Abstract
Mitochondrial aldehyde dehydrogenase-2 (ALDH2) has been shown to benefit myopathic changes following alcohol intake, although the precise mechanism is still unclear. This study was designed to evaluate the role of ALDH2 on chronic alcohol intake-induced myocardial geometric and functional damage with a focus on autophagic signalling. Wild-type friendly virus B (FVB) and transgenic mice overexpressing ALDH2 driven by chicken β-actin promoter were fed a 4% alcohol liquid diet for 12 weeks. Cardiac geometry and function were assessed using echocardiographic and IonOptix systems. Western blot analysis was used to evaluate the essential autophagy markers, Akt and AMP-dependent protein kinase (AMPK) as well as their downstream signalling mammalian target of rapamycin (mTOR) and signal transducer and activator of transcription 3 (STAT3). Alcohol intake altered cardiac geometry and function as demonstrated by lessened LV wall and septal thickness, enlarged end systolic and diastolic diameters, decreased fractional shortening and cell shortening, the effects of which were mitigated by ALDH2 transgene. Chronic alcohol intake triggered myocardial autophagy as shown by LC3B II isoform switch, as well as decreased phosphorylation of mTOR, the effects of which were ablated by ALDH2. Chronic alcohol intake suppressed phosphorylation of Akt and AMPK, which was reconciled by ALDH2. Levels of Notch1 and STAT3 phosphorylation were dampened by chronic alcohol intake in FVB but not ALDH2 myocardium. Moreover, the γ-secretase Notch inhibitor N-[N-(3,5-difluorophenacetyl)-1-alany1]-S-phenyglycine t-butyl ester exacerbated ethanol-induced cardiomyocyte contractile dysfunction, apoptosis and autophagy. In summary, these findings suggested that ALDH2 elicits cardioprotection against chronic alcohol intake-induced cardiac geometric and functional anomalies by inhibition of autophagy possibly via restoring the Akt-mTOR-STAT3-Notch signalling cascade.
Keywords: alcohol, ALDH2, myocardial dysfunction, autophagy, Notch
Introduction
Chronic alcoholism is a common medical, economical and social problem. Nearly 50% of alcoholics develop a unique form of dilated cardiomyopathy, namely alcoholic cardiomyopathy manifested by ventricular dysfunction, heart failure and ultimately cardiac death [1, 2]. Recent evidence from our group has unveiled a role of mitochondrial aldehyde dehydrogenase (ALDH), ALDH2, in the protection against alcohol-induced cardiac injury [3-5]. Similarly, ALDH2 was shown to benefit non-alcoholic cardiac myopathies such as ischemic and reperfusion arrhythmic injury [6-8]. However, the precise nature behind ALDH2-mediated cardioprotection is still elusive. Given that autophagy is an essential regulator in cardiomyocyte survival and death under both physiological and pathophysiological conditions [9] and our recent finding that ALDH2 protects against ischemia-reperfusion injury through regulation of autophagy [8], this study was designed to examine the role of autophagy and autophagic signalling mechanism in ALDH2-elicited protection against chronic alcohol intake-induced cardiac anomalies. Signalling cascades involved in the regulation of autophagy in particular AMP-dependent protein kinase (AMPK) and Akt, two essential protein kinases governing autophagy, myocardial survival and function [8, 10], were examined in wild-type friendly virus B (FVB) and ALDH2 overexpression transgenic mice. Activation of AMPK may promote whereas activation of Akt is known to suppress autophagy [8]. Meanwhile, the AMPK/Akt downstream target mammalian target of rapamycin (mTOR) plays a pivotal role in cell growth and survival. mTOR is a serine/threonine kinase present as both rapamycin-sensitive (mTOR complex 1) and rapamycin-insensitive multimeric protein complexes [11, 12]. More importantly, mTOR serves as an essential nutrient and energy sensor to regulate protein synthesis and autophagy thus to modulate cell growth and survival [12]. Recent evidence has depicted a role of the signal transducer and activator of transcription 3 (STAT3) as a downstream signal of mTOR governing regulation of autophagy [12, 13]. Further evidence has revealed close interactions between STAT3 and Notch receptors in a number of biological processes including proliferation, differentiation and apoptosis [14]. The biological effects of Notch are believed to be mediated through proteolytic cleavage of Notch to release the Notch intracellular domain, which participates in a transcriptional complex in the nucleus to regulate Notch-dependent gene expression [14]. Notch1 receptor has been speculated to control the cardiac response to injury by limiting cardiomyocyte hypertrophy, enhancing myocyte survival, promoting precursor proliferation and reducing interstitial fibrosis [15]. However, little is known with regard to the role of Notch signalling in the regulation of cardiac geometry and function under either normal or pathological conditions. To this end, the possible role of Notch and its potential cross-talk with STAT3 under alcohol challenge were scrutinized both in vivo following chronic alcohol intake and in vitro following acute ethanol challenge.
Material and Methods
Experimental animals and chronic alcohol intake
All animal procedures were approved by our Institutional Animal Care and Use Committee and were in accordance with the NIH guidelines. The ALDH2 transgenic mouse line was produced using the chicken β-actin promoter as described [4]. The non-specific chicken β-actin promoter was chosen over the cardiac-specific α-myosin heavy chain promoter to drive ALDH2 overexpression largely because cardiac overexpression of ALDH2 is unable to detoxify the alcohol metabolite acetaldehyde delivered to the heart through systemic blood perfusion. Adult male ALDH2 overexpression transgenic mice and FVB wild-type mice (weight-matched) were housed in a temperature-controlled room under a 12 hr/12 hr light/dark with access to tap water ad libitum. FVB and ALDH2 mice were introduced to a nutritionally complete liquid diet (Shake & Pour Bioserv, Inc., Frenchtown, NJ, USA) for a 1 week acclimation period. Upon completion of the acclimation period, half of the FVB and ALDH2 mice were maintained on the regular liquid diet (without ethanol), and the remaining half began a 12 week period of isocaloric 4% (vol/vol) ethanol diet feeding. An isocaloric pair-feeding regimen was employed to eliminate the possibility of nutritional deficits. Control mice were offered the same quantity of diet ethanol-consuming mice drank the previous day [4].
Echocardiographic assessment
Cardiac geometry and function were evaluated in anesthetized (Avertin 2.5%, 10 μl/g body weight, i.p.) mice using 2D guided M-mode echocardiography (Sonos, Duluth, GA, USA, 5500) equipped with a 15-6 MHz linear transducer. Left ventricular anterior and posterior wall dimensions during diastole and systole were recorded from three consecutive cycles in M-mode using methods adopted by the American Society of Echocardiography. Fractional shortening was calculated from LV end diastolic (EDD) [16] and end systolic (ESD) diameters using the equation (EDD – ESD)/EDD [4].
Cardiomyocyte isolation and in vitro drug treatment
After ketamine/xylazine (3:1, 1.32 mg/kg, i.p.) sedation, hearts were removed and perfused with Krebs-Henseleit Buffer (KHB) buffer containing (in mM): 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 11.1 glucose. Hearts were digested with 223 U/ml Liberase (Roche, Indianapolis, IN, USA, 1988476) for 20 min. Left ventricles were removed and minced before being filtered. Myocyte yield was approximately 75% which was not affected by either ethanol or ALDH2 transgene. Only rod-shaped cardiomyocytes with clear edges were used for mechanical evaluation [4]. To examine the role of Notch signalling on ethanol-induced cardiac response, cardiomyocytes from FVB mice were treated with ethanol (240 mg/dl) at 37°C for 4 hrs in the absence or presence of the γ-secretase Notch inhibitor N-[N-(3,5-difluorophenacetyl)-1-alany1]-S-phenyglycine t-butyl ester (DAPT, 20 μM) prior to the mechanical assessment.
Cell shortening and relengthening
Mechanical properties of cardiomyocytes were evaluated utilizing a SoftEdge MyoCam® system (IonOptix Corporation, Milton, MA, USA) [4]. Briefly, cardiomyocytes were visualized under an inverted microscope (IX-70; Olympus, Tokyo, Japan) and were stimulated with a voltage frequency of 0.5 Hz. The myocyte being observed was shown on a computer monitor using an IonOptix MyoCam camera. An IonOptix SoftEdge software was utilized to capture cell shortening and relengthening changes. The indices considered were peak shortening amplitude, time-to-peak shortening (TPS), time-to-90% relengthening (TR90), maximal velocity of shortening and relengthening (±dL/dt).
Western blot
Ventricular tissues were homogenzied and sonicated in a lysis buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ethylene glycol tetraacetic acid (EGTA), 1% Triton, 0.1% SDS and 1% protease inhibitor cocktail. Equal amounts (30 μg protein) of proteins were separated on 10% or 15% SDS-PAGE in a minigel apparatus (Mini-PROTEAN II; Bio-Rad, Hercules, CA, USA) and were then transferred electrophoretically to nitrocellulose membranes. The membranes were blocked with 5% milk in Tris-buffered saline before overnight incubation at 4°C with the anti-Beclin-1 (1:1000), anti-Akt (1:1000), anti-phosphorylated Akt (Ser473, 1:500), anti-AMPK (1:1000), anti-pAMPK (Thr172, 1:500), anti-mTOR (1:1000), anti-pmTOR (Ser2448, 1:1000), anti-LC3B (1:500), anti-STAT3 (1:1000), anti-pSTAT3 (Ser727, 1:500) and anti-Notch1 (1:1000) antibodies. Membranes were incubated for 1 hr with horseradish peroxidase conjugated secondary antibody (1:5000). After immunoblotting, films were scanned and the intensity of immunoblot bands was detected with a Bio-Rad Calibrated Densitometer. Either Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or α-tubulin was used as the loading control [4].
Caspase-3 assay
Caspase-3 is an enzyme activated during induction of apoptosis. Caspase-3 activity was determined according to published method [4]. In brief, myocytes were lysed in 100 μl of ice-cold cell lysis buffer {50 mM HEPES, 0.1% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 1 mM dithiothreitol, 0.1 mM EDTA, 0.1% NP40}. Following cell lysis, 70 μl reaction buffer and 20 μl caspase-3 colorimetric substrate (Ac-DEVD-p-nitroanilide) were added to cell lysate and incubated for 1 hr at 37°C, during which time, caspase enzyme in the sample was allowed to cleave the chromophore pNA from its substrate molecule. Absorbency was detected at 405 nm with caspase-3 activity being proportional to the colour reaction. Caspase-3 activity was expressed as picomoles of p-nitroanilide released per micrograms of protein per minute.
Statistical analysis
Data were expressed as mean ± S.E.M. Statistical significance (P < 0.05) for each variable was estimated by ANOVA followed by Tukey’s test for post- hoc analysis.
Results
Echocardiographic properties of FVB and ALDH2 mice fed with alcohol
Chronic alcohol intake led to a significantly increased body weight in FVB (FVB: 28.1 ± 2.9 g; FVB-ethanol: 36.8 ± 3.7 g, n = 8–10 mice, P < 0.05) but not ALDH2 mice (ALDH2: 28.3 ± 1.5 g; ALDH2-ethanol: 30.9 ± 1.4 g, n = 8–10 mice, P > 0.05). Heart rate was comparable among all groups. Chronic alcohol intake significantly lessened LV wall thickness and intraventricular septal thickness while enlarging LV EDD and LV ESD, the effects of which were ablated by ALDH2. Furthermore, chronic alcohol intake significantly decreased fractional shortening, which was abrogated by ALDH2 transgene. ALDH2 overexpression itself failed to alter any of the geometric or functional echocardiographic parameters tested (Fig. 1). These data suggest that ALDH2 is capable of alleviating chronic alcohol intake-induced myocardial remodelling.
Fig 1.
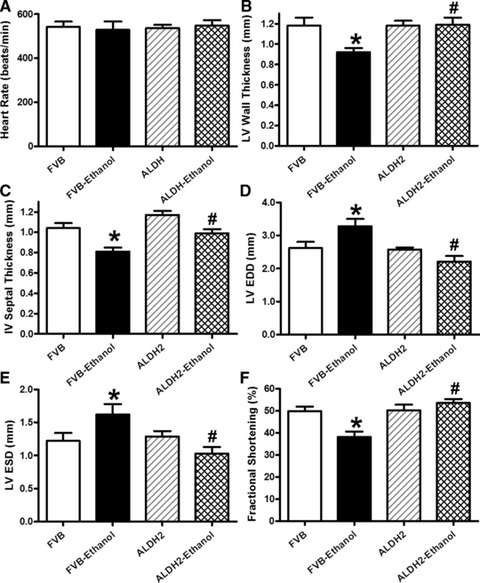
Effect of 12 week alcohol intake on echocardiographic indices in FVB and ALDH2 mice. (A) Heart rate; (B) LV wall thickness; (C) intraventricular (IV) septal thickness; (D) LV EDD; (E) LV ESD and (F) fractional shortening. Mean ± S.E.M., n = 6–11 mice per group, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
Effect of chronic alcohol intake on cardiomyocyte mechanics in FVB and ALDH2 mice
Our further assessment of cardiomyocyte mechanics revealed that chronic alcohol intake significantly depressed peak shortening and ±dL/dt as well as prolonged TR90 without affecting TPS. Although ALDH2 itself failed to alter these mechanical indices, it significantly attenuated chronic alcohol intake-induced cardiomyocyte mechanical abnormalities (Fig. 2).
Fig 2.
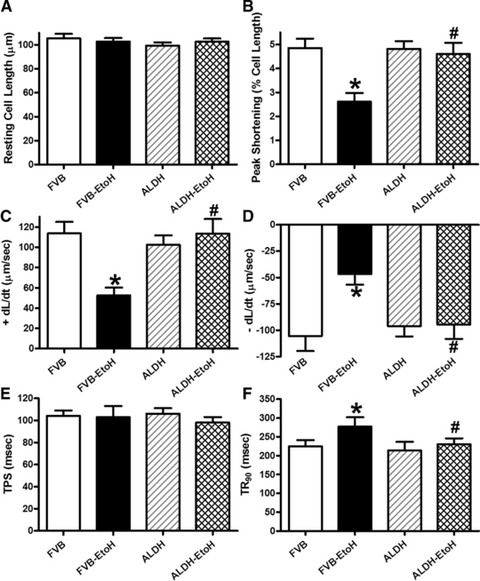
Effect of 12 week alcohol intake on cardiomyocyte shortening in myocytes from FVB and ALDH2 mice. (A) Resting cell length; (B) peak cell shortening (normalized to cell length); (C) maximal velocity of shortening (+dL/dt); (D) maximal velocity of relengthening (–dL/dt); (E) TPS and (F) TR90. Mean ± S.E.M., n = 66–67 cells from three mice per group, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
Effect of ALDH2 on alcohol intake-induced changes in protein markers for autophagy
Recent evidence has indicated a role of autophagy in alcohol-induced tissue damage [17]. Our data revealed that chronic alcohol intake up-regulated the expression of the key autophagic marker LC3B II (absolute or isoform ratio of LC3B II-to-LC3B I) but not Beclin-1, the effect of which was mitigated by ALDH2 (Fig. 3). These data suggested a possible role of autophagy in the alcohol intake-induced and ALDH2-elicited cardiac geometric and functional responses.
Fig 3.
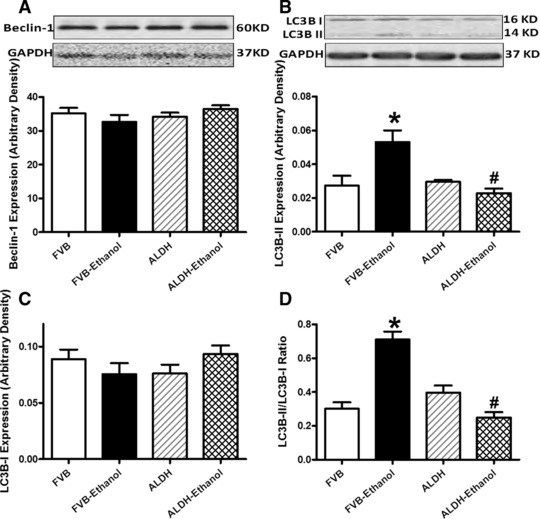
Effect of 12 week alcohol intake on autophagy markers in myocardium from FVB and ALDH2 mice. (A) Beclin-1; (B) LC3B-II; (C) LC3B-I and (D) LC3B-II-to-LC3B-I ratio. Insets: representative immunoblots of Belin-1, LC3B-I, LC3B-II and GAPDH (loading control) using specific antibodies. Mean ± S.E.M., n = 4–5, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
Effect of ALDH2 on alcohol intake-induced changes in Akt, AMPK and mTOR signalling
To examine the potential signalling pathways involved in alcohol and/or ALDH2 transgene-elicited cardiac autophagic, geometric and mechanical responses, we examined levels of the key cardiac surviving factor Akt and the cardiac energy fuel AMPK, two pivotal regulators for autophagy [8, 10, 18]. Our results indicated that chronic alcohol intake markedly depressed the phosphorylation of both Akt and AMPK (either absolute or normalized value) without affecting expression of pan Akt and AMPK. Although ALDH2 itself did not affect the expression of pan or phosphorylated Akt and AMPK, it effectively reconciled alcohol intake-induced change in the phosphorylation of Akt and AMPK (Fig. 4). These findings depicted that ALDH2 is capable of compensating for the alcohol intake-induced change in Akt and AMPK signalling.
Fig 4.
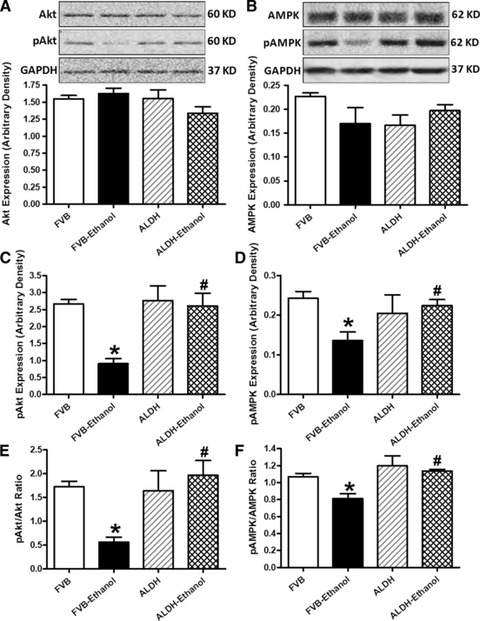
Effect of 12 week alcohol intake on Akt and AMPK signalling in myocardium from FVB and ALDH2 mice. (A) Akt; (B) AMPK; (C) phosphorylated Akt (pAkt); (D) Phosphorylated AMPK (pAMPK); (E) pAkt-to-Akt ratio and (F) pAMPK-to-AMPK ratio. Insets: representative gel blots of pan and pAkt and AMPK using specific antibodies (GAPDH as loading control). Mean ± S.E.M., n = 4–5, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
Phosphorylation of the key autophagy regulatory signalling molecule mTOR displays a reciprocal relationship with the induction of autophagy [19]. Our data indicated that chronic alcohol intake significantly dampened mTOR phosphorylation (absolute or normalized value) in FVB but not ALDH2 mice. ALDH2 itself did not affect the phosphorylation of mTOR. Furthermore, neither alcohol intake nor ALDH2 transgene altered the expression of pan mTOR (Fig. 5). These observations are in line with the possible role of autophagy in chronic alcohol intake-induced cardiac geometric and functional responses, as well as the ALDH2 transgene-exerted protection against alcoholic injury.
Fig 5.
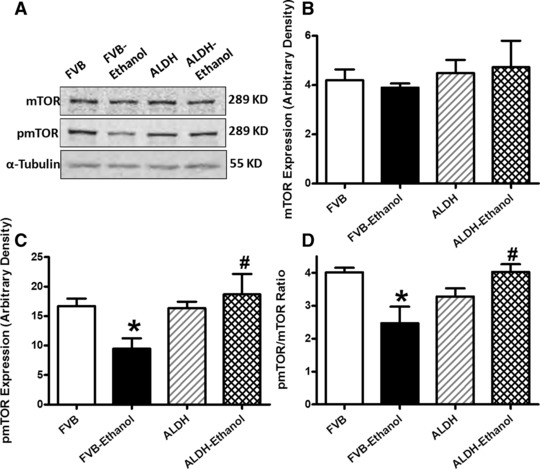
Effect of 12 week alcohol intake on mTOR signalling in myocardium from FVB and ALDH2 mice. (A) Representative gel blots depicting pan and phosphorylated mTOR as well as α-tubulin proteins using specific antibodies; (B) mTOR; (C) pmTOR and (D) pmTOR-to-mTOR ratio. Mean ± S.E.M., n = 4–5, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
Role of Notch and STAT3 signalling in alcohol-induced mechanical and autophagic responses
We further examined the potential cell signalling mechanisms involved in alcohol-induced cardiac mechanical and autophagic responses. Data shown in Figure 6 revealed that expression of Notch1 signal and phosphorylated STAT3 (absolute or normalized) were significantly dampened by chronic alcohol intake, the effect of which was reconciled by ALDH2 transgene with little effect by ALDH2 transgene itself. Neither ALDH2 overexpression nor alcohol intake overtly affected STAT3 expression (Fig. 6). Interestingly, inhibition of Notch1 using DAPT exacerbated ethanol-induced depression in peak shortening and ±dL/dt as well as prolongation in TR90 without affecting resting cell length and TPS. DAPT did not affect cardiomyocyte mechanics by itself (Fig. 7). Assessment of apoptosis and autophagy indicated that ethanol treatment facilitated apoptosis and autophagy (either absolute or isoform ratio of LC3B II-to-LC3B I), the effects of which were accentuated by Notch inhibition using DAPT. Last but not least, DAPT failed to alter either apoptosis or autophagy by itself (Fig. 8).
Fig 6.
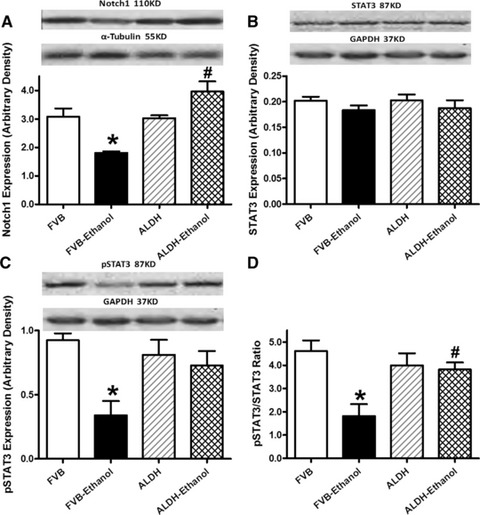
Effect of 12 week alcohol intake on Notch1 and STAT3 signalling in myocardium from FVB and ALDH2 mice. (A) Notch1; (B) STAT3; (C) phosphorylated STAT3 (pSTAT) and (D) pSTAT-to-STAT ratio. Representative gel blots depicting Notch1, STAT3, pSTAT3, α-tubulin and GAPDH (loading controls) using specific antibodies; Mean ± S.E.M., n = 4–5, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
Fig 7.
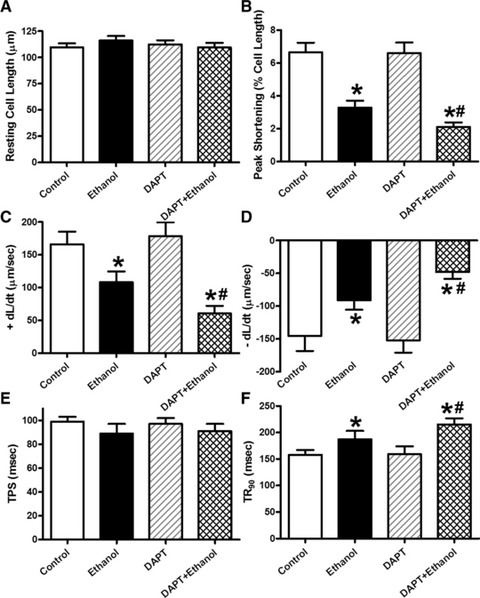
Effect of the Notch inhibitor DAPT on ethanol toxicity-induced cardiomyocyte contractile dysfunction. Cardiomyocytes from FVB mice were incubated with ethanol (240 mg/dl) for four hrs in the absence and presence of DAPT (20 μM) before mechanical function was assessed. (A) Resting cell length; (B) peak cell shortening (normalized to cell length); (C) maximal velocity of shortening (+dL/dt); (D) maximal velocity of relengthening (–dL/dt); (E) TPS and (F) TR90. Mean ± S.E.M., n = 46 cells per group, *P < 0.05 versus control group, #P < 0.05 versus ethanol group.
Fig 8.
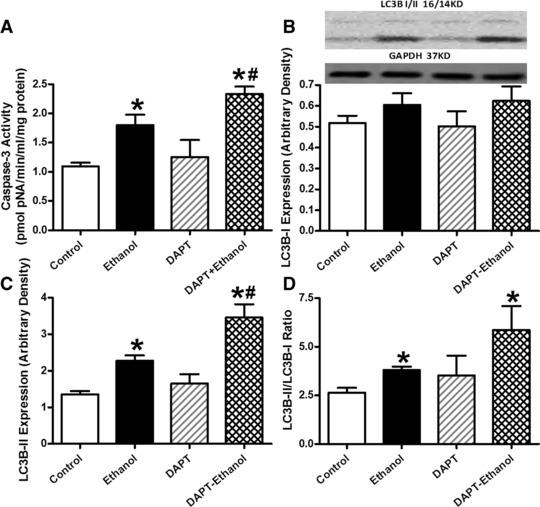
Effect of the Notch inhibitor DAPT on ethanol-induced apoptosis and autophagy. Cardiomyocytes from FVB mice were incubated with ethanol (240 mg/dl) for four hrs in the absence and presence of DAPT (20 μM) prior to assessment of apoptosis and autophagy. (A) Caspase-3 activity; (B) LC3B-1 expression; (C) LC3B-II expression and (D) LC3B-II-to-LC3B-I ratio. Insets: representative gel blots of LC3B-I, LC3B-II and GAPDH (loading control) using specific antibodies. Mean ± S.E.M., n = 3–4 mice per group, *P < 0.05 versus control, #P < 0.05 versus ethanol group.
Discussion
The salient findings of our study are that ALDH2 overexpression exerts protective effects against chronic alcohol intake-induced cardiac geometric and contractile anomalies likely through inhibition of autophagy. Our data indicated that chronic alcohol intake-induced change in cardiac mechanical and autophagic responses may be associated with dampened activation of Akt/AMPK and their downstream signal mTOR. ALDH2 appears to offer its protection in the heart by reversing alcohol-induced changes in AMPK, Akt and mTOR, en route to the ablation of alcohol intake-induced autophagy and contractile dysfunction. The ALDH2-elicited cardiac protection was also accompanied by restoration of alcohol-suppressed STAT3 phosphorylation and Notch1. This is supported by the fact that inhibition of Notch1 using DAPT exacerbates the ethanol-induced changes in contractile function and autophagy induction, denoting a role of Notch signalling in ethanol and/or ALDH2 overexpression-induced cardiac mechanical and autophagic responses. These findings revealed that ALDH2 may be protective against chronic alcohol intake-induced cardiac geometric and functional changes possibly through mitigating chronic alcohol intake-elicited autophagy which is dependent on mTOR-STAT3-Notch signalling.
Our data revealed that chronic alcohol intake triggered cardiac geometric and contractile dysfunction including lessened LV wall thickness and IV septal thickness, enlarged LV systolic and diastolic diameters, reduced fractional shortening, cardiomyocyte peak shortening, ±dL/dt and prolonged TR90. These findings are consistent with the previous findings of myopathic alteration following alcohol intake featured by compromised myocardial contractility [5, 20, 21]. Several hypotheses have been put forward with regards to chronic alcohol intake-induced cardiac anomalies including lipid peroxidation, oxidative damage [2], mitochondrial dysfunction [5] and altered membrane properties [22]. Although earlier findings from our lab demonstrated that overexpression of ALDH2 alleviates alcohol and acetaldehyde-induced cell injury both in vivo and in vitro [3, 5, 23], little is known with regard to the precise mechanism involved in ALDH2-offered protection against alcohol intake. Data from our present study revealed that ALDH2 overexpression counteracts cardiac geometric and functional anomalies following chronic alcohol exposure possibly related to regulation of autophagy, as supported by the autophagic markers. Chronic alcohol intake triggered autophagic responses in FVB and ALDH2 mice (LC3BII or LC3B isozyme ratio) in parallel with cardiac geometric and contractile responses. Beclin-1 is considered a marker for autophagy happening rather early on in autophagic pathway [24]. Our negative finding of Beclin-1 following chronic alcohol consumption in FVB and ALDH2 mice suggested a Beclin-1-independent mechanism in our current experimental setting.
Akt and AMPK are essential regulators of autophagy, survival, energy metabolism and contractile properties in the heart [25-27]. Our earlier study noted that acute ethanol exposure-induced cardiomyocyte contractile dysfunction is associated with a reduced Akt activity whereas an enhanced AMPK activation [27, 28]. Interestingly, data from this study revealed dampened phosphorylation of both Akt and AMPK along with facilitated autophagy in conjunction with cardiac contractile dysfunction following chronic alcohol intake, the effect of which was reconciled by ALDH2 transgene. It is quite possible that such discrepancy in AMPK signalling may be related to the difference in experimental settings (i.e. short versus chronic alcohol feeding). Given that activation of AMPK promotes whereas activation of Akt suppresses autophagy [8], our data favour a pivotal role of Akt- rather than AMPK-dependent regulation of autophagy in alcohol and ALDH2 transgene-elicited myocardial responses. In an effort to elucidate the complex signalling cascade, the upstream regulators of autophagy, mTOR and STAT3, were also examined in our study and our observations were in line with the lessened Akt phosphorylation following chronic alcohol intake. Our results revealed that alcohol intake-induced inhibition in phosphorylation of Akt, mTOR and STAT3 is restored by ALDH2 transgene. These observations favour the notion that overexpression of ALDH2 rescues alcohol intake-induced geometric and contractile anomalies via inhibition of alcohol-induced autophagy dependent on the Akt-mTOR-STAT3 signalling. Nonetheless, given that AMPK may regulate mTOR and autophagy via an Akt-dependent mechanism [18, 29], further study is warranted to understand the role of AMPK in alcohol-elicited regulation of autophagy, and subsequently, changes in myocardial geometry and function.
Perhaps the most important finding from our present study is the down-regulated Notch signalling following alcohol intake and the obliteration of this effect by ALDH2 overexpression. Consistently, inhibition of Notch1 with the γ-secretase inhibitor DAPT exaggerated ethanol exposure-induced cardiomyocyte contractile dysfunction, apoptosis and autophagy (LC3BII levels and LC3B isozyme ratio). These findings suggested a possible role of dampened Notch1 signalling in alcohol-induced contractile and autophagic anomalies. It is possible that ALDH2 may execute its cardioprotective role against alcoholic heart injury via restoring Notch signalling. This notion seems to be in line with the changes in mTOR and STAT3 signalling under both alcoholism and ALDH2 intervention as observed in our current study. In our hand, phosphorylation of mTOR and STAT3 was dampened by chronic alcohol intake in hearts from FVB mice, the effect of which was ablated by ALDH2 overexpression. mTOR complex 1 was shown to positively regulate Notch signalling through STAT3 in the regulation cell differentiation [12]. In addition, development of tumours as a result of hyperactive mTOR signalling is often associated with the aberrant high STAT3/Notch activity, whereas inhibition of Notch signalling extends survival [12]. Although a role of Notch has not been elucidated in the regulation of autophagy and alcoholic complications, the fact that the Notch pathway acts as a positive regulator of the PI3K/Akt/mTOR pathway favours its likely role in the regulation of autophagy and consequently cardiac function [30]. It is believed that Notch signalling may be of wider significance in the development, physiology and pathophysiology of the heart far beyond its originally identified cancer connection [12]. Last but not least, data from our in vitro study revealed that the Notch1 inhibitor DAPT exaggerated ethanol exposure-induced apoptosis and autophagy, suggesting a positive correlation between apoptosis and autophagy in cardiomyocytes following ethanol exposure. The interplay between apoptosis and autophagy is rather complex in the heart with damaged (apoptotic) cardiomyocytes exhibiting characteristics of autophagy in heart failure. However, it remains elusive whether autophagy is a sign of failing cardiomyocyte repair or is a suicidal pathway for the failing cardiomyocytes [31].
In summary, findings from our current study have provided rather convincing evidence of the involvement of the mTOR-STAT3-Notch signalling in the alcoholic cardiac injury and more importantly, ALDH2 overexpression-offered protection against such injury. It is plausible to speculate that ALDH2 overexpression protects against cardiac mechanical defect and apoptosis following alcohol intake through preserved phosphorylation of mTOR-STAT3 and subsequently Notch signalling, leading to inhibition of autophagy induction and restored cardiac homeostasis. Further study is warranted to unveil the precise role of ALDH2, Notch signalling and autophagy in the maintenance of cardiac geometry and function under both normal and alcoholic conditions.
Acknowledgments
This work was supported in part by NIAAA 1R01 AA013412.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Fernandez-Sola J, Estruch R, Grau JM, et al. The relation of alcoholic myopathy to cardiomyopathy. Ann Intern Med. 1994;120:529–36. doi: 10.7326/0003-4819-120-7-199404010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Ren J, Wold LE. Mechanisms of alcoholic heart disease. Ther Adv Cardiovasc Dis. 2008;2:497–506. doi: 10.1177/1753944708095137. [DOI] [PubMed] [Google Scholar]
- 3.Li SY, Gomelsky M, Duan J, et al. Overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells: role of ERK and p38 mitogen-activated protein kinase. The Journal of biological chemistry. 2004;279:11244–52. doi: 10.1074/jbc.M308011200. [DOI] [PubMed] [Google Scholar]
- 4.Doser TA, Turdi S, Thomas DP, et al. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119:1941–9. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Ma H, Li J, Gao F, et al. Aldehyde dehydrogenase 2 ameliorates acute cardiac toxicity of ethanol: role of protein phosphatase and forkhead transcription factor. J Am Coll Cardiol. 2009;54:2187–96. doi: 10.1016/j.jacc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- 6.Chen CH, Budas GR, Churchill EN, et al. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koda K, Salazar-Rodriguez M, Corti F, et al. Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation. 2010;122:771–81. doi: 10.1161/CIRCULATIONAHA.110.952481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma H, Guo R, Yu L, et al. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32:1025–38. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakai A, Yamaguchi O, Takeda T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–24. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Ren J. Autophagy in ALDH2-elicited cardioprotection against ischemic heart disease: slayer or savior. Autophagy. 2010;6:1212–3. doi: 10.4161/auto.6.8.13652. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Meng Y, Kwiatkowski DJ, et al. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J Clin Invest. 2010;120:103–14. doi: 10.1172/JCI37964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoyama T, Kondo Y, Kondo S. Roles of mTOR and STAT3 in autophagy induced by telomere 3’ overhang-specific DNA oligonucleotides. Autophagy. 2007;3:496–8. doi: 10.4161/auto.4602. [DOI] [PubMed] [Google Scholar]
- 14.Mazzone M, Selfors LM, Albeck J, et al. Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc Natl Acad Sci USA. 2010;107:5012–7. doi: 10.1073/pnas.1000896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Duan W, Jin Z, et al. New role of Notch-mediated signaling pathway in myocardial ischemic preconditioning. Med Hypotheses. 2011;76:427–8. doi: 10.1016/j.mehy.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Wu JH, Hagaman J, Kim S, et al. Aortic constriction exacerbates atherosclerosis and induces cardiac dysfunction in mice lacking apolipoprotein E. Arterioscler Thromb Vasc Biol. 2002;22:469–75. doi: 10.1161/hq0302.105287. [DOI] [PubMed] [Google Scholar]
- 17.Donohue TM., Jr Autophagy and ethanol-induced liver injury. World J Gastroenterol. 2009;15:1178–85. doi: 10.3748/wjg.15.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Rehman SK, Zhang W, et al. Autophagy is a therapeutic target in anticancer drug resistance. Biochim Biophys Acta. 2010;1806:220–9. doi: 10.1016/j.bbcan.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Parkhitko AA, Henske EP. Mammalian target of rapamycin signaling and autophagy: roles in lymphangioleiomyomatosis therapy. Proc Am Thorac Soc. 2010;7:48–53. doi: 10.1513/pats.200909-104JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Li SY, Brown RA, et al. Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stress. Alcohol. 2004;32:175–86. doi: 10.1016/j.alcohol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Spies CD, Sander M, Stangl K, et al. Effects of alcohol on the heart. Curr Opin Crit Care. 2001;7:337–43. doi: 10.1097/00075198-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Cederbaum AI, Wu D, Mari M, et al. CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Free Radic Biol Med. 2001;31:1539–43. doi: 10.1016/s0891-5849(01)00743-2. [DOI] [PubMed] [Google Scholar]
- 23.Li SY, Li Q, Shen JJ, et al. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006;40:283–94. doi: 10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;27:S137–48. doi: 10.1038/onc.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100:474–88. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 26.Clerk A, Cole SM, Cullingford TE, et al. Regulation of cardiac myocyte cell death. Pharmacol Ther. 2003;97:223–61. doi: 10.1016/s0163-7258(02)00339-x. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Ren J. Cardiac overexpression of metallothionein rescues chronic alcohol intake-induced cardiomyocyte dysfunction: role of Akt, mammalian target of rapamycin and ribosomal p70s6 kinase. Alcohol Alcohol. 2006;41:585–92. doi: 10.1093/alcalc/agl080. [DOI] [PubMed] [Google Scholar]
- 28.Guo R, Scott GI, Ren J. Involvement of AMPK in alcohol dehydrogenase accentuated myocardial dysfunction following acute ethanol challenge in mice. PLoS ONE. 2010;5:e11268. doi: 10.1371/journal.pone.0011268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurusamy N, Das DK. Autophagy, redox signaling, and ventricular remodeling. Antioxid Redox Signal. 2009;11:1975–88. doi: 10.1089/ars.2009.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan SM, Weng AP, Tibshirani R, et al. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood. 2007;110:278–86. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishida K, Yamaguchi O, Otsu K. Crosstalk between autophagy and apoptosis in heart disease. Circ Res. 2008;103:343–51. doi: 10.1161/CIRCRESAHA.108.175448. [DOI] [PubMed] [Google Scholar]


