Abstract
The pineal gland is a neuroendocrine organ of the brain. Its main task is to synthesize and secrete melatonin, a nocturnal hormone with diverse physiological functions. This review will focus on the central and pineal mechanisms in generation of mammalian pineal rhythmicity including melatonin production. In particular, this review covers the following topics: (1) local control of serotonin and melatonin rhythms; (2) neurotransmitters involved in central control of melatonin; (3) plasticity of the neural circuit controlling melatonin production; (4) role of clock genes in melatonin formation; (5) phase control of pineal rhythmicity; (6) impact of light at night on pineal rhythms; and (7) physiological function of the pineal rhythmicity.
The importance of the pineal gland in circadian physiology is evident in the more than 1,000 research articles published during the past decade. It is clearly a hopeless task to cover all of these articles in a single review. Fortunately, many of the published primary research papers have already been evaluated by a number of excellent review articles, which will be cited in this manuscript wherever appropriate to eliminate redundancy. While increasing numbers of studies support the role of pineal melatonin in a wide array of functions including seasonal reproduction (Goldman, 2003; Malpaux et al., 2001; Revel et al., 2009), sleep regulation (Cajochen et al., 2003; Pandi-Perumal et al., 2007; Pandi-Perumal et al., 2005; Turek and Gillette, 2004), cancer (Bartsch and Bartsch, 2006; Blask et al., 2002; Jung and Ahmad, 2006; Reiter et al., 2007) and diabetes (Nishida, 2005; Peschke, 2008; Peschke and Muhlbauer, 2010), these topics will not be covered in this manuscript. Readers who are interested in these research areas are strongly encouraged to consult the review articles cited above for in-depth discussion. In this review, we will focus on the central and local mechanisms regulating amplitude and timing of melatonin synthesis in the mammalian pineal gland. We will discuss emerging information related to these issues and contrast published studies with those from our own laboratory, aiming to identify gaps in our knowledge on in vivo regulation of pineal rhythmicity.
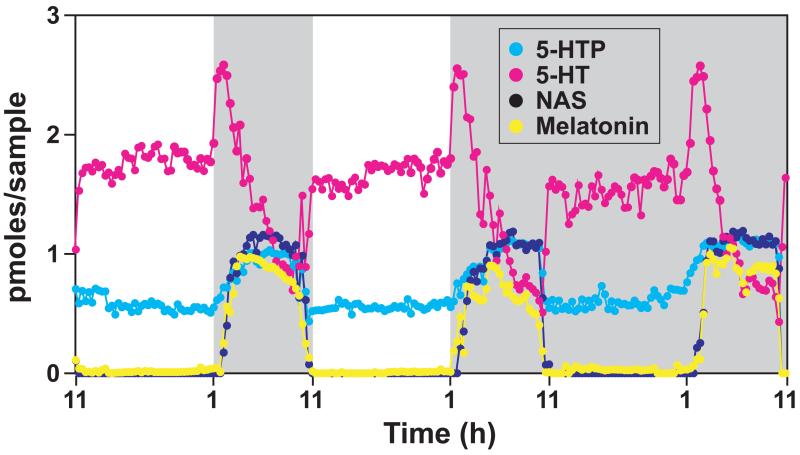
The pineal gland is an unpaired neuroendocrine organ situated in the midline of the brain. Its primary function is to transduce light and dark information to whole body physiology via the release of hormone melatonin (Arendt, 2005). Melatonin is synthesized from the amino acid tryptophan via 4 sequential enzymatic steps (Borjigin et al., 1999): conversion of dietary amino acid tryptophan to 5-hydroxytryptophan (5-HTP) by tryptophan hydroxylase 1 (TPH1); synthesis of 5-hydroxytryptamine (5-HT or serotonin) by aromatic amino acid decarboxylase; formation of N-acetylserotonin (NAS) by arylalkylamine N-acetyltransferase (AANAT), and production of melatonin by hydroxyindole-O-methyltransferase (HIOMT) (also termed N-acetylserotonin methyltransferase or ASMT). All 4 enzymatic derivatives of tryptophan display circadian rhythms of release in the rat pineal gland with higher levels at night (Figure 1). Unlike NAS and melatonin that are barely detectable during the daytime, both 5-HTP and serotonin are released at relatively high levels during the day and increase further at night.
Figure 1. Pineal gland rhythms.
Dietary tryptophan is sequentially converted to 5-hydroxytryptophan (5-HTP, light blue), 5-hydroxytryptamine (5-HT, pink), N-acetylserotonin (NAS, dark blue), and melatonin (yellow). Dark shaded areas represent dark period. Pineal rhythms over 3 consecutive days in one rat were monitored by pineal microdialysis.
Local control of pineal rhythmicity
Local control - serotonin formation
The pineal gland receives adrenergic innervation, which activates a cascade of circadian events that leads to the nightly formation of melatonin from serotonin. Serotonin is present at high levels in the pineal gland during the day and increases further at night in the absence of melatonin formation (Sun et al., 2002). In the presence of melatonin synthesis, however, 5-HTP (Bach et al.; Champney et al., 1984) and serotonin (Snyder and Axelrod, 1965; Snyder et al., 1965) content of the rat pineal gland is below their daytime levels due to their consumption by melatonin synthesis (Sun et al., 2002). The increased nocturnal synthesis of serotonin is driven by increased enzyme activity (Ehret et al., 1991; Shibuya et al., 1977; Sitaram and Lees, 1978) and protein levels (Huang et al., 2008) of TPH1, the rate-limiting enzyme of serotonin production. Post-translational control by phosphorylation of serine-58 stabilizes the TPH1 protein to elevate serotonin production at night (Huang et al., 2008).
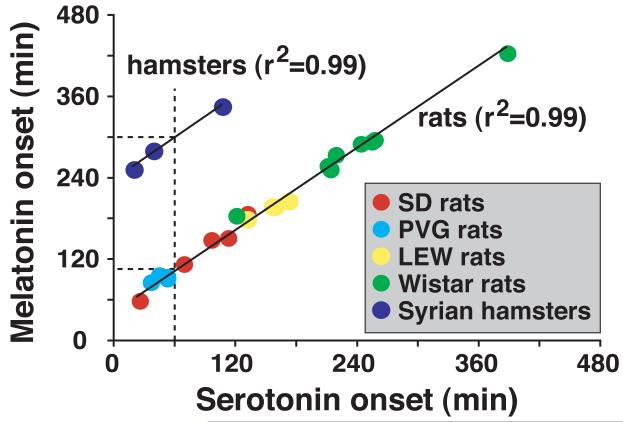
Serotonin secretion displays biphasic patterns at night with an early peak in amplitude, followed by sustained decrease in concentration for the remainder of the night [Figure 1; see (Sun et al., 2002)]. In both rats and hamsters where AANAT activation is transcriptionally controlled, serotonin release surges during early night and precedes melatonin onset [Figure 2; (Liu and Borjigin, 2006)]. In contrast to the rat pineal gland where serotonin release surges 1 hr earlier than melatonin onset, Syrian hamsters show a lag of 4 hrs between serotonin surge and melatonin induction [Figure 2; (Liu and Borjigin, 2006)]. These data indicate that mechanisms of transcriptional activation of melatonin synthesis downstream of adrenergic stimulation in the pineal differ between rats and hamsters, a finding consistent with studies conducted in other laboratories (Simonneaux and Ribelayga, 2003; Simonneaux et al., 2006). In the degu pineal gland where AANAT activation is regulated posttranscriptionally, however, nocturnal surge of serotonin release was undetectable (Lee et al., 2009). These data suggest that nightly stimulation of adrenergic signaling leads to an immediate activation of TPH1 (posttranscriptional) and a delayed stimulation of AANAT (transcriptional), which generates a sequential surge of secretion of serotonin and melatonin (Huang et al., 2008; Liu and Borjigin, 2006; Sun et al., 2002). When both TPH1 and AANAT are stimulated posttranscriptionally in degus, the time lag of secretion between serotonin and melatonin disappears (Chattoraj et al., 2009; Lee et al., 2009).
Figure 2. Time lag between serotonin and melatonin secretion in rodents.
Serotonin release precedes melatonin release by about 1 hr in rat and 4 hrs in hamsters. In rats, the time lag is constant, regardless the strain and individual differences in timing of melatonin release.
Local control - melatonin formation
Melatonin production from serotonin requires activities of both AANAT and HIOMT. In humans, decreased melatonin levels are found in patients with Alzheimer’s disease (Liu et al., 1999; Mishima et al., 1999), age-related macular degeneration (Rosen et al., 2009); or autism spectrum disorders (Melke et al., 2008; Nir et al., 1995; Tordjman et al., 2005). In addition, disrupted melatonin production in shift workers is considered a risk factor for increased breast cancer rates (Blask et al., 2009). It is therefore critical to define the key step of melatonin synthesis in vivo. Classically AANAT has been considered the rate-limiting enzyme of melatonin production (Klein, 2007) ever since the discovery of its striking diurnal rhythms in enzyme activity (Klein and Weller, 1970). During daytime, AANAT clearly limits the production of melatonin since its enzyme activity is low. For the bulk of night, however, AANAT does not limit the amplitudes of melatonin formation (Liu and Borjigin, 2005c). Several lines of evidence from the past few years support this view: (1) A strain of rats harboring H28Y mutation in AANAT sequence shows low levels of AANAT protein and activity in the night pineal (Huang et al., 2005). Despite this, melatonin levels are the same as control rats with normal AANAT (Liu and Borjigin, 2005c); (2) NAS, the enzymatic product of AANAT, is present in molar excess at night in rat pineal gland (Chattoraj et al., 2009; Liu and Borjigin, 2005c; Sun et al., 2003) and in human circulation (Attanasio et al., 1986); (3) AANAT protein level continues to increase in the second half of night, hours after melatonin production reached its peak (Liu and Borjigin, 2005c); and (4) Within the same individual animal, a change in NAS concentration is not associated with a corresponding change in melatonin concentration (Liu and Borjigin, 2005c). It is thus not surprising that no mutations in AANAT have been identified in any of the human disorders associated with low melatonin levels. The identification of mutations in HIOMT in patients with autism spectrum disorders (Melke et al., 2008), who possess very low levels of melatonin, points to a need to redirect our research effort to the last step of melatonin synthesis: the methylation of NAS by HIOMT.
Central control of pineal rhythmicity
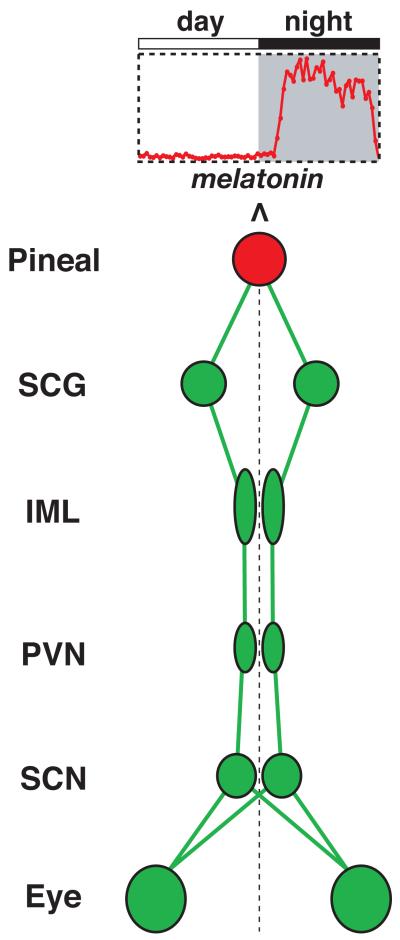
The pineal gland is connected to the central rhythm generator in the suprachiasmatic nucleus (SCN) of the hypothalamus via a multi-synaptic pathway (Figure 3) (Kalsbeek et al., 2006). Circadian signals from the SCN are transmitted sequentially to the paraventricular nuclei (PVN), intermediolateral nucleus of the spinal cord (IML), superior cervical ganglion (SCG), then finally the pineal gland (Borjigin et al., 1999). The pineal is also innervated by parasympathetic system (Larsen, 1999); the role and regulation of which are presently unclear.
Figure 3. The neuronal circuit that controls pineal rhythmicity.
SCG: superior cervical ganglion; IML: intermediolateral nucleus of the spinal cord; PVN: paraventricular nucleus of the hypothalamus; SCN: suprachiasmatic nucleus.
Central control – neurotransmitters involved
Diurnal rhythms of melatonin secretion are controlled by a complex cascade of neurotransmission originating in the SCN. During the daytime, a synaptic input from GABAergic SCN terminals to autonomic PVN neurons (Buijs et al., 2003; Teclemariam-Mesbah et al., 1999) permits the SCN to block the glutamatergic inputs from PVN. The resulting impact on the preganglionic sympathetic neurons in the IML (Kannan et al., 1989) suppresses melatonin production during the day (Kalsbeek et al., 2000). It is however worth noting that the experimental evidence for the glutamatergic signaling from PVN to IML to regulate melatonin output is indirect, since melatonin was not one of the outputs measured (including heart rate, arterial pressure, and renal sympathetic activity) in the study (Kannan et al., 1989). At night, glutamatergic SCN output innervating PVN (Hermes et al., 1996) stimulates melatonin synthesis in the pineal (Perreau-Lenz et al., 2004). Within the pineal gland, melatonin synthesis is activated by the release of norepinephrine from the SCG during the night (Drijfhout et al., 1996b; Drijfhout et al., 1996a). Norepinephrine release can be potently inhibited by light (Drijfhout et al., 1996b) via the release of glutamate in the SCN (Ebling, 1996) from the nerve endings of the retinohypothalamic tract – the axon bundles of the melanopsin-containing retinal ganglion cells (Guler et al., 2008).
Central control – neuroplasticity of the circuit
The SCN-pineal circuit is one of the most well understood neural circuits of the brain with clearly defined central components and a simple, robust, and predictable readout of the circuit activity (melatonin). Moreover, the neuronal activity that stimulates the physiological output (melatonin production) of the target organ (pineal gland) can be shut down with just a light switch. These advantages make the pineal gland an ideal system for understanding activity dependent neuronalplasticity. In addition, since the pineal is bilaterally innervated by two SCGs, a partial injury to the circuit can be reliably performed to allow investigation of functional recovery from partial nerve lesions (Zigmond et al., 1981). Studies from Zigmond laboratory have demonstrated that the pineal gland recovers fully within two days after the loss of 50% of its adrenergic input (Kuchel and Zigmond, 1991; Zigmond et al., 1981). Interestingly, the spontaneous and rapid functional recovery of the pineal gland following partial injury to the circuit (Dornay et al., 1985) is reproducible only after partial denervation of the pineal gland; no recovery is seen following a partial decentralization of the pineal by denervating one of the two SCGs (Zigmond et al., 1985). A detailed investigation of neuroplasticity within the SCN-pineal circuit is warranted to provide further clues to this important process.
Central control – role of the clock genes
Circadian rhythms of melatonin synthesis and secretion are abolished after bilateral SCN lesion, demonstrating that the SCN is the melatonin rhythm generator (Kalsbeek et al., 2000). The SCN generates circadian rhythms through a set of positive and negative feedback loops of transcription and translation (Mendoza and Challet, 2009; Welsh et al., 2010). The rhythm generation involves two PAS domain helix-loop-helix proteins, CLOCK and BMAL1, which form dimers to activate the transcription of three Period (Per1-3) and two Cryptochrome (Cry1-2) genes via the E-Box sequences in their promoter. PER-CRY dimers translocate to the nucleus and negatively interfere with CLOCK/BMAL1-dependent transcription (Reppert and Weaver, 2002).
CLOCK
Takahashi’s group demonstrated that the mutation of CLOCK protein abolishes behavioral rhythms (Vitaterna et al., 1994). Surprisingly, CLOCK-null mice continue to display robust circadian activity rhythms (Debruyne et al., 2006). The role of CLOCK in melatonin rhythm generation was investigated by Kennaway lab by crossing the melatonin-deficient CLOCK mutant mice with melatonin-proficient mice (Kennaway et al., 2006). The resultant CLOCK mutant mice possess normal melatonin rhythms, suggesting that a functional homolog of CLOCK exists in the SCN. More recently, a thought-provoking study has demonstrated that melatonin can suppress the behavioral phenotype of the CLOCK mutant by acting directly on the SCN (Shimomura et al., 2010). This finding reveals an important feedback role of melatonin in circadian timing.
CRY1/2
Cry 1 and Cry 2 double-deficient mice (Cry1/2 null) display arrhythmic behavior in constant darkness (van der Horst et al., 1999). Cry1/2 null mice, generated in melatonin-proficient background, do not display circadian rhythms of melatonin secretion under normal light and dark condition and show very weak rhythms in constant darkness (Yamanaka et al., 2010).
Per1
In Per1 null mice (Bae et al., 2001), the levels of aanat gene expression and nocturnal melatonin secretion are significantly increased compared to the wildtype mice (Christ et al., 2010), suggesting that PER1 normally functions to suppress melatonin synthesis.
It is widely accepted that most cell types possess the basic clock machinery and the tissues isolated in Petri dish can display dramatic oscillations of clock gene expression including per1 (Welsh et al., 2010). The pineal gland is certainly no exception (Maronde and Stehle, 2007; Yoshikawa et al., 2005). In vivo, pineal rhythms are abolished in the absence of the SCN-controlled sympathetic input, suggesting that the local clock machinery is not sufficient for pineal melatonin rhythm generation. Yet, one cannot rule out the possibility that the normal oscillation of pineal rhythms require one or more of these local clock components in addition to central input from the SCN. Role of clock genes within the pineal gland in modulation of SCN-regulated pineal rhythmicity remains to be explored.
Central control – timing of pineal rhythmicity
Advanced sleep phase syndrome (ASPS) and delayed sleep phase syndrome (DSPS) are circadian rhythm sleep disorders characterized by a change in the timing of the sleep episode [reviewed in (Ebisawa, 2007)]. Both ASPS and DSPS are associated with altered timings of melatonin secretion: individuals with ASPS show advanced melatonin onset (Jones et al., 1999), whereas those with DSPS display delayed timing of melatonin secretion (Chang et al., 2009). In recent years, circadian sleep phase disorders are becoming hot topics for investigation of circadian rhythm abnormalities in humans, and are commonly investigated by monitoring the phase of melatonin secretion (Kanathur et al., 2010; Reid and Zee, 2009). We now know that mutations in casein kinase 1, which alter degradation rates of core clock components, result in individuals with shortened circadian period and extremely early onset of melatonin secretion in humans (Ebisawa, 2007).
In spite of such findings in humans, there have been very few studies conducted in animal models that link circadian periods with phase angle of entrainment or connect phase of entrainment with sleep phase disorders. This may be due to the lack of a clear association between circadian period and phase angle of entrainment when behavioral rhythms are used as the marker of the circadian clock. For instance, mice with mutations in per1 (Zheng et al., 2001) and per2 (Zheng et al., 1999) display circadian periods shorter than 24h and yet show no changes in behavioral phase angle of entrainment. On the other hand, mice with Clock mutation that causes lengthening of circadian period from 24h to 28h possess phase angle of entrainment indistinguishable with that of wildtype mice (Vitaterna et al., 1994). These results suggest that behavior output may be inadequate for investigation of circadian chronotypes in animal models.
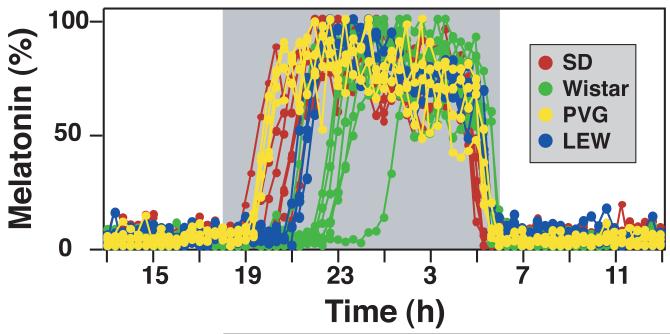
Because melatonin is the most reliable and robust marker of the circadian pacemaker and is used extensively in circadian chronotype studies, we have examined melatonin secretion profiles from various strains of laboratory rats (Liu and Borjigin, 2006). Results indicate that rats possess wide inter-individual and inter-strain variations in their phase of melatonin secretion (Figure 4). Expectedly, rats from outbred strains show larger inter-individual variations of melatonin onset compared with those from inbred strains. Importantly, different rat strains show distinct characteristics in melatonin onset. For instance, melatonin onset in Sprague Dawley rats is in general earlier than in Wistar rats (Figure 4). When circadian period (assayed by free-running period in constant darkness) and timing of melatonin onset were compared within the same animals, earlier melatonin onset was not always associated with shorter circadian period (data not shown). Existence of wide variations of melatonin onset in animal models is consistent with the wide range of circadian chronotypes found in humans, and shall allow further understanding on the impact of phase angle in circadian timing.
Figure 4. Inter-individual and inter-strain variation of the timing of melatonin secretion in rats.
Melatonin secretion, monitored from outbred Sprague Dawley (SD; n=6) and Wistar rats (n=8) and inbred PVG (n=4) and Lewis (LEW; n=4) rats, is normalized to the nocturnal peak values.
Central clock – impact of light on pineal rhythms
The clock driven melatonin production persists in constant dark conditions (Liu and Borjigin, 2005a) and is entrained to the solar environment by the light:dark cycle. Light entering the eyes stimulates photoreceptors in rods, cones, and melanopsin-expressing retina ganglion cells, which convert light signals to electric signals in the ganglion cells (Do and Yau, 2010). The circadian light signal from the melanopsin-positive ganglion cells transmits to the SCN via the retinohypothalamic tracts (Do and Yau, 2010). Under entrained conditions, melatonin secretion from the same individual is remarkably consistent from day to day with identical timings of onset and offset (Liu and Borjigin, 2005b, 2005a, 2006). When light pulse interrupts the lighting regime at night, the resultant rapid termination of norepinephrine secretion from the SCG (Drijfhout et al., 1996b) dramatically impacts pineal rhythm and suppresses melatonin production.
The light effect is mediated by the light-stimulated release of neurotransmitters. These include glutamate (Ebling, 1996) and pituitary adenylate cyclase-activating polypeptide (PACAP) (Hannibal, 2002); both of which are co-stored in the presynaptic terminals in the SCN (Hannibal, 2002). Earlier studies in hamsters showed that MK801, the non-competitive inhibitor of N-methyl-D-aspartate (NMDA) receptor, did not prevent light mediated inhibition of melatonin at night (Vuillez et al., 1998). In rats, it was shown that MK801 blocked suppression of melatonin by red light but not white light at night (Poeggeler et al., 1995; Rowe and Kennaway, 1996). In our own laboratory using pineal microdialysis, we were able to partially inhibit melatonin suppression by white light using MK801 (not shown). We find that the effect of MK801 on blockade of light mediated melatonin suppression is heterogeneous, and depends critically on the dose of MK801, light duration, light intensity, and time intervals between the MK801 administration and light pulse (manuscript in preparation). Nothing is currently known about the effect of PACAP in the SCN on pineal melatonin production.
Physiological function of the pineal rhythmicity
NAS
As shown in Figure 1, pineal gland secretes NAS, in addition to melatonin in a diurnal manner. At night, NAS levels in the circulation are higher than melatonin in both rats (Chattoraj et al., 2009) and humans (Attanasio et al., 1986). Increasing evidence support the role of NAS as an antioxidant, which is more effective than melatonin (reviewed in (Oxenkrug, 2005)). NAS was shown to protect against 6-hydroxydopamine-induced neurotoxicity (Aguiar et al., 2005) and against glutamine-induced lipid peroxidation in retinal samples (Tang et al., 2006). More recently, NAS was shown to activate TrkB (a receptor for brain derived neurotrophic factor – BDNF) leading to an antidepressant-like behavior (Jang et al., 2010). These data suggest that endogenous NAS secreted from the pineal gland may have physiological functions beyond its classical role as the precursor of melatonin production.
Melatonin
Melatonin acts centrally and peripherally through type 1 and type 2 melatonin receptors present in many tissues and cell types, including the SCN (Dubocovich, 2007).
In the periphery, melatonin is a well-known transducer of seasonal information, defining the length of the night (Borjigin et al., 1999; Reiter, 1993). Melatonin plays an essential role in reproduction rhythms of seasonal animals by acting on the pars tuberalis of the pituitary where melatonin receptor is highly expressed (Hazlerigg et al., 2001; Pevet et al., 2006). Similar to NAS, melatonin is a free radical scavenger and an effective antioxidant (Reiter et al., 2010). In the past few years, melatonin receptors have been identified in pancreatic islets, suggesting a possible direct role of melatonin in the regulation of insulin secretion (Bouatia-Naji et al., 2009). Genetic studies in humans have revealed a potential link between melatonin receptor allele variants and hyperglycemia and impaired insulin secretion (Bouatia-Naji et al., 2009; Lyssenko et al., 2009). Increased secretion of insulin in isolated islets from mice devoid of melatonin receptors further strengthen the link between melatonin signaling and insulin secretion (Muhlbauer et al., 2009).
Centrally, melatonin acts directly on the SCN and modulates the clock function (Challet, 2007; Pevet et al., 2006). In vitro, application of low concentration of melatonin on SCN slices results in phase shifts of the neuronal firing rate rhythms in a phase-dependent manner (McArthur et al., 1991). The direct effect of melatonin on SCN activities underscores the effectiveness of melatonin in humans as the prototype of chronobiotics, a class of drugs that influence the circadian system (Brown et al., 2009). Blind individuals with no circadian light perceptions were entrained by timed administration of melatonin (Arendt and Broadway, 1987; Lockley et al., 2006; Sack et al., 1991). Melatonin has also been used widely in normal subjects to reduce jet lag (Brown et al., 2009). More recently, melatonin was shown to suppress the phenotype of the Clock mutant and to interact with Clock to affect the circadian system in mice (Shimomura et al., 2010). As almost all genetic analyses of the circadian system have been performed in melatonin-deficient mouse strains, there is a need to reevaluate the role of melatonin in circadian timing in melatonin-proficient animals.
Acknowledgement
This work was supported by NIH grant NS057583 and DoD grants FA9550-08-0149 and FA9550-09-0352.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguiar LM, Macedo DS, de Freitas RM, de Albuquerque Oliveira A, Vasconcelos SM, de Sousa FC, de Barros Viana GS. Protective effects of N-acetylserotonin against 6-hydroxydopamine-induced neurotoxicity. Life Sci. 2005;76:2193–2202. doi: 10.1016/j.lfs.2004.09.035. [DOI] [PubMed] [Google Scholar]
- Arendt J. Melatonin and the Mammalan Pineal Gland. Chapman & Hill; London: 2005. [Google Scholar]
- Arendt J, Broadway J. Light and melatonin as zeitgebers in man. Chronobiol Int. 1987;4:273–282. doi: 10.3109/07420528709078534. [DOI] [PubMed] [Google Scholar]
- Attanasio A, Rager K, Gupta D. Ontogeny of circadian rhythmicity for melatonin, serotonin, and N-acetylserotonin in humans. J Pineal Res. 1986;3:251–256. doi: 10.1111/j.1600-079x.1986.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Bach AG, Muhlbauer E, Peschke E. Adrenoceptor expression and diurnal rhythms of melatonin and its precursors in the pineal gland of type 2 diabetic goto-kakizaki rats. Endocrinology. 2010;151:2483–2493. doi: 10.1210/en.2009-1299. [DOI] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Bartsch C, Bartsch H. The anti-tumor activity of pineal melatonin and cancer enhancing life styles in industrialized societies. Cancer Causes Control. 2006;17:559–571. doi: 10.1007/s10552-005-9011-8. [DOI] [PubMed] [Google Scholar]
- Blask DE, Dauchy RT, Brainard GC, Hanifin JP. Circadian stage-dependent inhibition of human breast cancer metabolism and growth by the nocturnal melatonin signal: consequences of its disruption by light at night in rats and women. Integr Cancer Ther. 2009;8:347–353. doi: 10.1177/1534735409352320. [DOI] [PubMed] [Google Scholar]
- Blask DE, Dauchy RT, Sauer LA, Krause JA, Brainard GC. Light during darkness, melatonin suppression and cancer progression. Neuro Endocrinol Lett. 2002;23(Suppl 2):52–56. [PubMed] [Google Scholar]
- Borjigin J, Li X, Snyder SH. The pineal gland and melatonin: molecular and pharmacologic regulation. Annu Rev Pharmacol Toxicol. 1999;39:53–65. doi: 10.1146/annurev.pharmtox.39.1.53. [DOI] [PubMed] [Google Scholar]
- Bouatia-Naji N, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- Brown GM, Pandi-Perumal SR, Trakht I, Cardinali DP. Melatonin and its relevance to jet lag. Travel Med Infect Dis. 2009;7:69–81. doi: 10.1016/j.tmaid.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J Endocrinol. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Krauchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- Challet E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–5655. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- Champney TH, Holtorf AP, Steger RW, Reiter RJ. Concurrent determination of enzymatic activities and substrate concentrations in the melatonin synthetic pathway within the same rat pineal gland. J Neurosci Res. 1984;11:59–66. doi: 10.1002/jnr.490110107. [DOI] [PubMed] [Google Scholar]
- Chang AM, Reid KJ, Gourineni R, Zee PC. Sleep timing and circadian phase in delayed sleep phase syndrome. J Biol Rhythms. 2009;24:313–321. doi: 10.1177/0748730409339611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj A, Liu T, Zhang LS, Huang Z, Borjigin J. Melatonin formation in mammals: in vivo perspectives. Rev Endocr Metab Disord. 2009;10:237–243. doi: 10.1007/s11154-009-9125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ E, Pfeffer M, Korf HW, von Gall C. Pineal melatonin synthesis is altered in Period1 deficient mice. Neuroscience. 2010;171:398–406. doi: 10.1016/j.neuroscience.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornay M, Gilad VH, Gilad GM. Compensatory changes in contralateral sympathetic neurons of the superior cervical ganglion and in their terminals in the pineal gland following unilateral ganglionectomy. J Neurosci. 1985;5:1522–1526. doi: 10.1523/JNEUROSCI.05-06-01522.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drijfhout WJ, van der Linde AG, Kooi SE, Grol CJ, Westerink BH. Norepinephrine release in the rat pineal gland: the input from the biological clock measured by in vivo microdialysis. J Neurochem. 1996a;66:748–755. doi: 10.1046/j.1471-4159.1996.66020748.x. [DOI] [PubMed] [Google Scholar]
- Drijfhout WJ, van der Linde AG, de Vries JB, Grol CJ, Westerink BH. Microdialysis reveals dynamics of coupling between noradrenaline release and melatonin secretion in conscious rats. Neurosci Lett. 1996b;202:185–188. doi: 10.1016/0304-3940(95)12245-1. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8(Suppl 3):34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Ebisawa T. Circadian Rhythms in the CNS and Peripheral Clock Disorders: Human Sleep Disorders and Clock Genes. Journal of Pharmacological Sciences. 2007;103:150–154. doi: 10.1254/jphs.fmj06003x5. [DOI] [PubMed] [Google Scholar]
- Ebling FJ. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- Ehret M, Pevet P, Maitre M. Tryptophan hydroxylase synthesis is induced by 3′,5′-cyclic adenosine monophosphate during circadian rhythm in the rat pineal gland. J Neurochem. 1991;57:1516–1521. doi: 10.1111/j.1471-4159.1991.tb06346.x. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Pattern of melatonin secretion mediates transfer of photoperiod information from mother to fetus in mammals. Sci STKE. 2003;2003:PE29. doi: 10.1126/stke.2003.192.pe29. [DOI] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J. Neurotransmitters of the retino-hypothalamic tract. Cell Tissue Res. 2002;309:73–88. doi: 10.1007/s00441-002-0574-3. [DOI] [PubMed] [Google Scholar]
- Hazlerigg DG, Morgan PJ, Messager S. Decoding photoperiodic time and melatonin in mammals: what can we learn from the pars tuberalis? J Biol Rhythms. 2001;16:326–335. doi: 10.1177/074873001129002042. [DOI] [PubMed] [Google Scholar]
- Hermes ML, Coderre EM, Buijs RM, Renaud LP. GABA and glutamate mediate rapid neurotransmission from suprachiasmatic nucleus to hypothalamic paraventricular nucleus in rat. J Physiol. 1996;496(Pt 3):749–757. doi: 10.1113/jphysiol.1996.sp021724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Deng J, Borjigin J. A novel H28Y mutation in LEC rats leads to decreased NAT protein stability in vivo and in vitro. J Pineal Res. 2005;39:84–90. doi: 10.1111/j.1600-079X.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- Huang Z, Liu T, Chattoraj A, Ahmed S, Wang MM, Deng J, Sun X, Borjigin J. Posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland. J Pineal Res. 2008;45:506–514. doi: 10.1111/j.1600-079X.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SW, Liu X, Pradoldej S, Tosini G, Chang Q, Iuvone PM, Ye K. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc Natl Acad Sci U S A. 2010;107:3876–3881. doi: 10.1073/pnas.0912531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CR, Campbell SS, Zone SE, Cooper F, DeSano A, Murphy PJ, Jones B, Czajkowski L, Ptacek LJ. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- Jung B, Ahmad N. Melatonin in cancer management: progress and promise. Cancer Res. 2006;66:9789–9793. doi: 10.1158/0008-5472.CAN-06-1776. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Garidou ML, Palm IF, Van Der Vliet J, Simonneaux V, Pevet P, Buijs RM. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur J Neurosci. 2000;12:3146–3154. doi: 10.1046/j.1460-9568.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- Kanathur N, Harrington J, Lee-Chiong T., Jr. Circadian rhythm sleep disorders. Clin Chest Med. 2010;31:319–325. doi: 10.1016/j.ccm.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol. 1989;256:R1325–1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ, Owens JA, Voultsios A, Varcoe TJ. Functional central rhythmicity and light entrainment, but not liver and muscle rhythmicity, are Clock independent. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1172–1180. doi: 10.1152/ajpregu.00223.2006. [DOI] [PubMed] [Google Scholar]
- Klein DC. Arylalkylamine N-acetyltransferase: “the Timezyme”. J Biol Chem. 2007;282:4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- Klein DC, Weller JL. Indole metabolism in the pineal gland: a circadian rhythm in N-acetyltransferase. Science. 1970;169:1093–1095. doi: 10.1126/science.169.3950.1093. [DOI] [PubMed] [Google Scholar]
- Kuchel GA, Zigmond RE. Functional recovery and collateral neuronal sprouting examined in young and aged rats following a partial neural lesion. Brain Res. 1991;540:195–203. doi: 10.1016/0006-8993(91)90507-r. [DOI] [PubMed] [Google Scholar]
- Larsen PJ. Tracing autonomic innervation of the rat pineal gland using viral transneuronal tracing. Microsc Res Tech. 1999;46:296–304. doi: 10.1002/(SICI)1097-0029(19990815/01)46:4/5<296::AID-JEMT6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Liu T, Chattoraj A, Zhang SL, Wang L, Lee TM, Wang MM, Borjigin J. Posttranscriptional regulation of pineal melatonin synthesis in Octodon degus. J Pineal Res. 2009;47:75–81. doi: 10.1111/j.1600-079X.2009.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RY, Zhou JN, van Heerikhuize J, Hofman MA, Swaab DF. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. J Clin Endocrinol Metab. 1999;84:323–327. doi: 10.1210/jcem.84.1.5394. [DOI] [PubMed] [Google Scholar]
- Liu T, Borjigin J. Free-running rhythms of pineal circadian output. J Biol Rhythms. 2005a;20:430–440. doi: 10.1177/0748730405277868. [DOI] [PubMed] [Google Scholar]
- Liu T, Borjigin J. Reentrainment of the circadian pacemaker through three distinct stages. J Biol Rhythms. 2005b;20:441–450. doi: 10.1177/0748730405279388. [DOI] [PubMed] [Google Scholar]
- Liu T, Borjigin J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J Pineal Res. 2005c;39:91–96. doi: 10.1111/j.1600-079X.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- Liu T, Borjigin J. Relationship between nocturnal serotonin surge and melatonin onset in rodent pineal gland. J Circadian Rhythms. 2006;4:12. doi: 10.1186/1740-3391-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FA, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- Lyssenko V, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpaux B, Migaud M, Tricoire H, Chemineau P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J Biol Rhythms. 2001;16:336–347. doi: 10.1177/074873001129002051. [DOI] [PubMed] [Google Scholar]
- Maronde E, Stehle JH. The mammalian pineal gland: known facts, unknown facets. Trends Endocrinol Metab. 2007;18:142–149. doi: 10.1016/j.tem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- McArthur AJ, Gillette MU, Prosser RA. Melatonin directly resets the rat suprachiasmatic circadian clock in vitro. Brain Res. 1991;565:158–161. doi: 10.1016/0006-8993(91)91748-p. [DOI] [PubMed] [Google Scholar]
- Melke J, et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol Psychiatry. 2008;13:90–98. doi: 10.1038/sj.mp.4002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Challet E. Brain clocks: from the suprachiasmatic nuclei to a cerebral network. Neuroscientist. 2009;15:477–488. doi: 10.1177/1073858408327808. [DOI] [PubMed] [Google Scholar]
- Mishima K, Tozawa T, Satoh K, Matsumoto Y, Hishikawa Y, Okawa M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol Psychiatry. 1999;45:417–421. doi: 10.1016/s0006-3223(97)00510-6. [DOI] [PubMed] [Google Scholar]
- Muhlbauer E, Gross E, Labucay K, Wolgast S, Peschke E. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol. 2009;606:61–71. doi: 10.1016/j.ejphar.2009.01.029. [DOI] [PubMed] [Google Scholar]
- Nir I, Meir D, Zilber N, Knobler H, Hadjez J, Lerner Y. Brief report: circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. J Autism Dev Disord. 1995;25:641–654. doi: 10.1007/BF02178193. [DOI] [PubMed] [Google Scholar]
- Nishida S. Metabolic effects of melatonin on oxidative stress and diabetes mellitus. Endocrine. 2005;27:131–136. doi: 10.1385/endo:27:2:131. [DOI] [PubMed] [Google Scholar]
- Oxenkrug G. Antioxidant effects of N-acetylserotonin: possible mechanisms and clinical implications. Ann N Y Acad Sci. 2005;1053:334–347. doi: 10.1196/annals.1344.029. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Zisapel N, Srinivasan V, Cardinali DP. Melatonin and sleep in aging population. Exp Gerontol. 2005;40:911–925. doi: 10.1016/j.exger.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Srinivasan V, Spence DW, Cardinali DP. Role of the melatonin system in the control of sleep: therapeutic implications. CNS Drugs. 2007;21:995–1018. doi: 10.2165/00023210-200721120-00004. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Kalsbeek A, Pevet P, Buijs RM. Glutamatergic clock output stimulates melatonin synthesis at night. Eur J Neurosci. 2004;19:318–324. doi: 10.1111/j.0953-816x.2003.03132.x. [DOI] [PubMed] [Google Scholar]
- Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 2008;44:26–40. doi: 10.1111/j.1600-079X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- Peschke E, Muhlbauer E. New evidence for a role of melatonin in glucose regulation. Best Pract Res Clin Endocrinol Metab. 2010;24:829–841. doi: 10.1016/j.beem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Pevet P, Agez L, Bothorel B, Saboureau M, Gauer F, Laurent V, Masson-Pevet M. Melatonin in the multi-oscillatory mammalian circadian world. Chronobiol Int. 2006;23:39–51. doi: 10.1080/07420520500482074. [DOI] [PubMed] [Google Scholar]
- Poeggeler BH, Barlow-Walden LR, Reiter RJ, Saarela S, Menendez-Pelaez A, Yaga K, Manchester LC, Chen LD, Tan DX. Red-light-induced suppression of melatonin synthesis is mediated by N-methyl-D-aspartate receptor activation in retinally normal and retinally degenerate rats. J Neurobiol. 1995;28:1–8. doi: 10.1002/neu.480280102. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Zee PC. Circadian rhythm disorders. Semin Neurol. 2009;29:393–405. doi: 10.1055/s-0029-1237120. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia. 1993;49:654–664. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Fuentes-Broto L. Melatonin: a multitasking molecule. Prog Brain Res. 2010;181:127–151. doi: 10.1016/S0079-6123(08)81008-4. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Korkmaz A, Erren TC, Piekarski C, Tamura H, Manchester LC. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog. 2007;13:303–328. doi: 10.1615/critrevoncog.v13.i4.30. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Revel FG, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Melatonin controls seasonal breeding by a network of hypothalamic targets. Neuroendocrinology. 2009;90:1–14. doi: 10.1159/000219588. [DOI] [PubMed] [Google Scholar]
- Rosen R, Hu DN, Perez V, Tai K, Yu GP, Chen M, Tone P, McCormick SA, Walsh J. Urinary 6-sulfatoxymelatonin level in age-related macular degeneration patients. Mol Vis. 2009;15:1673–1679. [PMC free article] [PubMed] [Google Scholar]
- Rowe SA, Kennaway DJ. Effect of NMDA receptor blockade on melatonin and activity rhythm responses to a light pulse in rats. Brain Res Bull. 1996;41:351–358. doi: 10.1016/s0361-9230(96)00189-x. [DOI] [PubMed] [Google Scholar]
- Sack RL, Lewy AJ, Blood ML, Stevenson J, Keith LD. Melatonin administration to blind people: phase advances and entrainment. J Biol Rhythms. 1991;6:249–261. doi: 10.1177/074873049100600305. [DOI] [PubMed] [Google Scholar]
- Shibuya H, Toru M, Watanabe S. A circadian rhythm of tryptophan hydroxylase in rat pineals. Brain Res. 1977;138:364–368. doi: 10.1016/0006-8993(77)90754-5. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Lowrey PL, Vitaterna MH, Buhr ED, Kumar V, Hanna P, Omura C, Izumo M, Low SS, Barrett RK, LaRue SI, Green CB, Takahashi JS. Genetic suppression of the circadian Clock mutation by the melatonin biosynthesis pathway. Proc Natl Acad Sci U S A. 2010;107:8399–8403. doi: 10.1073/pnas.1004368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55:325–395. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- Simonneaux V, Sinitskaya N, Salingre A, Garidou ML, Pevet P. Rat and Syrian hamster: two models for the regulation of AANAT gene expression. Chronobiol Int. 2006;23:351–359. doi: 10.1080/07420520500521962. [DOI] [PubMed] [Google Scholar]
- Sitaram BR, Lees GJ. Diurnal rhythm and turnover of tryptophan hydroxylase in the pineal gland of the rat. J Neurochem. 1978;31:1021–1026. doi: 10.1111/j.1471-4159.1978.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Axelrod J. Circadian Rhythm in Pineal Serotonin: Effect of Monoamine Oxidase Inhibition and Reserpine. Science. 1965;149:542–544. doi: 10.1126/science.149.3683.542. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Zweig M, Axelrod J, Fischer JE. Control of the Circadian Rhythm in Serotonin Content of the Rat Pineal Gland. Proc Natl Acad Sci U S A. 1965;53:301–305. doi: 10.1073/pnas.53.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Deng J, Liu T, Borjigin J. Circadian 5-HT production regulated by adrenergic signaling. Proc Natl Acad Sci U S A. 2002;99:4686–4691. doi: 10.1073/pnas.062585499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Liu T, Deng J, Borjigin J. Long-term in vivo pineal microdialysis. J Pineal Res. 2003;35:118–124. doi: 10.1034/j.1600-079x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Tang Q, Hu Y, Cao Y. Neuroprotective effect of melatonin on retinal ganglion cells in rats. J Huazhong Univ Sci Technolog Med Sci. 2006;26:235–237. 253. doi: 10.1007/BF02895825. [DOI] [PubMed] [Google Scholar]
- Teclemariam-Mesbah R, Ter Horst GJ, Postema F, Wortel J, Buijs RM. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J Comp Neurol. 1999;406:171–182. [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Pichard N, Charbuy H, Touitou Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol Psychiatry. 2005;57:134–138. doi: 10.1016/j.biopsych.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Turek FW, Gillette MU. Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists. Sleep Med. 2004;5:523–532. doi: 10.1016/j.sleep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, Bootsma D, Hoeijmakers JH, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillez P, Jacob N, Teclemariam-Mesbah R, Van Rossum A, Vivien-Roels B, Pevet P. Effect of NMDA receptor antagonist MK-801 on light-induced Fos expression in the suprachiasmatic nuclei and on melatonin production in the Syrian hamster. J Neuroendocrinol. 1998;10:671–677. doi: 10.1046/j.1365-2826.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Suzuki Y, Todo T, Honma K, Honma S. Loss of circadian rhythm and light-induced suppression of pineal melatonin levels in Cry1 and Cry2 double-deficient mice. Genes Cells. 2010;15:1063–1071. doi: 10.1111/j.1365-2443.2010.01443.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Yamazaki S, Menaker M. Effects of preparation time on phase of cultured tissues reveal complexity of circadian organization. J Biol Rhythms. 2005;20:500–512. doi: 10.1177/0748730405280775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- Zigmond RE, Baldwin C, Bowers CW. Rapid recovery of function after partial denervation of the rat pineal gland suggests a novel mechanism for neural plasticity. Proc Natl Acad Sci U S A. 1981;78:3959–3963. doi: 10.1073/pnas.78.6.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE, Baldwin C, Bowers CW. Rapid recovery of pineal function after partial denervation: a possible role for heteroneuronal uptake of transmitter in modulating synaptic efficacy. J Neurosci. 1985;5:142–150. doi: 10.1523/JNEUROSCI.05-01-00142.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]