order of magnitude lower than in circumneutral mesotrophic and eutrophic lakes (Pöhler et al., 2002; Nixdorf and Jander, 2003). The phytoplankton of acid mining lakes is generally dominated by flagellates belonging to the Chlorophyta (Chlamydomonas), Chrysophyta (Ochromonas), Cryptophyta, and Euglenophyta (Lessmann et al., 2000). Chrysophytes and chlamydomonds are pioneer colonists of these extreme habitats (Nixdorf et al., 1998). Ciliates do not play a major role in the food web. The zooplankton typically comprises a few rotifers, the pioneer crustacean Chydorus sphaericus, and the heliozoan Actinophrys sol that may act as top predator (Wollmann et al., 2000). Fish do not occur in very and extremely acidic lakes.
In central Europe, acid pit lakes are known from Poland, the Czech Republic, and Germany. In Lusatia, east Germany, more than 400 of these lakes exist (Nixdorf et al., 1998). This region harbours one of the best investigated acidic lakes worldwide (ML 111) (Kamjunke et al., 2004). Some highly acidified lakes are also found in Austria. The most acidic Austrian lake (pH ∼2.6) is located in the community of Langau, Lower Austria, where three ML originated in the course of lignite mining activities. About 25 years ago, the largest of the three lakes was flooded with water of a redirected small stream, the Langaubach, for neutralization. At present, flooding is restricted mainly to periods of heavy rainfall, and pH in near-surface waters of this lake ranges from 4.3 to 8.0 (this study).
While the food web structure and diversity of ML have been investigated in some detail (Tittel et al., 1998; Wollmann et al., 2000; Gaedke and Kamjunke, 2006), studies describing changes in the plankton community as a consequence of neutralization of ML are rare (Rönicke et al., 2010). This study presents a comparison of the species diversity and the food web structure of the neutralized lake with its neighbouring acidic lake. Chlorophyll fluorescence was measured as a proxy for phytoplankton biomass and production. We also report pH, temperature, oxygen, conductivity, and heavy metal concentrations to characterize the physicochemical environment of both lakes with respect to flooding. Because these two lakes are of identical origin and only separated from each other by a 7-m wide dam, the effects of neutralization on the physicochemical and biological properties of a ML can be deduced from a comparative analysis of both lakes. Weithoff et al. (2010) already compared some general characteristics of the acidic lake at Langau to a similar AML in the Lusatian area (ML 130). Both lakes were similar in their chemical characteristics but differed in their morphometry and mixing type. The wind exposed Lake 130 exhibited higher species richness. Weithoff et al. (2010) concluded that lake morphometry in combination with wind-induced mixing strongly influences the community structure in these ML. The present article focuses on the biological properties and the food web structure of both lakes at Langau, with emphasis on changes in planktonic biodiversity induced by neutralization. Our sampling frequency was too low to account for seasonal plankton dynamics in detail. The main question was if the current, non-permanent flooding is enough to sustain a plankton community similar to that of natural, circumneutral lakes.
Study sites and methods
The mining lakes at Langau
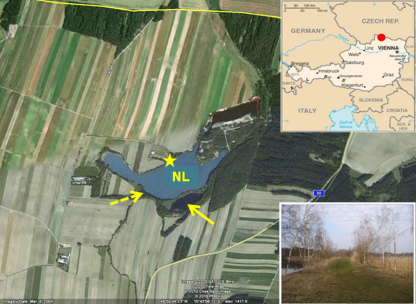
The two mining lakes studied are located at Langau (Lower Austria 48°50′N, 15°43′E), close to the border of the Czech Republic and originated 45 years ago when open cast lignite mining was finished. The neutralized lake (NL in the following) is about 15 ha large, with a maximum depth of 14.5 m. The unchanged acidic lake (AML in the following) has a surface area of 2 ha and a maximum depth of 9.7 m. The lakes are separated by a 7-m wide dam (Fig. 1). The dam was constructed shortly after filling of the pits with groundwater in the 1970s. The NL is more exposed to westerly and northerly winds than the more sheltered AML.
Fig. 1.
Location of the neutralized pit lake (NL) and the acid mining lake (arrow). The inflow of the Langaubach into the NL is indicated by the broken arrow, that of the drainage at its northern shore by the 5-point star. The lakes are located in northern Austria, close to the border with the Czech Republic (upper insert), and separated from each other by a 7-m wide dam (lower insert).
The Langaubach, which was redirected to flood the NL in the mid 1980s, has its source approximately 4 km west of the NL. The man-made connection to the NL (overflow) is indicated by the broken arrow in Fig. 1. After a period of 10 years the inflow was manipulated in a way to ensure that only during periods of heavy rainfall (>15–20 mm d−1) the overflow would reach the ML. This happened, on average, four to eight times during recent years (measured by F. Neunteufl at Langau, unpubl., and by the Austrian meteorological service, ZAMG, at the neighbouring station Retz, Lower Austria; available at www.zamg.ac.at/klima/klima_monat/klimawerte/). Additionally, run-off from the surrounding farmland reaches the lake via drainage at its northern shore (Fig. 1). The outflow of the lake into the Langaubach, which is activated only if the lake level rises after heavy precipitation, is located at the eastern shore of the NL. The Langaubach flows into the River Thaya 12 km NE of the NL. The catchment area of the stream west of the NL is approximately 400 ha. Chemical, physical and biological properties of the Langaubach in the vicinity of its inflow into the NL were investigated by an authorized water laboratory by order of the local authorities in November 2009 (T. Lendenfeld, unpubl. technical report).
Sampling and profile measurements
Concentrations of soluble reactive phosphorus (SRP), total phosphorus (TP), and nitrate were measured on two occasions during a preliminary survey conducted in the AML and the NL during September and October 2005. All other data reported refer to sampling in both lakes that was performed four times per year from April to November in 2007 and 2008. Additionally, an under ice sampling was conducted in January 2010 when both lakes were frozen. On the first two sampling dates in April and June 2007, samples were taken with a water bottle (vol 5 L) in 1-m intervals from surface to bottom. Physical and chemical parameters (pH, oxygen, conductivity and temperature) were measured using a multiparameter probe (Hach Lange HQ 40d multi). For chlorophyll a measurements, 0.25–2 L were filtered through a 0.2 μm GF/C filter and kept frozen until analysis several days later. Chlorophyll concentration was measured photometrically (SHIMADZU UV-1202, UV–vis spectrophotometer) after hot ethanol (90%, v/v) extraction.
From August 2007 onwards, depth profiles including temperature, pH, conductivity, oxygen and chlorophyll fluorescence were measured with a different multiparameter probe (YSI Environmental, Yellow Spring, Ohio, USA). The probe was lowered at a speed of 0.08 m s−1. Electrical conductivity is reported as specific conductance (κ25) corrected for a reference temperature of 25 °C according to:
where X is the measured conductivity and T is the measured temperature in °C (YSI Incorporated, 2009). Dissolved oxygen was measured by a fast responding optical sensor (YSI ROX) that measures luminescence after excitation with blue light.
Additional to the chlorophyll profiles derived from the fluorescence signal of the probe, two epilimnic samples and one sample from the deep chlorophyll maximum were taken for photometric chlorophyll measurements to compare results with the relative fluorescence data derived from the probe. Since chlorophyll a estimates from both methods were significantly linearly correlated (R2 = 0.91, n = 34) and only the probe yielded continuous chlorophyll profiles, we report data from the latter only.
The underwater light intensity (photosynthetically active radiation, PAR) was recorded with a spherical light sensor (Li-Cor 193, Li-Cor, Lincoln, NE, USA) every 0.5 m on each sampling occasion. Secchi depth was measured by a white disk of 30 cm diameter.
Chemical analyses
Heavy metal analyses including iron, manganese, aluminium, copper, zinc, cadmium, and mercury, as well as sulphate and nitrate measurements, were performed by the Institute for Water and Waste water treatment of the Linz AG using standard analytical procedures on October 25, 2005 and April 21, 2008. Additionally, a full set of >50 elements was measured using inductively coupled plasma (ICP) analysis by the Centro de Astrobiologia, Madrid with samples taken on September 10, 2009. Dissolved organic carbon (DOC) concentrations in the lakes were measured once, in September 2008. Samples were gently filtered through precombusted GF/F filters (Whatman) and DOC was measured in an infrared gas analyzer (Kamjunke et al., 2004).
Biological analyses
To determine the plankton community composition two or three samples were taken with a 10-l water sampler from the AML on each occasion, i.e. one or two epilimnic samples and one sample from the deep chlorophyll maximum (DCM). The NL was sampled twice in the mixolimnion (at 3 m and 5–8 m) and once in the monimolimnion (12–13 m). Sampling depths varied slightly because of seasonal water level fluctuations; sampling was adjusted according to the fluorescence profile to ensure that chlorophyll peaks were sampled.
For bacteria, 30 mL unfiltered water samples were formaldehyde fixed to yield a final concentration of 1%. Bacterial abundance was determined by epifluorescence microscopy after DAPI staining in the laboratory (Porter and Feig, 1980). Phytoplankton samples were fixed with acid Lugol's solution (2 mL/100 mL sample) in 100 mL brown glass bottles. The abundance and community composition of the phytoplankton was assessed by the Utermöhl procedure (Utermöhl, 1958) using an inverted microscope (Nikon). Since unequivocal species identification is impossible in fixed samples for many phytoplankton taxa, we assessed the phytoplankton composition at the genus level. The cell sizes of bacteria and phytoplankton species were determined by an image analysis system (NIS Elements, Nikon). From these measurements cell volume of bacteria and phytoplankton was calculated assuming appropriate geometric shapes. Carbon biomass was converted from cell volume assuming conversion factors used by Kamjunke et al. (2004), i.e. 230 fg C μm3 for Chlamydomonas and Ochromonas and the allometric function of cell volume according to Simon and Azam (1989) for coccoid bacteria. For filaments (>5 μm length) a mean value of 220 fg C μm–3 (Bratbak and Dundas, 1984) was used.
On several occasions, the phytoplankton samples were also analysed by flow cytometry (Facs Calibur, Becton Dickinson, San Jose, CA, USA). The flow cytometric cell counts were important for the assessment of the phytoplankton abundance in the DCM, because detritus and inorganic particles hindered the microscopic analysis of these samples. The taxon identity of the organisms in the DCM and of the two most abundant phytoflagellates in the epilimnic samples of the AML was verified by molecular taxonomy. After PCR amplification and sequencing of the SSU rDNA, a BLAST search was conducted to identify the phylogenetic position of the respective species (T. Berendonk et al., unpubl.).
To quantify and determine the zooplankton composition, 10 L from each sampling depth were filtered through an 11-μm net and concentrated to 100 mL. The filtrate was then formaldehyde-fixed (1%, v/v, final concentration). The samples from the DCM were pre-filtered through 100 μm to remove the high detritus and inorganic particle concentrations. All zooplankton samples were analysed under an inverted microscope (Nikon) using the Utermöhl procedure (Utermöhl, 1958). Body length and width were measured with an image analysing system (NIS Elements, Nikon). Rotifer biomass was calculated from geometric formulas established by Ruttner-Kolisko (1977). To convert biovolume into carbon biomass we used the conversion factors from Telesh et al. (1998). The biomass of crustaceans was calculated via length–weight-relations provided by Bottrell et al. (1976). For carbon content estimates of crustaceans, we assumed a C content of 4% of wet weight, i.e. a fresh to dry weight ratio of 10:1 and a carbon content of 40% of dry weight (e.g. Dumont et al., 1975; Manca and Comoli, 2000).
In addition to the discrete samples, an integrated sample from the whole water column was taken with an 11-μm plankton net. This sample was divided into two parts; 100 mL were formalin-fixed and the remaining part was kept in a Dewar vessel for transportation to the laboratory and then used for live observations under a stereo microscope.
Diversity of phyto- and zooplankton was calculated according to Shannon's index, i.e.:
where pi is the relative abundance of each species, calculated as the proportion of individuals of a given species to the total number of individuals in the community.
Results
Temperature, pH, and mixing
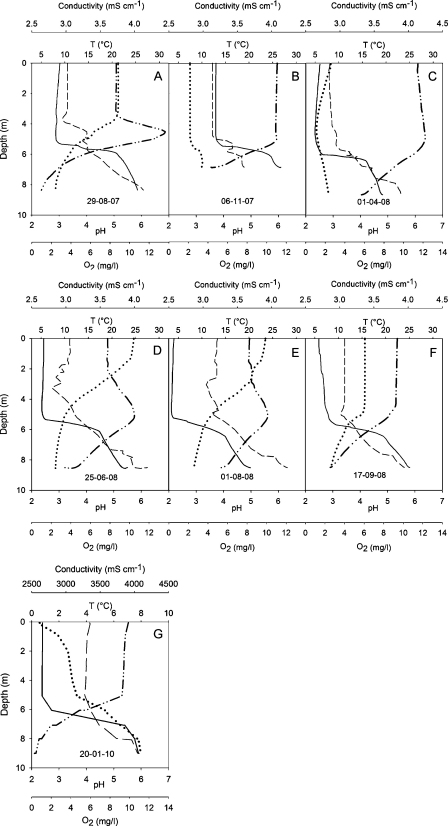
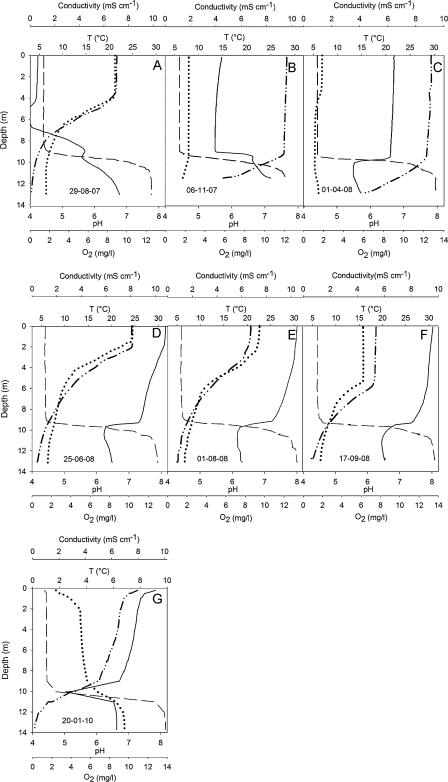
The temperature profiles of both lakes varied seasonally, but showed relatively stable seasonal patterns during two consecutive years (Figs. 2 and 3). During summer, both lakes were strongly stratified, and their temperature profiles differed little. The metalimnion was usually 0.5–1 m deeper in the NL than in the AML (Figs. 2A, E, F and 3A, E, F). Although the temperature profiles of both lakes were overall similar, the AML differed from the NL in the deep water layer. Temperature profiles measured in spring (Figs. 2C and 3C) and autumn (Figs. 2B and 3B) suggest that the AML does not mix to the bottom. Temperature recorded under ice in January 2010 (Fig. 2G) shows the existence of a relatively warm (>5 °C) monimolimnion in the AML more clearly. Temperature measured in the NL on the same occasion (Fig. 3G) suggests that this lake was also meromictic in this year. However, nearly isothermal conditions in the NL were recorded in autumn 2007 (Fig. 3B) and spring 2008 (Fig. 3C). Accordingly, meromixis was less obvious in the NL from the temperature profiles and may be restricted to some years.
Fig. 2.
Depth profiles recorded on 7 sampling dates in the acid mining lake including pH (solid line), conductivity (dashed line), temperature (dotted line), and oxygen (dashed and double dotted line).
Fig. 3.
Depth profiles recorded on 7 sampling dates in the neutralized pit lake including pH (solid line), conductivity (dashed line), temperature (dotted line), and oxygen (dashed and double dotted line).
Profiles of pH differed markedly between the two adjacent lakes (Figs. 2 and 3). With the exception of late summer and autumn 2007 (Fig. 3A and B), pH in the epilimnion of the NL ranged from 6 to 8. The reason for the unusually low pH (∼4) recorded in the NL in 6–9 m depth during June (data not shown) and closer to the surface in late August 2007 (Fig. 3A) remained unknown. We cannot rule out that there is some water flow from the AML into the NL. However, the vertical conductivity profiles measured in both lakes during summer 2007 suggest otherwise. The pH gradient between 9 and 11 m was less marked on August 29 (Fig. 3A) than on the following (Fig. 3B) sampling occasion. In the deep water layer (>10 m), pH was relatively constant and close to 6. The pH profile, therefore, enhances the conjecture derived from the thermal profile that the NL is meromictic. This is certainly the case for the AML; pH increased from 5 to 6 m to the lake bottom on each occasion, indicating two different water masses. Similar to the NL, pH was close to 6 in the deep water layer. Total acidity (base capacity KB 4.3) measured on two occasions in autumn ranged from 8 to 9 mmol L−1 in the AML.
Conductivity and oxygen
Similar to pH, conductivity increased with depth from 2.8 to 3.2 mS cm−1 at the surface to 3.6–4.1 mS cm−1 close to the lake bottom in the AML (Fig. 2). Oxygen concentration showed an inverse pattern, decreasing sharply beneath 5–6 m. Suboxic conditions (<3 mg O2 L−1) were recorded under ice in winter (Fig. 2G), when oxygen saturation dropped to 2.4% above ground, and in both years during summer (Fig. 2A and F). Oxygen depletion in the deep water layer of the AML was more pronounced in 2007 than in the following year. Oxygen concentrations < 0.6 mg O2 L−1, corresponding to saturation levels ≤5%, were recorded in 7–9 m depth during June 2007 (data not shown), when the highest surface water temperature was measured during the investigation period.
Oxygen deplete conditions persisted in the NL beneath 10 m in both study years (Fig. 3A and D–F). Anaerobic conditions were recorded at the lake bottom when the lake was frozen and in both years during summer (Fig. 3A and D–G). Conductivity in the NL increased sharply at 10 m throughout the investigation period, separating an upper layer with remarkably constant and, relative to the AML, reduced conductivity of ∼1 mS cm−1 from a deep water layer with 10-fold higher conductivity.
The conductivity and oxygen profiles indicate a strong and stable chemocline in the NL, i.e. the persistence of a monimolimnion during the study period. A monimolimnion was also present in the AML, but the chemocline was less steep than in the NL.
Chemical composition
The chemical composition of the water in the AML was dominated by sulphate, iron, and calcium (Table 1). Further, the AML was characterized by high concentrations of Mg, Al and Mn throughout the water column. The concentration of Sr exceeded 1 mg L−1 in the deep water layer during September 2009. Concentration of Zn, Cu, Cd and Hg (Table 1) and of >20 other heavy metals were all <1 mg L−1 (data not shown). The DOC concentration in 0–5 m water depth was 6.5 mg L−1 in September 2009.
Table 1.
Major chemical constituents in the acid mining lake and the neutralized pit lake at 3 different depths (means of three measurements, if not stated otherwise; SO4 in g L−1, all other in mg L−1).
| Acid mining lake |
Neutralized lake |
|||||
|---|---|---|---|---|---|---|
| Depth | 0–3 m | 5 m | 7–8 m | 0–3 m | 4–6 m | 12–13 m |
| Fe | 81 ± 30 | 95 ± 30b | 382 ± 133 | 0.63 ± 0.51 | 1.09 ± 0.38 | 1673 ± 547 |
| SO4 | 1.29 ± 0.27 | 1.64 ± 0.01b | 1.44 ± 087 | 0.45 ± 0.42 | 0.69 ± 0.44 | 3.33 ± 1.13 |
| Mg | 64a | n.d. | 133a | 29a | 46a | 57a |
| Ca | 198a | n.d. | 341a | 25a | 187a | 362a |
| NO3 | 0.46 ± 0.19 | 0.47 ± 0.22 | 0.53 ± 0.31 | 0.94 ± 0.57 | 4.02 ± 3.85 | 0.72 ± 0.30 |
| NH4 | 3.14 ± 0.09c | 3.18 ± 0.13c | 5.46 ± 0.16c | 0.54 ± 0.21c | 0.50 ± 0.17c | 2.58 ± 0.39c |
| Mn | 7.7 ± 1.8 | 8.3 ± 2.2b | 8.9 ± 1.0 | 1.6 ± 0.6 | 2.3 ± 0.9 | 6.6 ± 1.4 |
| Al | 14.4 ± 4.3 | 16.4 ± 3.4b | 5.3 ± 7.9 | <0.03 | 0.15 ± 0.21 | 0.07 ± 0.01 |
| Cu | 0.04 ± 0.04 | 0.06 ± 0.06b | <0.02 | <0.02 | <0.01 | <0.02 |
| Zn | 0.49 ± 0.15 | 0.55 ± 0.15b | 0.23 ± 0.29 | 0.03 ± 0.02 | 0.05 ± 0.02 | 0.06 ± 0.02 |
| Sr | 0.863a | n.d. | 1.387a | 0.374a | 0. 498a | 3.506a |
| Cd | <0.001 | <0.003b | <0.001 | <0.002 | <0.001 | <0.001 |
| Hg | <0.0002 | <0.0002b | <0.0002 | <0.0002 | <0.0002 | <0.0002 |
Single measurement on September 10, 2009.
Measured on October 25, 2005 and April 1, 2008.
Measured on September 29 and October 25, 2005.
The chemical composition of the NL differed distinctly from that of the AML. Fe concentration in 0–6 m was two orders of magnitude lower than in the AML, while in deep water the concentrations of Fe and SO4 even exceeded those measured in the AML (Table 1). Concentrations of Mg, Ca, and Mn were all somewhat lower in the NL. Similar to Fe, Al was two orders of magnitude lower in the NL, relative to the AML. With the exception of Sr, which peaked in the deep water of the NL, the other constituents were of minor quantitative importance. The DOC concentration in the mixolimnion of the NL ranged from 7.3 to 8.4 mg L−1 in September 2009.
Nitrate concentrations were generally higher in the NL than in the AML (Table 1). The peak NO3 concentration recorded in the NL is mainly due to one exceptionally high value (9.4 mg L−1) measured at 4.5 m water depth in early April 2008. Ammonia levels were reduced in the mixolimnion of the NL (Table 1) and exceeded 3 mg L−1 throughout the water column of the AML during autumn 2005. In the epilimnion of the NL, SRP ranged from 1.65 to 3.66 μg L−1 (n = 4), TP from 11.3 to 16.4 μg L−1 (n = 4). In the deep water of the NL and in all depths of the AML, dissolved iron interfered with the colorimetric phosphorus assays, yielding unreliable results. We therefore refrained from continuing TP and SRP measurements. The ICP analysis performed in September 2009 yielded P concentrations below the detection limit (<0.001 μg P L−1) in surface and deep water layers of both lakes.
Chlorophyll profiles
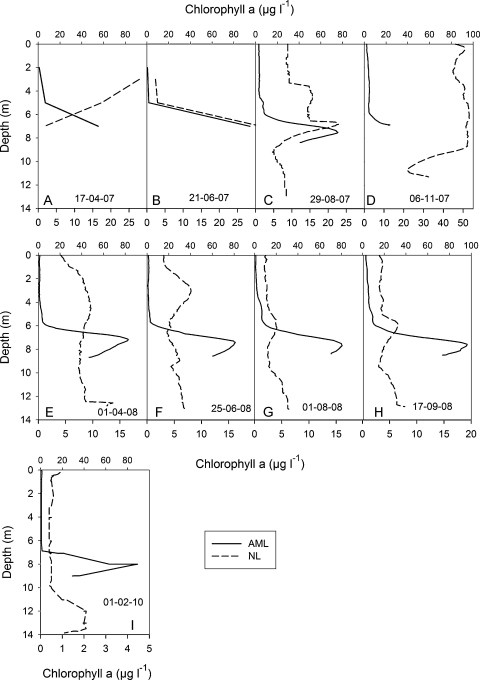
Chlorophyll a profiles were remarkably different between the two study lakes. In the mixolimnion, chlorophyll levels were always higher in the NL than in the AML (Fig. 4; note the different X-scales for the lakes). Chlorophyll peaked in the NL in autumn 2007, when concentrations close to 50 μg L−1 were measured in the mixolimnion (Fig. 4D). During summer, the chlorophyll maximum was recorded mostly in 6–7 m water depth (Fig. 4B, G, and H). From early to late summer 2008, the chlorophyll peak migrated downward in the NL (Fig. 4F–H). Under ice cover, highest chlorophyll concentrations were measured in the deep water of the NL (Fig. 4I).
Fig. 4.
Chlorophyll a profiles recorded on 9 occasions in the acid mining lake (solid line, top x-axis) and in the neutralized pit lake (dashed line, bottom x-axis).
In the AML, we recorded a stable deep chlorophyll maximum (DCM) at 7–8 m (Fig. 4A–I). In the DCM, chlorophyll concentrations were relatively constant, ranging from 80 to 96 μg L−1. The DCM was also found when the lake was ice covered (Fig. 4I). In the upper 5 m of the water column in the AML, chlorophyll levels ranged from 0.6 to 6.8 μg L−1.
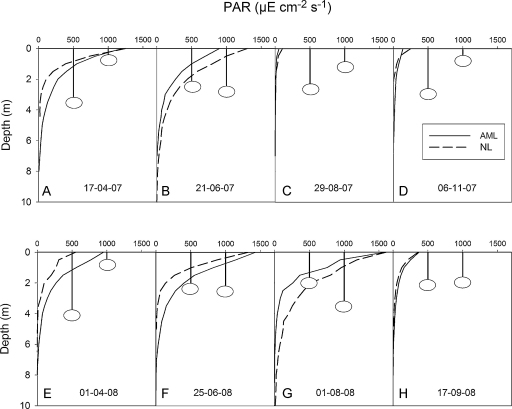
Optical properties
Seasonal and interannual variation of optical properties was less pronounced in the AML than in the NL. Vertical profiles of photosynthetically active radiation (PAR) are shown in Fig. 5, together with Secchi disc readings. The extinction coefficient ɛ, averaged over the PAR spectrum and the euphotic zone (≥1% surface light intensity), ranged from 0.627 m−1 (on 17 April 2007) to 0.841 m−1 (on 1 August 2008) in the AML. The average extinction coefficient was more variable in the NL, with a minimum of 0.498 m−1 recorded on 1 August 2008 and a maximum of 1.432 m−1, measured on 11 November 2007. On five of the eight sampling occasions shown in Fig. 5, ɛ exceeded 1.0 m−1 in the NL. The depth of the euphotic zone, zeu, was calculated as:
where 100 and 1 correspond to the relative light intensities (in %) measured at the surface and at the depth where surface light intensity was reduced to 0.01. The depth of the euphotic zone ranged from 5.5 to 7.3 m in the AML and from 3.2 to 9.2 m in the NL. On most occasions, Secchi disc readings corresponded closely to these estimates. Secchi depth was lowest in the NL in spring (Fig. 5A and E) and autumn (Fig. 5D) and peaked in the AML in early spring (Fig. 5E). The minimum ɛ and the maximum zeu coincided with the peak of the Secchi disc readings (3.5 m, Fig. 5G) in the NL.
Fig. 5.
PAR profiles and Secchi depth recorded on 8 sampling dates in the acid mining lake (PAR: solid line; Secchi: left vertical bar) and in the neutralized pit lake (PAR: dashed line; Secchi: right vertical bar).
Bacteria
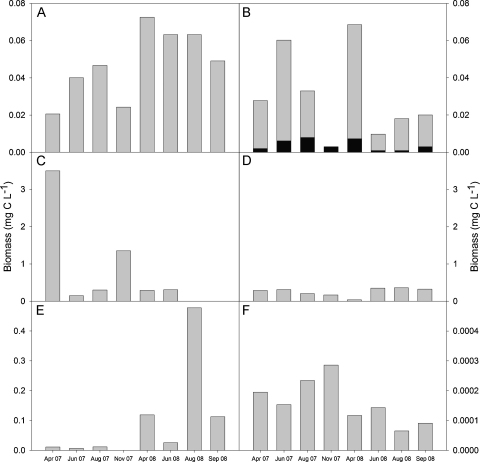
Bacterial abundance in the NL was evenly distributed over all water layers, mostly ranging from 1 to 2 × 109 cells L−1. The maximum, close to 2.6 × 109 cells L−1, was recorded in 3 m depth in April 2008, the minimum of 7.4 × 108 cells L−1 in April 2007. This range corresponded to a bacterial carbon content of 20–73 μg C L−1 (Fig. 6A). In the AML, the abundance of coccal bacteria was unevenly distributed, with 1–6 × 109 cells L−1 in the monimolimnion and an order of magnitude lower cell numbers in the mixolimnion. Only ∼4 × 107 cells L−1 were recorded during June and August 2008, corresponding to a carbon biomass of 0.9 μg C L−1 (Fig. 6B). The carbon biomass of the coccal bacteria in the mixolimnion of the AML was usually in the range of 2–7.5 μg C L−1. Filamentous bacteria appeared with 2 × 106–107 cells L−1 in the upper water layer of the AML downward to a depth of ∼5 m. Filaments were absent only in November 2007. Although the abundance of filamentous bacteria was one order of magnitude lower than the cell number of the coccoid bacteria, the carbon content of the former was approximately one order of magnitude higher than that of the latter, ranging from 10 to 60 μg C L−1 (Fig. 6B). Accordingly, the total bacterial carbon content was similar in both lakes during April 2007 to April 2008. Thereafter, the bacterial carbon content in the NL was 2.5–4-fold higher than in the AML (Fig. 6A and B).
Fig. 6.
Plankton biomass in the mixolimnion of the NL (left panels) and the AML (right panels). The bars in (A) and (B) denote bacterial biomass; the stacked bars in (B) are divided into biomass of single celled (black part) and filamentous bacteria (grey part). (C and D) Phytoplankton biomass; zooplankton biomass is illustrated in (E) and (F).
During the first year of investigation, picocyanobacteria were recorded at low numbers of 4.5 × 106 cells L−1 only in the NL during June. In 2008, they were present in the NL on all occasions except in April, in cell numbers ranging from 2 to 3.5 × 107 L−1.
Phytoplankton
Morphological determination under the microscope and sequencing of the ssu rDNA identified the two most abundant phytoflagellates in the mixolimnion of the AML as the osmomixotrophic Chlamydomonas acidophila and the phagomixotrophic Ochromonas sp. Flow cytometric measurements confirmed that the phytoplankton was composed of only two abundant species. The euglenid Lepocinclis buetschlii contributed to the phytoplankton in the mixolimnion of the AML with low abundances ranging from 1.0 to 13.1 × 104 cells L−1 during April, June, and November 2007. C. acidophila occurred with 1.1–2.9 × 107 cells L−1, i.e. exceeded the abundance of Ochromonas sp. usually by a factor of 2–3. We recorded lower cell numbers of C. acidophila (2.1 × 106 cells L−1) than of Ochromonas sp. (2.4 × 106 cells L−1) only in April 2007. These low cell numbers of the two dominant phytoflagellates were equivalent to a carbon content of 37 μg C L−1 (Fig. 6D). On the other sampling occasions, phytoplankton biomass varied seasonally from 168 μg C L−1 in November 2007 to 360 μg C L−1 recorded in summer 2008. The low species number in the AML was reflected by a low Shannon index with little seasonal variation, ranging from 0.23 to 0.29 in the AML.
The monimolimnion of the AML was free of C. acidophila and Ochromonas sp. The euglenid L. buetschlii thrived in the monimolimnion and formed a stable DCM of 80–96 μg chl a L−1 (Fig. 4). Flow cytometric analyses revealed abundances of 7.8 × 107 cells L−1 at 7.8 m depth, respectively 11.0 × 107 cells L−1 at 9 m depth. With an estimated L. buetschlii cell volume of 5900 μm3, this abundance yields an exceptionally high biomass of 50–70 mg C L−1 (Weithoff et al., 2010). Although we cannot rule out that some rare species were present in the monimolimnion of the AML, the flow cytometric histograms confirmed that no other eukaryotic organism occurred at abundances >5 × 104 cells L−1. With the instrument's settings used, the detection limit of the flow cytometer was close to 1.5 × 104 cells L−1.
The phytoplankton composition in the NL was more diverse than in the AML (Table 2). At four of the six sampling dates, the phytoplankton consisted of 13 taxa or more (Table 2) Dominant species were present within the families Chlorophyceae, Chrysophyceae, Dinophyceae, Euglenophyceae, Bacillariophyceae, and Cryptophyceae. Reduced diversity, with only 4 or 5 phytoplankton taxa, was recorded in spring and autumn 2007. Phytoplankton biomass peaked with 3.5 mg C L−1 in spring 2007 and showed a second maximum (1.35 mg C L−1) in autumn of the same year (Fig. 6C). The peak in April 2007 was mainly composed of Ochromonas sp., which reached abundances of 5.4 × 108 cells L−1. Accordingly, the Shannon index was very low on this occasion (0.007, Table 2). On the other sampling occasions, the diversity index ranged from 0.49 to 0.78, i.e. it was significantly higher than in the AML. Even in November 2007, when only 5 taxa were present, Shannon's index reached 0.49 (Table 2), because the abundance of all taxa was similar.
Table 2.
Phytoplankton composition in the NL from April 2007 to June 2008. Taxa with abundances higher than 50 cells mL−1 were considered abundant and denoted with “xx”. All other genera or species that occurred were marked with a single “x”.
| April 2007 | June 2007 | August 2007 | November 2007 | April 2008 | June 2008 | |
|---|---|---|---|---|---|---|
| Ochromonas | xx | xx | xx | xx | xx | x |
| Chorella | xx | xx | x | xx | xx | |
| Tetramites | x | x | xx | xx | xx | |
| Cryptomonas | x | x | xx | xx | ||
| Dinobryon | xx | xx | ||||
| Fragilaria | xx | x | x | xx | ||
| Scenedesmus | x | x | ||||
| Peridinium | x | xx | x | x | x | |
| Gynodinium | x | xx | x | x | x | |
| Chlamydomonas | x | xx | xx | xx | x | |
| Synedra | x | x | ||||
| Nitzschia | x | x | x | x | ||
| Hyalogonium | x | x | x | x | ||
| Pinnularia | x | |||||
| Lepocinclis | x | x | ||||
| Rhizosolenia | x | x | ||||
| Navicula | x | |||||
| Achnanthes | x | |||||
| Chroococcus | xx | x | ||||
| Asterococcus | xx | x | ||||
| Euglena | x | |||||
| Ankistrodesmus | x | |||||
| Number of species | 5 | 14 | 13 | 5 | 13 | 16 |
| Shannon diversity | 0.007 | 0.784 | 0.620 | 0.488 | 0.657 | 0.663 |
Zooplankton
Similar to phytoplankton, zooplankton in the AML was reduced to only two species: the ciliate Oxytricha sp. and the rotifer Cephalodella sp. The Oxytricha sp. from Langau is a new species that will be described in detail elsewhere (Berger et al., in prep.). The new species is approximately 70 μm × 35 μm in length and width, and morphologically most similar to Oxytricha longa. The rotifer Cephalodella sp. from Langau is also an as yet undescribed species, closely related to Cephalodella delicata. Total length of females of the new species varied from 134 to 161 μm. A detailed species description including morphological and molecular identification is in preparation (Jersabek et al. in prep.). Cephalodella sp. was uniformly distributed in the water column, while Oxytricha sp. appeared only in the mixolimnion with abundances between 20 and 40 individuals L−1 in April and June 2007. No ciliates were detected on the following sampling dates. The abundances of Cephalodella sp. ranged from 5 individuals in August 2008 to 22 individuals L−1 in autumn 2007. Zooplankton abundance was higher in the year 2007 than in 2008. The carbon biomass of the zooplankton, i.e. mainly of Cephalodella sp., was low (see Fig. 6F). The heliozoan A. sol was found occasionally in live samples (pers. observation). Crustaceans and insect larvae could neither be detected in the formalin fixed samples nor in live samples or field observations.
The NL harboured more zooplankton species than the AML. In 2007, the zooplankton was dominated by various rotifers (Table 3). Calanoid copepods and their nauplii occurred in negligible numbers. Daphnia were found in spring 2007. Not even a single specimen of any zooplankton species was observed in November 2007. In the subsequent spring, rotifers did not play a quantitative role. Nauplii and calanoid copepods occurred at abundances of 57, respectively 20 individuals L−1. Two months later zooplankton was dominated by rotifers, as in the previous summer. We could unequivocally identify 5 different taxa (Table 3). The rotifer Polyarthra sp. reached high abundances up to more than 1400 individuals L−1. In late summer 2008, zooplankton of the NL was most diverse (Table 3). Keratella sp. and Polyarthra sp. appeared with 690, respectively 860 individuals L−1 during this period. Calanoid copepods, their nauplii, daphnids, and Bosmina sp. were present at abundances ranging from 10 to 87 individuals L−1. Due to the higher abundance of crustaceans in 2008, the total zooplankton biomass was significantly higher in 2008 than in 2007. It peaked with 0.48 mg C L−1 in summer 2008 (Fig. 6E). Although stocking is officially forbidden at Langau (pers. comm. by the mayor, F. Linsbauer), we observed some pike, perch, carp, rudd, roach, and barbel during our sampling in the NL.
Table 3.
Zooplankton composition in the NL over the years 2007 and 2008. The occurrence of a species at the sampling dates was marked with a single “x”.
| April 2007 | June 2007 | August 2007 | November 2007 | April 2008 | June 2008 | August 2008 | September 2008 | |
|---|---|---|---|---|---|---|---|---|
| Keratella | x | x | x | x | x | |||
| Brachionus | x | x | x | |||||
| Filinia | x | x | ||||||
| Asplanchna | x | x | ||||||
| Polyarthra | x | x | x | x | ||||
| Vorticella | x | x | x | x | ||||
| Ciliate | x | x | x | x | x | x | ||
| Bosmina | x | x | ||||||
| Daphnia | x | x | ||||||
| Copepods | x | x | x | x | x | x | ||
| Nauplia | x | x | x | x | x | x | x | |
| Number of species | 3 | 5 | 6 | 0 | 6 | 8 | 8 | 8 |
| Shannon diversity | 0.053 | 0.091 | 0.394 | 0 | 0.265 | 0.130 | 0.458 | 0.655 |
Discussion
Chemical constituents and oxygen profiles point to meromixis in both lakes
The vertical profiles of conductivity, pH and oxygen indicate a distinct chemocline in both lakes. The high iron levels recorded in the deep water of both lakes suggest that they are primarily iron-meromictic (Boehrer et al., 2009). This is certainly the case for the NL, in which the Fe levels increased more than thousand fold from lake surface to bottom. The Fe concentration of ∼1.7 g L−1 measured in 12–13 m deep water exceeds the highest concentration measured in similar Fe-buffered acid pit lakes in the Lusatian mining district by a factor of three (Nixdorf et al., 2003). Meromixis is enhanced by the extremely high SO4 concentration (3.3 g L−1) in the deep water of the NL. Sulphate is less important for the mixing regime in the AML, because SO4 levels were high in the whole water column and did not increase with depth.
Isothermy does not necessarily induce mixing in acid mining lakes (Lessmann et al., 1999). Relative to the dissolved ions, temperature differences are of minor importance for the water circulation. This is because only 10 mg L−1 of salt have the same effect on the density of water as the temperature difference between 5 and 4 °C (Wetzel, 2001). In our study lakes, the total concentration of dissolved solids amounted to several g L−1 (Table 1). Since oxygen depletion was more severe in both lakes during summer 2007 than in the following year, the extent of meromixis may vary from year to year. In 2008, oxygen depletion was more apparent in the deep water of the NL than in the shallower AML. The (near-to-) anoxic conditions measured under ice in winter 2010 further point to the existence of a relatively stable monimolimnion in both lakes.
The magnesium concentration of >100 mg L−1 measured close to the lake bottom in the AML is also higher than in the Lusatian ML (Nixdorf et al., 2003). Conductivity, pH, and the other major chemical constituents of our study lakes fall all into the range known from the Fe-buffered Lusatian lakes (pH range 2.2–3.4, Nixdorf et al., 2003).
The effect of flooding with alkaline river water was restricted to the mixolimnion in the NL. Concentrations of iron, sulphate, manganese, aluminium and zinc were all strongly reduced in the upper 6 m of the NL, relative to the levels measured in the AML (Table 1). Conductivity amounted to 1 mS cm−1, and pH was close to neutral or even in the alkaline range in the epilimnion of the NL. The fact that the former remained stable while the latter varied seasonally, suggest that only pH interacts significantly with biological processes (photosynthesis, respiration) in the NL.
Nutrients and DOC
In the AML, ammonium was the prevailing nitrogen species, while nitrate concentrations were close to 0.5 mg L−1 at all depths (Table 1). This was different in the mixolimnion of the NL, where nitrate levels were higher than ammonium levels. Accordingly, nitrification was likely inhibited in the AML and in the deep water of the NL, as it is usually the case in Fe-buffered acid mining lakes (Nixdorf et al., 2003), but not in the mixolimnion of the NL. Nitrate concentrations measured by the local authorities in the Langaubach during November 2009 were >3 mg L−1, PO4–P levels close to 0.1 mg L−1 (T. Lendenfeld, unpubl. report). In summary, the somewhat cursory nutrient analyses suggest that the supply with nutrients is enhanced in the mixolimnion of the NL, providing improved conditions for primary production, relative to the AML.
The DOC concentrations were higher than those measured in comparable acid pit lakes in Lusatia (maximum reported 5.9 mg L−1, Lessmann et al., 1999; usually <3 mg L−1, Kamjunke et al., 2006; Weithoff et al., 2010). However, as the latter authors pointed out, since DOC quality is more important for bacterial uptake than total DOC concentration, these values have little explanatory power. The composition of DOC in our study lakes awaits further research.
Optical properties
Extinction was generally high in both study lakes. This is typical of iron-rich acid mining lakes with unusually high absorption in the UV range and transmission shifted to the red light, relative to circumneutral lakes (Kamjunke et al., 2004; Gerloff-Elias et al., 2005; Tittel et al., 2005). Optical properties indicated by PAR profiles and Secchi depth (Fig. 5) were more stable in the AML than in the NL. Since in the latter, the ionic composition was less variable than chlorophyll a concentration, we infer that phytoplankton biomass significantly affected optical properties in the NL. This is obvious from comparing PAR profiles recorded during relatively low phytoplankton biomass (Figs. 5F, G, cf. Figs. 6F, G) with those obtained during phytoplankton peaks (Figs. 5C–E and 6C–E).
In contrast to the NL, phytoplankton had only little effect on optical properties in the AML, in spite of the extremely high phytoplankton biomass measured in the DCM.
Bacterial composition and biomass
Although bacteria oxidizing iron and sulphur compounds play a major role in acid mining lakes (Wendt-Potthoff and Neu, 1998; Wendt-Potthoff and Koschorreck, 2002), the bacterial abundance in the mixolimnion of the AML was one order of magnitude lower than in circumneutral mesotrophic lakes (Pöhler et al., 2002). A remarkable finding was the relatively high abundance of filamentous bacteria, ranging from 0.2 to 2.0 × 107 cells L−1 in the AML. Since the individual cell size of the filamentous bacteria was 50-fold higher than that of the coccal bacteria, the total bacterial carbon content in the mixolimnion of the AML was relatively high and comparable to that of the NL. This high content of filamentous bacteria is characteristic of extremely acidic mining lakes and tends to increase with decreasing pH (Wendt-Potthoff and Koschorreck, 2002; Nixdorf and Jander, 2003).
In contrast to the AML, filamentous bacteria were virtually lacking in the NL. Bacterial abundance and bacterial carbon biomass in the NL (Fig. 6A) were in the range known from circumneutral mesotrophic lakes (Bird and Kalff, 1984; Cole et al., 1988; Simon et al., 1992). Picocyanobacteria were first recorded in June 2007, with low abundances of 4.5 × 106 cells L−1, and showed stable populations with 2.0–3.5 × 107 cells L−1 from June to September 2008. The question arises why picocyanobacteria did not occur during the first half of the year 2007 and in spring 2008. When picocyanobacteria occurred, pH in the mixolimnion of the NL was always >6.0; it was even in the alkaline range close to 8.0 during 2008. We recorded a decrease of pH in the NL in August 2007, when pH declined close to 4.0 and remained <6.0 until November 2007 (Fig. 3A and B). The mayor of Langau reported a dramatic decrease of pH to values below 3.0 in the course of heavy precipitation and a sudden increase of the lake level in June 2006, leading to mass mortality of fish (F. Linsbauer, pers. comm.). Since our sampling program started in spring 2007 and, to our knowledge, no other measurements are available for the meantime the persistence of the reduced pH remained unknown. The distribution of picocyanobacteria that we observed is, however, in accordance with the fact that they are sensitive to chemical stress and acidic conditions (Brock, 1973; Munawar and Weisse, 1989; Munawar et al., 1994; Weisse and Mindl, 2002).
Protists and the pelagic food web
Our findings from the AML generally agree with results reported in the literature from similar acid pit lakes in Lusatia, Germany. The low species diversity and the dominance of mixotrophic chrysophytes and chlamydomonads that we observed in the AML are characteristic for acid mining lakes (Nixdorf et al., 1998; Lessmann et al., 2000). We have no direct evidence that C. acidophila is osmomixotrophic in the AML, as it has been reported for an isolate of this species from Lusatia (Tittel and Kamjunke, 2004). However, because the C. acidophila strains isolated from Lusatia and Langau are identical in their ssu rDNA (Moser and Weisse, 2011b) and DOC concentrations are high in the AML (see Section “Nutrients and DOC”, above), we conclude that osmomixotrophy of C. acidophila is likely in the AML. Bacterial ingestion of the Ochromonas sp. isolate from Langau was demonstrated experimentally (Moser and Weisse, 2011a). A top predator in AML, the heliozoan A. sol, was observed only sporadically in live samples at low abundances in the AML at Langau. The mere presence of A. sol does not fully explain the generally low abundance of rotifers and ciliates in the AML. Taken together, they contributed less than 1% to the total plankton biomass (Fig. 6B, D and F). Laboratory experiments with the Oxytricha sp. from Langau revealed that each ciliate ingests <100 C. acidophila cells d−1 at pH 2.6 (Weisse et al., in prep.), i.e. the ciliate population can remove 0.04 μg C L−1 d−1 at the most. Similarly, feeding of Cephalodella sp. on C. acidophila at food concentrations that were comparable to the ambient levels in the AML was negligible (Weithoff, 2005). The threshold carbon concentration for positive population growth of Cephalodella measured in the laboratory (0.34 mg C L−1; Weithoff, 2005) was close to the peak phytoplankton biomass (0.36 mg C L−1) recorded in the AML during our investigation. In the same laboratory experiments, Cephalodella did not ingest Ochromonas sp. (Weithoff, 2005). The latter is known to feed on the larger green alga Chlamydomonas sp. in German acidic ML (Kamjunke et al., 2004; Tittel et al., 2003). However, in the AML at Langau the abundance of C. acidophila usually exceeded that of Ochromonas sp. by a factor of 2–3. Secondly, laboratory experiments conducted with the Ochromonas sp. isolate from Langau revealed that this strain primarily ingests bacteria (Moser and Weisse, 2011a). We conclude that the grazing pressure on phytoplankton is low in the AML. In accordance with this interpretation, phytoplankton cell numbers, carbon content, and chlorophyll a concentrations did not change much in the course of the year (Figs. 4 and 6D).
Lepocinclis is another common genus in acid mining lakes (Steinberg et al., 1999). The high abundance of L. buetschlii (∼108 cells L−1) created the permanent DCM of more than 80 μg chl a L−1 (Fig. 4). To our knowledge, this has never been reported in any comparable acid mining lake. A similar permanently high DCM was found in the monimolimnion of Lake Waldsee in Lusatia, but this was not caused by Lepocinclis but by Chlamydomonas sp. (Nixdorf et al., 1998). Since no larger consumers were present in the AML and Lepocinclis is too large (∼35 μm) for most rotifers, Lepocinclis did not suffer from large grazing losses, which contribute to the formation of a DCM in many circumneutral lakes (Weithoff et al., 2010). Due to the absence of grazers Lepocinclis required only low growth rates to maintain its high abundance. In the monimolimnion of the AML, L. buetschlii has to withstand low oxygen and low light conditions. The fact that the DCM was close to or even below the compensation depth, where surface PAR intensity was reduced to ∼1%, may suggest a mixotrophic nutrition for Lepocinclis. Several species of the genus Lepocinclis are known to prefer iron-rich pools, ditches, and acidic ponds (Preisfeld, 2009). The suboxic conditions, the further increased iron and sulphate content in the monimolimnion of the AML compared to the already high values measured its mixolimnion, provided an environment that no other eukaryotic organism than L. buetschlii can tolerate.
Species richness and diversity
Even with our limited taxonomic resolution (i.e., mostly at the genus level), it is obvious that the phytoplankton in the NL was more diverse than in the AML (Table 2). The reduced diversity that we recorded in spring and autumn 2007 was, most likely, due to the pH fluctuations mentioned above. During periods with pH consistently >6.0, phytoplankton consisted of 13–16 species belonging to 6 different classes. We recorded at least 7 different diatom species at this time (Table 2). The occurrence of diatoms correlated inversely with silicate concentration, which was approximately 5-fold higher in the AML than in the NL. High silicate concentrations of more than 10 mg L−1 are common for acid mining lakes (Geller and Schultze, 2009). As a consequence of uptake by diatoms, the silicate concentration usually decreases over a period of some years after neutralization (Rönicke et al., 2010).
A few calanoid copepods and mostly rotifers contributed to the zooplankton biomass of the NL in 2007. Generally, zooplankton biomass was low in 2007 (Fig. 6E), and zooplankton species composition was not comparable to natural lakes. It is known that rotifers predominate over crustaceans in acid mining lakes with pH up to 4.5. Copepods are rare at this pH and cladocerans become more important at higher pH (Deneke, 2000). Probably due to the stable neutral to alkaline conditions in the NL in 2008, a richer and more diverse (Table 3) zooplankton fauna could develop. The rotifer Polyarthra sp. occurred in the NL on all sampling dates. Several species of the genus Polyarthra appear in moderately acidic environments close to pH 5.0, but have their optimum at pH ∼7.0 (Berzins and Pejler, 1987). Furthermore, daphnids, Bosmina, and calanoid copepods contributed to the zooplankton in 2008 at higher abundances than in the previous year. Daphnids are an indicator for neutral lakes, requiring pH values >6.0 (Rönicke et al., 2010). Copepods can tolerate a moderately low pH (Steinberg et al., 1998). Environmental parameters must fit within a relatively narrow margin for daphnids (Rönicke et al., 2010). We conclude that the episodic changes of the proton concentrations that we measured in the NL in 2007 were too high for daphnids.
Remarkably, not a single specimen of the rotifer Cephalodella sp., which was common in the AML, was observed in the NL during our sampling. This is in accordance with recent experimental results showing that the new Cephalodella species from the AML cannot reproduce at neutral conditions (Laufenstein, 2010). Altogether, the zooplankton composition and biomass of the NL was more similar to that of natural circumneutral lakes in 2008 than in 2007.
General conclusions
Our investigation revealed that the mixolimnion of the AML remained in its original, extremely acidic state, almost 50 years after its man-made creation and is comparable to younger acid pit lakes known from the Lusatian area in Germany (Weithoff et al., 2010). We did not observe any signs of natural eutrophication. In spite of some peculiarities, we portrayed the AML as a typical, Fe-buffered acid pit lake.
Taken together, the results reported in this work demonstrate that (i) neutralization of the NL was successful, but that (ii) the effect of flooding was restricted to the mixolimnion of this lake. As a side effect of flooding, the chemocline was enhanced and mixing, therefore, further reduced. This is in contrast to the natural situation. The larger NL is more wind exposed than the smaller AML (Fig. 1), which is illustrated by the somewhat deeper epilimnion of the former (cf. temperature profiles, Figs. 2 and 3). The benefits of flooding in the mixolimnion were reached, to some extent, at the expense of the ecological situation in the monimolimnion. The monimolimnion of the NL provided even more adverse conditions for eukaryotic organisms than that of the AML. Since recreational activities are restricted to the (near-to)-surface waters, flooding was economically successful.
From an ecological point of view, flooding was also successful. The plankton composition in the NL was more diverse than in the AML and established a community similar to circumneutral lakes in 2008. The NL achieved the status of ‘good ecological potential’ requested by the European Water Framework Directive for artificial water bodies (for details, see Borja and Elliott, 2007). However, biotic parameters responded sensitive to pH fluctuations recorded in the previous year. Accordingly, non-permanent flooding with alkaline water, as it has been practised for the past 15 years, does not guarantee sustainable, circumneutral conditions. Overall, the biotic parameters responded more sensitive to periodic disturbance than the abiotic parameters. More frequent and more intense sampling is necessary to analyse the major forces causing the pH fluctuations in the NL and their consequences for the biota.
Acknowledgements
We thank U. Scheffel and P. Stadler for their assistance during the sampling at Langau. The cooperation by the mayor of Langau, F. Linsbauer, who provided unpublished data on precipitation at Langau and chemical measurements in the Langaubach, is gratefully acknowledged. We thank our colleagues from the University of Potsdam, G. Weithoff and U. Gaedke, for providing DOC measurements and exchanging data and ideas in the course of this project. Erik Zettler (Sea Education Association, Woods Hole, USA) and Angeles Aguilera (Centro de Astrobiologia, Madrid) provided ICP analyses of chemical elements.
Dana Barth and Thomas Berendonk provided the molecular identification of the flagellates. This work was supported by the Austrian Science Fund (FWF Project P20118-B17).
References
- Berzins B., Pejler B. Rotifer occurrence in relation to pH. Hydrobiologia. 1987;147:107–116. [Google Scholar]
- Bird D.F., Kalff J. Empirical relationships between bacterial abundance and chlorophyll concentration in fresh and marine waters. Can. J. Fish. Aquat. Sci. 1984;41:1015–1023. [Google Scholar]
- Boehrer B., Dietz S., von Rohden C., Kiwel U., Jöhnk K.D., Naujoks S., Ilmberger J., Lessmann D. Double-diffusive deep water circulation in an iron-meromictic lake. Geochem. Geophys. Geosyst. 2009;10:1–7. [Google Scholar]
- Borja A., Elliott M. What does ‘good ecological potential’ mean, within the European Water Framework Directive? Mar. Pollut. Bull. 2007;54:1559–1564. doi: 10.1016/j.marpolbul.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Bratbak G., Dundas I. Bacterial dry matter and biomass estimations. Appl. Environ. Microbiol. 1984;48:755–757. doi: 10.1128/aem.48.4.755-757.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottrell H.H., Duncan A., Gliwicz Z.M., Herzig G.E., Hillbricht A., Ilkowska H., Larrson A., Weglenska T. A review of some problems in zooplankton production studies. Norw. J. Zool. 1976;24:419–456. [Google Scholar]
- Brock T.D. Lower pH limit for the existence of blue-green algae: evolutionary and ecological implications. Science. 1973;179:480–483. doi: 10.1126/science.179.4072.480. [DOI] [PubMed] [Google Scholar]
- Cole J.J., Findlay S., Pace M.L. Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Mar. Ecol. Prog. Ser. 1988;43:1–10. [Google Scholar]
- Deneke R. Review of rotifers and crustaceans in highly acidic environments of pH values <3. Hydrobiologia. 2000;433:167–172. [Google Scholar]
- Dumont H.J., Van de Velde I., Dumont S. The dry weight estimate of biomass in a selection of cladocera, copepoda and rotifera from the plankton, periphyton and benthos of continental waters. Oecologia. 1975;19:75–97. doi: 10.1007/BF00377592. [DOI] [PubMed] [Google Scholar]
- Gaedke U., Kamjunke N. Structural and functional properties of low- and high-diversity planktonic food webs. J. Plankton Res. 2006;28:707–718. [Google Scholar]
- Geller W., Klapper H., Salomons W., editors. Acidic Mining Lakes: Acid Mine Drainage, Limnology and Reclamation. Springer; New York: 1998. [Google Scholar]
- Geller W., Schultze M. Acidification. In: Likens G., editor. vol. 3. Academic Press; Oxford: 2009. pp. 1–12. (Encyclopedia of Inland Waters). [Google Scholar]
- Gerloff-Elias A., Spijkerman E., Pröschold T. Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila Negoro, isolated from an extremely acid lake (pH 2.6) Plant Cell Environ. 2005;28:1218–1229. [Google Scholar]
- Kamjunke N., Gaedke U., Tittel J., Weithoff G., Bell E.M. Strong vertical differences in the plankton composition of an extremely acidic lake. Arch. Hydrobiol. 2004;161:289–306. [Google Scholar]
- Kamjunke N., Bohn C., Grey J. Utilisation of dissolved organic carbon from different sources by pelagic bacteria in an acidic mining lake. Arch. Hydrobiol. 2006;165:355–364. [Google Scholar]
- Laufenstein, N., 2010. Lebenszyklus einer acidophilen Cephalodella-Art in Abhängigkeit von verschiedenen Futterkonzentrationen, pH-Werten und Temperaturen. Master's thesis. Paris-Lodron-University Salzburg, Salzburg.
- Lessmann D., Deneke R., Ender R., Hemm M., Kapfer M., Krumbeck H., Wollmann K., Nixdorf B. Lake Plessa 107 (Lusatia Germany)—an extremely acidic shallow mining lake. Hydrobiologia. 1999;408/409:293–299. [Google Scholar]
- Lessmann D., Fyson A., Nixdorf B. Phytoplankon of the extremely acidic mining lakes of Lusatia (Germany) with pH <3. Hydrobiologia. 2000;433:123–128. [Google Scholar]
- Manca M., Comoli P. Biomass estimates of freshwater zooplankton from length-carbon regression equations. J. Limnol. 2000;59:15–18. [Google Scholar]
- Moser M., Weisse T. The outcome of competition between the two chrysomonads Ochromonas sp. and Poterioochromonas malhamensis depends on pH. Eur. J. Protistol. 2011;47 doi: 10.1016/j.ejop.2011.01.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M., Weisse T. Combined stress effect of pH and temperature narrows the niche width of flagellates in acid mining lakes. J. Plankton Res. 2011;33 doi: 10.1093/plankt/fbr014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munawar M., Weisse T. Is the “microbial loop” an early warning indicator of anthropogenic stress? Hydrobiologia. 1989;188/189:163–174. [Google Scholar]
- Munawar M., Munawar I.F., Weisse T., Leppard G.G., Legner M. The significance and future potential of using microbes for assessing ecosystem health: the Great Lakes example. J. Aquat. Ecosyst. Health. 1994;3:230–295. [Google Scholar]
- Nixdorf B., Jander J. Bacterial activities in shallow lakes—a comparison between extremely acidic and alkaline eutrophic hard water lakes. Hydrobiologia. 2003;506–509:697–705. [Google Scholar]
- Nixdorf B., Mischke U., Leßmann D. Chrysophytes and chlamydomonads: pioneer colonists in extremely acidic ming lakes (pH > 3) in Lusatia (Germany) Hydrobiologia. 1998;369/370:315–327. [Google Scholar]
- Nixdorf B., Lessmann D., Steinberg C.E.W. The importance of chemical buffering for pelagic and benthic colonization of acidic waters. Water Air Soil Pollut. 2003;3:27–46. [Google Scholar]
- Nixdorf B., Lessmann D., Deneke R. Mining lakes in a disturbed landscape: application of the EC Water Framework Directive and future management strategies. Ecol. Eng. 2005;24:67–73. [Google Scholar]
- Pöhler I., Wenderoth D.F., Wendt-Potthoff K., Höfle M.G. Bacterioplankton community structure and dynamics in enclosures during bioremediation experiments in an acid mining lake. Water Air Soil Pollut. Focus. 2002;2:111–121. [Google Scholar]
- Porter K.G., Feig Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980;25:943–948. [Google Scholar]
- Preisfeld A. Euglenida (Euglenophyta) Protozool. Monogr. 2009;4:47–59. [Google Scholar]
- Rönicke H., Schultze M., Neumann V., Nitsche C., Tittel J. Changes of the plankton community composition during chemical neutralisation of the Bockwitz pit lake. Limnologica. 2010;40:191–198. [Google Scholar]
- Ruttner-Kolisko A. Suggestions for biomass calculation of planktonic rotifers. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1977;8:71–76. [Google Scholar]
- Simon M., Azam F. Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Prog. Ser. 1989;51:201–213. [Google Scholar]
- Simon M., Cho B.C., Azam F. Significance of bacterial biomass in lakes and the ocean: comparison to phytoplankton biomass and biogeochemical implications. Mar. Ecol. Prog. Ser. 1992;86:103–110. [Google Scholar]
- Steinberg C., Fyson A., Nixdorf B. Extrem saure Seen in Deutschland. Biologie unserer Zeit. 1999;29:98–109. [Google Scholar]
- Steinberg C.E.W., Schäfer H., Tittel J., Beisker W. Phytoplankton composition and biomass spectra created by flow cytometry and zooplankton composition in mining lakes of different states of acidification. In: Geller W., Klapper H., Salomons W., editors. Acidic Mining Lakes. Springer; Berlin: 1998. pp. 127–145. [Google Scholar]
- Telesh I.V., Rahkola M., Viljanen M. Carbon content of some freshwater rotifers. Hydrobiologia. 1998;387/388:355–360. [Google Scholar]
- Tittel J., Kamjunke N. Metabolism of dissolved organic carbon by planktonic bacteria and mixotrophic algae in lake neutralization experiments. Freshw. Biol. 2004;49:1062–1071. [Google Scholar]
- Tittel T., Zippel B., Geller W., Seeger J. Relationships between plankton community structure and plankton size distribution in lakes of northern Germany. Limnol. Oceanogr. 1998;43:1119–1132. [Google Scholar]
- Tittel J., Bissinger V., Zippel B., Gaedke U., Bell E., Lorke A., Kamjunke N. Mixotrophs combine resource use to outcompete specialists: implications for aquatic food webs. Proc. Natl. Acad. Sci. 2003;100:12776–12781. doi: 10.1073/pnas.2130696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittel J., Bissinger V., Gaedke U., Kamjunke N. Inorganic carbon limitation and mixotrophic growth in Chlamydomonas from an acidic mining lake. Protist. 2005;156:63–75. doi: 10.1016/j.protis.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Utermöhl H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt. Int. Ver. Limnol. 1958;9:1–38. [Google Scholar]
- Weisse T., Mindl B. Picocyanobacteria—sensitive bioindicators of contaminant stress in an alpine lake (Lake Traunsee Austria) Water Air Soil Pollut. Focus. 2002;2:191–210. [Google Scholar]
- Weithoff G. On the ecology of the rotifer Cephalodella hoodi from an extremely acidic lake. Freshw. Biol. 2005;50:1464–1473. [Google Scholar]
- Weithoff G., Moser M., Kamjunke N., Gaedke U., Weisse T. Lake morphometry strongly shapes the plankton community structure in acidic mining lakes. Limnologica. 2010;40:161–166. doi: 10.1016/j.limno.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Potthoff K., Koschorreck M. Mikrobiologie saurer Tagebaurestseen. Wasser und Boden. 2002;54:6–10. [Google Scholar]
- Wendt-Potthoff K., Neu T.R. Microbial processes for potential in situ remediation of acid lakes. In: Geller W., Klapper H., Salomons W., editors. Acid Mining Lakes. Springer; Berlin: 1998. pp. 269–284. [Google Scholar]
- Wetzel R.G. 3rd ed. Academic Press; San Diego: 2001. Limnology—Lake and River Ecosystems. [Google Scholar]
- Wollmann K., Deneke R., Nixdorf B., Packroff G. Dynamics of planktonic food webs in three mining lakes across a pH gradient (pH 2–4) Hydrobiologia. 2000;433:3–14. [Google Scholar]
- YSI Incorporated . Revision F. Yellow Springs; Ohio, USA: 2009. 6-Series Multiparameter Water Quality Sondes User Manual. [Google Scholar]