Abstract
Background & Aims
Dental erosion is a complication of gastroesophageal reflux (GER) in adults; in children, it is not clear if GER has a role in dental pathologic conditions. Dietary intake, oral hygiene, high bacterial load, and decreased salivary flow might contribute independently to GER development or dental erosion, but their potential involvement in dental erosion from GER is not understood. We investigated the prevalence of dental erosion among children with and without GER symptoms, and whether salivary flow rate or bacterial load contribute to location-specific dental erosion.
Methods
We performed a cross-sectional study of 59 children (ages 9–17 y) with symptoms of GER and 20 asymptomatic children (controls); all completed a questionnaire on dietary exposure. Permanent teeth were examined for erosion into dentin, erosion locations, and affected surfaces. The dentist was not aware of GER status, nor was the gastroenterologist aware of dental status. Stimulated salivary flow was measured and salivary bacterial load was calculated for total bacteria, Streptococcus mutans and Lactobacilli.
Results
Controlling for age, dietary intake, and oral hygiene, there was no association between GER symptoms and dental erosion, by tooth location or affected surface. Salivary flow did not correlate with GER symptoms or erosion. Erosion location and surface were independent of total bacteria and levels of Streptococcus mutans and Lactobacilli.
Conclusions
Location-specific dental erosion is not associated with GER, salivary flow, or bacterial load. Prospective studies are required to determine the pathogenesis of GER-associated dental erosion and the relationship between dental caries to GER and dental erosion.
Keywords: tooth wear, mechanism, risk assessment, pediatrics
INTRODUCTION
We report a single-blinded cross-sectional study of the prevalence and location of dental erosion in children with or without gastroesophageal reflux (GER) symptoms. Our primary hypothesis was that the pathogenesis of dental erosion is related to GER symptoms. Our aims were to examine whether dietary factors, oral hygiene, salivary flow, or salivary bacterial load contribute to GER-associated dental erosion.
Dental erosion is loss of dental hard tissue by chemical processes without involvement of bacteria (Figure).1,2 Dental erosion can originate from extrinsic factors, including carbonated or acidic beverages and acidic foods,3,4 and intrinsic factors such as GER. Regurgitated intrinsic acid has a pH of approximately 1–2, significantly lower than pH 5.5, the critical threshold for tooth enamel dissolution.5,6 In individuals with GER, chronic exposure to extrinsic or intrinsic acid can increase the solubility of dental hard tissue, resulting in dental erosion.5,7 Consequently, erosion sites act as a focus for carious tooth damage, to cause further detrimental injury to the tooth. Gastroesophageal reflux (GER) has long been suspected to cause dental erosion.
Figure.
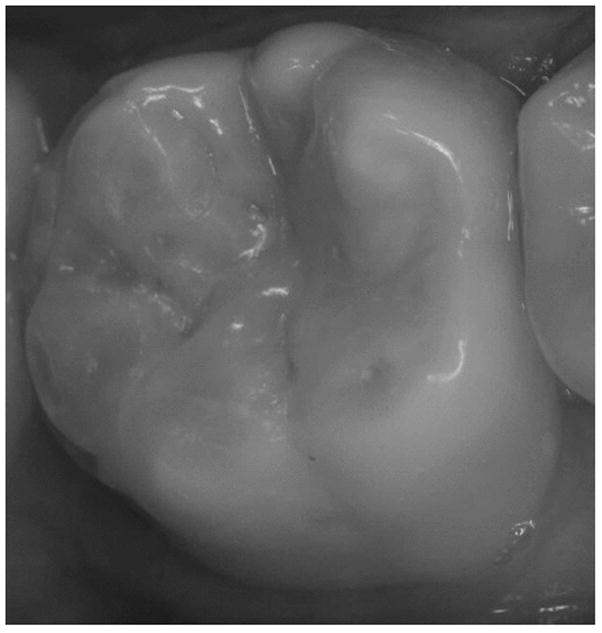
Clinical photograph of dental erosion (S-T.W.I. score 1 = Dentin just visible, including cupping, or dentin exposed for less than 1/3 of surface) on facial and lingual surfaces of the lower right first molar in a 14-year old Hispanic male with loss of anatomical contour and rounding of enamel edges.
Scope of the Problem: GER and Dental Erosion
Bargen and Austin first proposed the association between GER and dental erosion in an adult in 1937.8 Later reports on the relationship between GER and dental erosion are inconsistent and conflicting. Several investigators have observed a positive correlation between GER and dental erosion in adults.9–14 In contrast, Silva found only one dental erosion in 31 adults with GER.15
Reports of dental erosion in children as an extraesophageal manifestation of GER are also contradictory. A positive correlation between GER symptoms and dental erosion in children is observed by several investigators,16–21 while others record low prevalence of dental erosion and no correlation with erosion in primary or permanent teeth in children with GER symptoms.22, 23
Dietary Intake and Dental Erosion
Dietary intake may independently influence the development of GER symptoms or dental erosion. Increased consumption of cholesterol, saturated fatty acids and a higher percentage of calories from fats have been reported to be associated with an increased likelihood of GER events.24 Additionally, population studies have shown a direct correlation between consumption of carbonated drinks, fruit juices and dental erosion. Excessive intakes of acidic drinks and food have been implicated as the most important extrinsic factors contributing to dental erosion.25–29 O’Sullivan and Curzon found that dental erosion was increased in children who exhibited specific drinking habits – in particular, those that swished, sucked or held drinks in their mouth.30 Therefore, an evaluation of the association between GER symptoms and dental erosion in children must consider the dietary history and eating or drinking habits.
Salivary Flow Rate and Salivary Bacterial Load as Potential Mechanisms for GER-Associated Erosion
Mechanisms of dental erosion associated with GER have been inadequately investigated. The pathophysiology of dental caries in GER is clearer and may serve as a starting point in attempts to evaluate the role of salivary flow rate and salivary bacterial load in GER-associated erosion and consequent caries-related tooth injury.
The Caries Management by Risk Assessment (CAMBRA) document published in 2002 concluded that salivary flow rates in excess of 0.7 mL/minute protect against caries by facilitating oral clearance and neutralization of acids produced by cariogenic bacteria, most notably Streptococcus mutans and Lactobacillus species.31 Jarvinen et al reported that adults with low salivary flow displayed a 5-fold risk of developing dental erosion compared with individuals who registered normal flow,32 suggesting that adults with GER, in conjunction with low salivary flow rates, may be at risk for extensive tooth destruction from exposure to intrinsic acid, confounded by caries. In contrast, Gudmundsson et al reported a lack of statistically significant association between salivary volume and GER in adults.23
Few studies have examined the relationship between salivary flow rate and erosion in children. Salivary flow rates were comparable in GERD and control subjects according to Ersin et al, but the salivary flow rate had an impact on erosion, with lower salivary flow rates associated with increased dental erosion.16
Another possible mechanism for GER-associated dental erosion may be salivary bacteria in conjunction with acid that reduces the local pH below the critical value of 5.5, resulting in dissolution of tooth surface enamel and dentin, and eventual cavity formation.33–35 However, evidence for bacterial involvement in dental erosions is relatively limited. Increased salivary concentrations of Streptococcus mutans (S. mutans) and Lactobacilli have been shown to accelerate progression of a compromised surface to a cavity.19,34,36 Translocating ATPase facilitates survival of these organisms at the low pH levels following ingestion of fermentable carbohydrates.33 More specifically related to a possible involvement of GER in bacterial oral flora, Linnett et al described a higher concentration of salivary S. mutans in GER subjects compared with the normal population.19 Similarly, Ersin et al positively correlated the level of S. mutans and Lactobacilli with caries in a population of children with GERD.16 Taken together, these observations support the possibility that patients with GER may maintain an oral environment favorable for caries, with decreased salivary flow rates, possibly increased salivary bacteria, and oral pH below 5.5.5
METHODS
Patient Population and Study Sample
Pediatric subjects 9 to 17 years of age with symptoms of GER, and a control group of children with no symptoms of GER, were recruited from the Pediatric Gastroenterology Clinics and General Pediatric Clinics at the University of California, San Francisco (UCSF) Benioff Children’s Hospital from November 2005 through October 2008. This age range was chosen to examine the effects of GER on erosion in predominantly permanent teeth with potentially long-term deleterious effects.
Medical chart reviews identified eligible symptomatic subjects. Symptomatic subjects were characterized as having one or more of the following self-reported chronic (>3 months) symptoms: abdominal pain, chest pain or heartburn, difficulty swallowing, nausea and/or vomiting, regurgitation, bitter acid taste, burping or belching, choking while swallowing food, upper abdominal pain after eating. Parents of eligible subjects received a study flyer describing the study and a written request for participation. Non-respondents were not contacted further. A description of the study and an offer of inclusion of eligible children were mailed to pediatricians and dentists in the San Francisco Bay Area. Asymptomatic control subjects were recruited from the General Pediatric Clinics at UCSF and surrounding San Francisco Bay Area. Children with systemic diseases or a history of conditions potentially affecting oral health or flora, such as diabetes, HIV, or heart conditions that require antibiotic prophylaxis, were excluded, as were children younger than 9 or older than 17 years of age.
The Committee on Human Research at UCSF approved the study protocol (CHR Approval Number: H2302-27207). University of California “Permission to Use Personal Health Information for Research” forms (HIPAA certification) were authorized by parents. Informed consent was obtained from parents, and informed assent (for those under 12 years of age) or consent was obtained from children and adolescents before enrollment.
Sample Size and Power Calculation
The sample of 59 symptomatic and 20 asymptomatic participants provided 80% power in 2-sided tests with a type-I error rate of 5% to detect between-group differences of 24–37 percentage points in prevalence of binary outcomes including presence of at least one erosion, as well as differences of 0.77 SDs in the mean of continuous outcomes including erosions per tooth.
GER Assessment
Each symptomatic subject underwent a 24-hour pH probe test for the presence of acid reflux. All proton pump inhibitors and/or H2-receptor antagonists were discontinued 2 weeks prior to pH probe study. Correct placement of the pH probe was confirmed by chest radiograph, with the pH probe placed 7/8 of the distance from the nares to the distal esophagus, assumed to be at the diaphragm. The pH probe test was performed ± 2 weeks from the date of the dental exam. One pediatric gastroenterologist blinded to the subject’s erosion status interpreted all pH probe results. A positive pH probe study was defined as meeting at least one of the following four criteria:
≥ 4.2% of time pH < 4 (upright, recumbent, and a 24-hour tabulation)
≥ 50 total reflux episodes over a 24-hour period
> 3 reflux episodes lasting ≥ 5 minutes
a single episode lasting ≥ 9.2 minutes 37
Dental Erosion Assessment
Each subject underwent a dental assessment of permanent teeth to determine the presence of erosion according to tooth location and surfaces (occlusal/incisal, facial and lingual). Surfaces that aid in chewing are known as occlusal/incisal on posterior teeth and incisal on anterior teeth. Using 2× magnifying glasses, each tooth was assessed for dental erosion by the Simplified Tooth Wear Index (S-T.W.I.): 38
-
Score 0 –
No wear into dentine
-
Score 1 -
Dentine just visible (including cupping) or dentine exposed for less than 1/3 of surface
-
Score 2 –
Dentine exposure greater than 1/3 of surface
-
Score 3 –
Exposure of pulp or secondary dentine
The examining dentist was blinded to GER categorization of subjects and the results of the 24-hour pH probe testing (for symptomatic subjects).
We considered two outcomes summarizing dental erosion. First, because erosion scores >1 were very uncommon, we defined a binary indicator of any erosion for each tooth. To summarize the prevalence of erosion accounting for variability in number of permanent teeth (12–28 teeth per subject), we calculated the proportion of teeth with erosion overall, by tooth location (upper, lower, anterior, posterior) and by surface affected (occlusal/incisal, lingual, facial).
Food Analysis
Each subject completed a self-administered multiple-choice questionnaire on dietary exposure. The self-administered dietary intake and oral hygiene questionnaire was developed with reference to a previously validated questionnaire, the Oral Health Assessment portion of the UK National Diet and Nutrition Survey of Children.39, 40 This initial questionnaire was piloted in the UK and recently in Brazil for content and face validity, and designed to investigate the types and frequencies of consumption of foods and drinks. Dietary liquids were recorded for daily quantity, method of drinking, drinking speed, liquid temperature and time of day of consumption. Intake frequency of various foods was also recorded. Variables were coded by assigning numeric values to each answer choice and by transcribing the numeric equivalent of the checked box into an Excel spreadsheet.
Oral Hygiene
Oral hygiene practices were also documented in a multiple-choice questionnaire. Subjects documented the number of mouth fillings and history of dental trauma history or tooth pain. Detailed accounts of daily activities were also recorded, including parts of the mouth brushed, brushing frequency, brushing length of time and time of day, type of toothbrush and toothpaste and other daily oral hygiene routines. Subjects were specifically asked about chewing, swallowing and sucking behaviors, and level of satisfaction with one’s mouth, gums, breath, tooth health and color were also recorded. Lastly, dental visits and sources of dental advice were documented. Variables were coded by assigning numeric values to each answer choice and by transcribing the numeric equivalent of the checked box into an Excel spreadsheet.
Stimulated Salivary Volume
To measure stimulated salivary volume, subjects chewed a standard piece of paraffin wax, and then expectorated directly into a 15ml tube (1 ml scale) over a 2-minute collection period.
Salivary Bacterial Load
A 0.3 mL saliva sample obtained from saliva collected for stimulated salivary flow rate measurement was immediately plated onto selective media (standard microbiological procedure). The saliva sample was tested for number of colony forming units (CFU) of S. mutans and Lactobacillus species, and total viable bacteria flora with conventional plating on selective media.
Statistical Analysis
Data are expressed as mean±standard deviation (SD). We used Wilcoxon and Fisher’s exact test to compare symptomatic and asymptomatic study participants. We also used Fisher’s exact test to assess the associations between binary dietary and oral hygiene variables. Salivary volume was compared between groups using T-tests. Bacterial loads were not normally distributed and so were log-transformed and compared using Wilcoxon rank-sum test.
To assess the relationship between GER symptoms and prevalence of erosion, we first identified potential confounders associated with both GER symptoms and erosions. Then, the potential influence of GER symptoms on location-specific prevalence of dental erosion was assessed using linear regression, controlling for age, gender and the dietary and oral hygiene confounders identified in the first step. Because of the non-normality of the erosion prevalence summary measures, we used bias-corrected percentile bootstrap confidence intervals and robust standard errors for inference.
We considered p-values <0.05 to be statistically significant. Stat Version 11 software (StataCorp, College Station, TX) was used for all analyses.
RESULTS
Eighty-four children were recruited. Five were excluded due to incomplete pH probe tests (3 subjects), inherited developmental enamel disorder (1 subject), and a follow-up diagnosis of eosinophilic esophagitis (1 subject). The remaining 79 pediatric subjects 9–17 years old met the inclusion and exclusion criteria. Symptomatic children were older than asymptomatic children (p=0.0004), and symptomatic females were older than asymptomatic females (p=0.0004). No statistically significant differences were found for gender, body mass index (BMI) Z-score, or teeth present between groups. (Table 1)
Table 1.
Demographic data
| Symptomatic (N=59) | Asymptomatic (N=20) | p-value | |
|---|---|---|---|
| Females, n(%) | 35 (59%) | 10 (50%) | 0.47 |
| Males, n(%) | 24 (41%) | 10 (50%) | 0.47 |
| Age in years – mean (SD) | 14.0 (2.4) | 11.9 (1.4) | 0.0004 |
| Male Age | 13.1 (2.5) | 12.1 (1.1) | 0.22 |
| Female Age | 14.5 (2.3) | 11.6 (1.7) | 0.0004 |
| BMI Z-score (SD) | 0.45 (1.3) | 0.71 (1.2) | 0.46 |
| Teeth present (mean) | |||
| Total teeth (SD) | 24.1 (5.8) | 21.9 (5.9) | 0.13 |
| Upper teeth (SD) | 11.2 (3.0) | 10.8 (3.3) | 0.15 |
| Lower teeth (SD) | 12.2 (2.7) | 11.1 (2.7) | 0.12 |
GER Symptoms and pH Probe
Of the 59 pH probe tests performed on symptomatic children, 45 were positive and 14 were negative for GER. Children with negative pH probe results had 33.8±12.1 (range: 7–49) reflux episodes during pH probe testing; the fraction of time at pH <4 was 1.5±0.9% (range: 0.4–4.0%). Children with positive pH probe results had 122±79 (range: 41–362) reflux episodes, and the fraction of time at pH <4 was 9.9±15.2% (range: 0.8–72.5%). Among these 59 symptomatic children, we found no statistically significant relationship between the frequency of reflux episodes by pH probe results and erosion prevalence.
Unadjusted Association of GER with Dental Erosion By Tooth Location and Surface
Examination of erosion by tooth surface of all erosions in our sample study revealed that 15 tooth surfaces had STWI Score of 2 (0.2% of total surfaces) and zero surfaces had STWI Score of 3. Because of the low percentage of Scores 2–3, we simplified the statistical analysis and coded dental erosion as a binary variable (0 = absence of dental erosion, 1 = presence of any dental erosion). Comparable percentages of symptomatic and asymptomatic children had at least one dental erosion (85% vs. 70%, p=0.15). However, the number of teeth with erosion was statistically higher in the symptomatic group than in the control group, both overall (p=0.017) and by specific tooth locations. Symptomatic children had more erosion on upper compared with lower teeth (p=0.005) than children in the control group. Similarly, symptomatic children had more erosion on posterior compared with anterior teeth (p=0.016) than children in the control group. (Table 2) Erosion rates in symptomatic compared with asymptomatic children were higher on occlusal/incisal surfaces (p=0.01) but similar on facial and lingual surfaces. (Table 2)
Table 2.
Unadjusted Association between GER and Teeth With Erosion by Location and Surface
| Mean Number of Erosions per Tooth | 95% Confidence Interval | |||||
|---|---|---|---|---|---|---|
| Tooth Location or Surface | GER | Asymptomatic | Adjusted difference of means | Lower CI | Upper CI | p-value |
| Total Teeth | 0.19 | 0.11 | 0.074 | −0.013 | −0.157 | 0.017 |
| LOCATION | ||||||
| Upper | 0.15 | 0.04 | 0.104 | 0.031 | 0.176 | 0.005 |
| Lower | 0.24 | 0.17 | 0.054 | −0.070 | 0.178 | 0.393 |
| Anterior | 0.23 | 0.14 | 0.061 | −0.051 | 0.174 | 0.284 |
| Posterior | 0.18 | 0.08 | 0.115 | 0.022 | 0.209 | 0.016 |
| SURFACE | ||||||
| Facial | 0.04 | 0.03 | 0.011 | −0.033 | 0.055 | 0.630 |
| Occlusal/Incisal | 0.14 | 0.05 | 0.080 | 0.019 | 0.141 | 0.010 |
| Lingual | 0.04 | 0.05 | −0.001 | −0.047 | 0.046 | 0.975 |
Dietary Intake and Oral Hygiene and Health
All dietary intake and oral hygiene and health variables were examined between groups and in relationship to location-specific dental erosion (Tables 3, 4). Four variables confounded the relationship between GER symptoms and location-specific dental erosion. Citrus or sour/tart candies were associated with GER symptoms (p=0.03) and to increased upper teeth erosion (p=0.06) and occlusal/incisal surface erosion (p=0.03). Chocolate intake was associated with GER symptoms (p=0.034) and with increased erosion on anterior teeth (p=0.02) and occlusal/incisal tooth surfaces (p=0.01). Individuals symptomatic with GER were more dissatisfied with their tooth (p=0.004) and gum (p=0.03) health. Individuals with more dental erosion on lower teeth were also found to trend toward more dissatisfaction with their tooth (p=0.05) and gum (p=0.04) health.
Table 3.
Dietary Intake and Dental Hygiene Variables Associated with GER
| Dietary or Oral Hygiene Variable | GER Symptoms - Fisher’s Exact (p-value) |
|---|---|
| Drinking grape juice | 0.029 |
| Eating citrus or sour/tart candy | 0.032 |
| No gum chewing | 0.001 |
| Eating chocolate | 0.034 |
| Eating cookies | 0.008 |
| Tooth pain | 0.021 |
| Decreased frequency of chewing gum | 0.024 |
| No fluoride in mouthwash | 0.009 |
Table 4.
Dietary Intake and Dental Hygiene Variables Associated with Dental Erosion by Location
| Location of Dental Erosion - Fisher’s Exact (p-value) | |||||
|---|---|---|---|---|---|
| Dietary or Oral Hygiene Variable | All Teeth | Upper Teeth | Lower Teeth | Anterior Teeth | Posterior Teeth |
| Sport drinks | 0.03 | NS | 0.03 | NS | 0.02 |
| Drinking water from bottle | NS | NS | 0.03 | NS | NS |
| Milk temperature | 0.03 | NS | NS | 0.01 | NS |
| Eating mints | NS | 0.04 | NS | NS | NS |
| Eating chocolate | NS | NS | NS | 0.02 | NS |
GER and Dental Erosion, Adjusted for Diet
Regression analyses showed no relationship between GER symptoms and dental erosion by tooth location or surface after adjustment for dietary intake, oral hygiene, gender and age.
Salivary Volume
Comparable stimulated salivary volume was found between symptomatic and asymptomatic children (2.76 ± 0.99 vs. 2.96 ± 1.44 mL, p=0.50).
Salivary Bacterial Load
Neither total salivary bacteria load nor the presence of either S. mutans or Lactobacillus differed between groups. Rank-sum analyses showed similar bacterial loads of S. mutans and Lactobacillus in symptomatic and control groups (p=0.70 and p=0.08, respectively). T-test analysis of total bacterial loads between groups also showed no difference (symptomatic mean load: 8.56 ± 0.41 cfu; control mean load: 8.43 ± 0.36 cfu; p-value 0.24). To create a statistically more robust sample, we doubled the various measured bacterial loads and ran similar analyses between groups. However, doubling the bacterial loads did not differentially associate with erosion in the two groups, focusing on particular erosion locations and surfaces. All statistical tests for interaction among these variables (bacterial load, erosion surface or tooth location and GER symptoms) were NS, with the exception of the association of total bacteria with GER symptoms and increased erosion. This finding was likely an artifact of multiple comparisons, and not clinically insightful. Total, S. mutans and Lactobacillus bacterial loads also appeared to have similar associations with tooth erosion in symptomatic and asymptomatic children. Therefore, total bacteria, S. mutans and Lactobacillus bacterial loads did not differentially associate with erosion tooth location or surface.
DISCUSSION
In this blinded study of children with and without symptoms of GER, the proportion of teeth affected by dental erosion was similar in symptomatic compared with asymptomatic children controlled for age, dietary intake and oral hygiene. Although initial analysis suggested increased prevalence of overall dental erosion in symptomatic children, and location-specific erosion in children with GER symptoms on upper teeth compared to lower teeth, posterior teeth compared to anterior teeth, and on occlusal/incisal tooth surfaces, these differences disappeared when controlled for dietary intake and oral hygiene.
Our data support the results of previous studies, which showed no differences in prevalence of dental erosion in children with and without GER. A study in 53 younger children (mean 4.9 years old) found that only 9 (17%) showed signs of dental erosion; only 1 displayed erosion involving dentin.22 Larger sample size and inclusion of older children increased the statistical power of the present study. Prolonged exposure to potentially harmful dietary and hygienic variables that might contribute to GER symptoms or dental erosion offered an additional investigational advantage.
An additional potential confounder of the association of GER with dental erosion is diet, which differed between the symptomatic and asymptomatic groups, and was associated with erosion. Specifically, we found increased site-specific dental erosion in children who consumed sport drinks, room temperature milk, chocolate or mints. After adjusting for these dietary factors, we found no independent relationship between GER and dental erosion.
Unlike previous studies, exposure to other acidic, sugar-laden and caffeinated juices and beverages showed no relationship to dental erosion. Dental students who consumed grapefruit juice, orange juice or soda daily for 5 weeks developed signs of erosion on their labial incisors, most evident with grapefruit juice.41 According to Stabholz, et al., children who receive orange juice in school daily for 10–18 months develop mild dental demineralization.42 Consumption of citrus fruits two or more times per day, soft drinks once per day, vinegar or sports drinks more than once weekly has been associated with dental erosion.32 No relationship between dental erosion and intake of citrus fruit juice or whole fruit, regular or diet soda, or sugar-laden beverages was observed in our subjects. These differences between our findings and dietary studies reported by others may be due to small sample size, limiting the subjects’ answers to those listed in the self-administered questionnaire, or unanswered questions during the questionnaire process.
In our study, only 76% of children with symptoms had significant GER documented by pH probe, in contrast with reports by Aine et al and Ersin et al.16, 21 However, the pH probe results were not predictive of dental erosion among symptomatic children in our series. Although the statistical power was low, this finding is consistent with the possibility that other factors, such as dietary intake and oral hygiene practices may contribute to dental erosion in children with symptoms of GER.
No association was found between salivary flow or salivary bacterial load with dental erosion, decreasing the likelihood these independent variables may contribute to mechanisms responsible for GER-associated erosion. Salivary flow rates obtained in our cohort support previously reported negative results obtained in adult and pediatric patients. Silva et al failed to demonstrate a relationship between GER and salivary flow tests in a cohort of 31 adults with esophagitis.15 Moazzez et al reported in a cohort of adults with and without gastro-esophageal reflux disease that 13% of GERD patients complaining of hoarseness had a lower salivary flow rate than controls. Overall, however, GERD and control subjects had similar salivary flow rates.10,16
The relationship between salivary bacteria, GERD symptoms and dental erosion remains unclear. We found no correlations between these variables. Higher salivary colonization rates with S. mutans have been reported in children with GERD compared with healthy children (p<0.02).16 In addition, Linnett et al noted only a trend that did not reach significance for colonization of S. mutans in children with GERD versus controls (42% vs. 25%).19 The role of salivary bacterial load in GER-associated erosion has not been previously assessed.
POTENTIAL LIMITATIONS
Inclusion of older children in the present study provided data on permanent teeth. This unique feature may also represent a potential limitation, because older children are generally more likely to have experienced prolonged exposure to acid from extrinsic sources (i.e. food and soft drinks) in addition to exposure from GER-related acid. Our statistical analysis was age adjusted to account for this difference, given the older age of symptomatic children compared with asymptomatic children. Moreover, our subjects were selected for the presence of GER symptoms, whereas previous studies focused on children with GER determined by pH probe. Therefore, our data may reflect a population with less severe GER than previously reported in other studies.
Though we measured pH probe parameters and used established criteria by DeMeester to define a positive pH probe study for children with symptomatic GER.37 we did not collect data on the defined amount of acid exposure. The lack of this data prevents us from analyzing the relationship between a defined amount of acid exposure with the number of dental erosion per tooth.
A further limitation is possible misclassification of children into the symptomatic group; however, we found statistically significant differences in prevalence of erosion in symptomatic compared with asymptomatic groups. The observed association between GER symptoms and dental erosion suggests that children with clinical evidence of GER are at risk for increased dental erosion, regardless of the results of pH probe studies. Thus, while our results do not reveal a positive relationship, future investigations of GER symptoms should include routine examinations for evidence of dental erosion. Additionally, techniques to specifically address the degree of proximal reflux in subjects with symptoms of GER may better predict the risk of tooth exposure.
Lastly, the cross-sectional study design may represent a potential limitation. Dental erosion is a gradual process, and dietary as well as hygienic factors may impact teeth for several years before achieving the degree of damage observed in our older study subjects.
In summary, our findings indicate that children with symptoms of GER are not at increased risk for dental erosion. Future longitudinal investigations should explore the progression of dental erosion in the context of a dietary and dental hygiene history as a guide to pediatricians, pediatric gastroenterologists and dentists involved in preventive assessment and management of children with GER and dental erosion.
Supplementary Material
Acknowledgments
Grant Support:
This project was supported in part by NIH Grants DK007762 (YKW, MBH) and DK060617 (MBH) and an unrestricted research grant from Takeda Pharmaceuticals North America, Inc.
List of Abbreviations
- GER
Gastroesophageal reflux
- GERD
Gastroesophageal reflux disease
- UCSF
University of California, San Francisco
Footnotes
Disclosures:
All authors have no disclosures of potential conflicts (financial, professional, or personal) that are relevant to the manuscript.
Writing Assistance:
The authors also appreciate the contributions, comments, and suggestions from M. Michael Thaler and Elizabeth Garnett.
Author Contributions:
1. Yvette K. Wild, M.D., M.P.H. – Analysis and interpretation of data, statistical analysis, drafting of the manuscript
2. Melvin B. Heyman, M.D., M.P.H. – Study concept and design, obtained funding, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision
3. Eric Vittinghoff, Ph.D., M.P.H. – Analysis and interpretation of data, statistical analysis
4. Deepal H. Dalal, B.S. – Analysis and interpretation of data, technical, drafting of the manuscript
5. Janet M. Wojcicki, Ph.D., M.P.H. – Statistical analysis
6. Ann L. Clark, B.S. - Acquisition of data, drafting of the manuscript
7. Beate Rechmann - Acquisition of data, Administrative, technical
8. Peter Rechmann, D.D.S., Ph.D. - Study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pace F, Pallotta S, Tonini M, Vakil N, Bianchi Porro G. Systematic review: Gastro-oesophageal reflux disease and dental lesions. Aliment Pharmacol Ther. 2008;27(12):1179–1186. doi: 10.1111/j.1365-2036.2008.03694.x. [DOI] [PubMed] [Google Scholar]
- 2.Linnett V, Seow WK. Dental erosion in children: A literature review. Pediatr Dent. 2001;23(1):37–43. [PubMed] [Google Scholar]
- 3.Jensdottir T, Arnadottir IB, Thorsdottir I, et al. Relationship between dental erosion, soft drink consumption, and gastroesophageal reflux among icelanders. Clin Oral Investig. 2004;8(2):91–96. doi: 10.1007/s00784-003-0252-1. [DOI] [PubMed] [Google Scholar]
- 4.Barron RP, Carmichael RP, Marcon MA, Sandor GK. Dental erosion in gastroesophageal reflux disease. J Can Dent Assoc. 2003;69(2):84–89. [PubMed] [Google Scholar]
- 5.Cengiz S, Cengiz MI, Sarac YS. Dental erosion caused by gastroesophageal reflux disease: A case report. Cases J. 2009;2:8018. doi: 10.4076/1757-1626-2-8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfaro EV, Aps JK, Martens LC. Oral implications in children with gastroesophageal reflux disease. Curr Opin Pediatr. 2008;20(5):576–583. doi: 10.1097/MOP.0b013e32830dd7df. [DOI] [PubMed] [Google Scholar]
- 7.Reis A, Higashi C, Loguercio AD. Re-anatomization of anterior eroded teeth by stratification with direct composite resin. J Esthet Restor Dent. 2009;21(5):304–316. doi: 10.1111/j.1708-8240.2009.00281.x. [DOI] [PubMed] [Google Scholar]
- 8.Bargen J, Austin L. Decalcification of teeth as a result of obstipation with long continued vomiting: Report of a case. J Am Dent Assoc. 1937;24:1271–1273. [Google Scholar]
- 9.Jarvinen V, Meurman JH, Hyvarinen H, Rytomaa I, Murtomaa H. Dental erosion and upper gastrointestinal disorders. Oral Surg Oral Med Oral Pathol. 1988;65(3):298–303. doi: 10.1016/0030-4220(88)90113-2. [DOI] [PubMed] [Google Scholar]
- 10.Moazzez R, Bartlett D, Anggiansah A. Dental erosion, gastro-oesophageal reflux disease and saliva: How are they related? J Dent. 2004;32(6):489–494. doi: 10.1016/j.jdent.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Smith WA, Marchan S, Rafeek RN. The prevalence and severity of non-carious cervical lesions in a group of patients attending a university hospital in trinidad. J Oral Rehabil. 2008;35(2):128–134. doi: 10.1111/j.1365-2842.2007.01763.x. [DOI] [PubMed] [Google Scholar]
- 12.Oginni AO, Agbakwuru EA, Ndububa DA. The prevalence of dental erosion in nigerian patients with gastro-oesophageal reflux disease. BMC Oral Health. 2005;5(1):1. doi: 10.1186/1472-6831-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregory-Head BL, Curtis DA, Kim L, Cello J. Evaluation of dental erosion in patients with gastroesophageal reflux disease. J Prosthet Dent. 2000;83(6):675–680. [PubMed] [Google Scholar]
- 14.Munoz JV, Herreros B, Sanchiz V, et al. Dental and periodontal lesions in patients with gastro-oesophageal reflux disease. Dig Liver Dis. 2003;35(7):461–467. doi: 10.1016/s1590-8658(03)00215-9. [DOI] [PubMed] [Google Scholar]
- 15.Silva MA, Damante JH, Stipp AC, Tolentino MM, Carlotto PR, Fleury RN. Gastroesophageal reflux disease: New oral findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(3):301–310. doi: 10.1067/moe.2001.111139. [DOI] [PubMed] [Google Scholar]
- 16.Ersin NK, Oncag O, Tumgor G, Aydogdu S, Hilmioglu S. Oral and dental manifestations of gastroesophageal reflux disease in children: A preliminary study. Pediatr Dent. 2006;28(3):279–284. [PubMed] [Google Scholar]
- 17.Su JM, Tsamtsouris A, Laskou M. Gastroesophageal reflux in children with cerebral palsy and its relationship to erosion of primary and permanent teeth. J Mass Dent Soc. 2003;52(2):20–24. [PubMed] [Google Scholar]
- 18.Dahshan A, Patel H, Delaney J, Wuerth A, Thomas R, Tolia V. Gastroesophageal reflux disease and dental erosion in children. J Pediatr. 2002;140(4):474–478. doi: 10.1067/mpd.2002.123285. [DOI] [PubMed] [Google Scholar]
- 19.Linnett V, Seow WK, Connor F, Shepherd R. Oral health of children with gastro-esophageal reflux disease: A controlled study. Aust Dent J. 2002;47(2):156–162. doi: 10.1111/j.1834-7819.2002.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 20.Shaw L, Weatherill S, Smith A. Tooth wear in children: An investigation of etiological factors in children with cerebral palsy and gastroesophageal reflux. ASDC J Dent Child. 1998;65(6):484–6. 439. [PubMed] [Google Scholar]
- 21.Aine L, Baer M, Maki M. Dental erosions caused by gastroesophageal reflux disease in children. ASDC J Dent Child. 1993;60(3):210–214. [PubMed] [Google Scholar]
- 22.O’Sullivan EA, Curzon ME, Roberts GJ, Milla PJ, Stringer MD. Gastroesophageal reflux in children and its relationship to erosion of primary and permanent teeth. Eur J Oral Sci. 1998;106(3):765–769. doi: 10.1046/j.0909-8836.1998.eos106302.x. [DOI] [PubMed] [Google Scholar]
- 23.Gudmundsson K, Kristleifsson G, Theodors A, Holbrook WP. Tooth erosion, gastroesophageal reflux, and salivary buffer capacity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(2):185–189. doi: 10.1016/s1079-2104(05)80280-x. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro M, Green C, Bautista JM, et al. Assessment of dietary nutrients that influence perception of intra-oesophageal acid reflux events in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007;25(1):93–101. doi: 10.1111/j.1365-2036.2006.03170.x. [DOI] [PubMed] [Google Scholar]
- 25.Luo Y, Zeng XJ, Du MQ, Bedi R. The prevalence of dental erosion in preschool children in china. J Dent. 2005;33(2):115–121. doi: 10.1016/j.jdent.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Harding MA, Whelton H, O’Mullane DM, Cronin M. Dental erosion in 5-year-old irish school children and associated factors: A pilot study. Community Dent Health. 2003;20(3):165–170. [PubMed] [Google Scholar]
- 27.Al-Majed I, Maguire A, Murray JJ. Risk factors for dental erosion in 5–6 year old and 12–14 year old boys in saudi arabia. Community Dent Oral Epidemiol. 2002;30(1):38–46. doi: 10.1034/j.1600-0528.2002.300106.x. [DOI] [PubMed] [Google Scholar]
- 28.Al-Malik MI, Holt RD, Bedi R. The relationship between erosion, caries and rampant caries and dietary habits in preschool children in saudi arabia. Int J Paediatr Dent. 2001;11(6):430–439. [PubMed] [Google Scholar]
- 29.Johansson AK, Sorvari R, Birkhed D, Meurman JH. Dental erosion in deciduous teeth--an in vivo and in vitro study. J Dent. 2001;29(5):333–340. doi: 10.1016/s0300-5712(01)00029-x. [DOI] [PubMed] [Google Scholar]
- 30.O’Sullivan EA, Curzon ME. A comparison of acidic dietary factors in children with and without dental erosion. ASDC J Dent Child. 2000;67(3):186–92. 160. [PubMed] [Google Scholar]
- 31.Featherstone JD, Adair SM, Anderson MH, et al. Caries management by risk assessment: Consensus statement, april 2002. J Calif Dent Assoc. 2003;31(3):257–269. [PubMed] [Google Scholar]
- 32.Jarvinen VK, Rytomaa II, Heinonen OP. Risk factors in dental erosion. J Dent Res. 1991;70(6):942–947. doi: 10.1177/00220345910700060601. [DOI] [PubMed] [Google Scholar]
- 33.Bowen WH. Do we need to be concerned about dental caries in the coming millennium? Crit Rev Oral Biol Med. 2002;13(2):126–131. doi: 10.1177/154411130201300203. [DOI] [PubMed] [Google Scholar]
- 34.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369(9555):51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 35.Shaw JH. Causes and control of dental caries. N Engl J Med. 1987;317(16):996–1004. doi: 10.1056/NEJM198710153171605. [DOI] [PubMed] [Google Scholar]
- 36.Hamada S, Slade HD. Biology, immunology, and cariogenicity of streptococcus mutans. Microbiol Rev. 1980;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson LF, DeMeester TR. Development of the 24-Hour Intraesophageal pH Monitoring Composite Scoring System. J Clin Gastroenterology. 1998;8(Suppl 1):52–28. doi: 10.1097/00004836-198606001-00008. [DOI] [PubMed] [Google Scholar]
- 38.Margaritis V, Mamai-Homata E, Koletsi-Kounari H, Polychronopoulou A. Evaluation of three different scoring systems for dental erosion: A comparative study in adolescents. J Dent. 2011;39(1):88–93. doi: 10.1016/j.jdent.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Waterhouse PJ, Auad SM, Nunn JH, Steen IN, Moynihan PJ. Diet and dental erosion in young people in south-east Brazil. Int J Paediatr Dent. 2008;18(5):353–360. doi: 10.1111/j.1365-263X.2008.00919.x. [DOI] [PubMed] [Google Scholar]
- 40.Walker A, Gregory J, Bradnock G, Nunn JH, White D. Report of the Oral Health Survey. Vol. 2. London: The Stationery Office; 2000. National Diet and Nutrition Survey: Young People Aged 4 to 18 Years; p. 292. [Google Scholar]
- 41.Thomas AK. Further observations on the influence of citrus fruit juices on human teeth. 1957;23:424–430. [Google Scholar]
- 42.Stabholz A, Raistein J, Markitziu A, et al. Tooth enamel dissolution from erosion or etching and subsequent caries development. J Pedod. 1983;7(2):100–108. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


