Abstract
Purposes
To compare oxygen uptake before and after the onset of claudication in subjects with peripheral artery disease (PAD) during a 6-minute walk test, and to identify predictors of the change in oxygen uptake following the onset of claudication pain
Methods
Fifty subjects with PAD were studied, in which 33 experienced claudication (Pain Group) during a 6-minute walk test, and 17 were pain-free during this test (Pain-Free Group). Oxygen uptake and ambulatory cadence were primary outcomes obtained during the 6-minute walk test.
Results
The Pain Group experienced onset of claudication pain at 179 ± 45 meters (mean ± standard deviation) and continued to walk to achieve a 6-minute walk distance of 393 ± 74 meters, which was similar (p = 0.74) to the Pain-Free Group (401 ± 76 meters). Oxygen uptake increased (p < 0.0001) after the onset of pain in the Pain Group, and this change was greater (p = 0.025) than the increase in oxygen uptake from the second to fifth minute of walking in the Pain-Free Group. Furthermore, ambulatory cadence decreased after the onset of pain in the Pain Group (p = 0.0003). The change in oxygen uptake was associated with metabolic syndrome (p = 0.0023), 6-minute walk distance (p = 0.0037), age, (p = 0.0041), and the oxygen uptake during the second minute of the test (p = 0.012).
Conclusion
Claudication increases oxygen uptake of self-paced, over-ground ambulation despite a decrease in cadence. The pain-mediated increase in oxygen uptake was blunted in subjects with metabolic syndrome, suggesting that they have an impaired ability to increase oxygen uptake during ambulation. The clinical significance is that claudication increases metabolic cost of ambulation, thereby increasing the relative intensity of exercise and reducing the tolerance to sustain ambulation.
INTRODUCTION
Peripheral artery disease (PAD) is prevalent in more than 12% of the US population 65 years of age and older,(1) and is associated with elevated rates of mortality(2–5) and morbidity,(6) as over 60% of subjects have concomitant cardiovascular and/or cerebrovascular disease.(7) In addition to having high cardiovascular risk, many of those with PAD are physically limited by ambulatory leg pain. Claudication is prevalent in more than 6% of those at least 65 years of age,(1;7) which results in impaired ambulation,(8;9) reduced physical function,(10;11) and lower daily physical activity.(12)
Less is known about how the onset of claudication acutely affects ambulation. Oxygen uptake is increased with the onset of claudication during treadmill exercise at constant load, thereby increasing the relative metabolic cost of painful ambulation and reducing exercise tolerance.(13) It is not clear whether the increase in oxygen uptake following pain onset during treadmill walking is evident during self-paced, over-ground ambulation because subjects may compensate by slowing their pace(14;15) to maintain a given metabolic cost. However, slowing freely-chosen velocity by too much results in less economical ambulation,(16–18) which may increase oxygen uptake even further during painful ambulation. The oxygen uptake of self-paced, over-ground ambulation during pain-free and painful ambulation has not been previously examined, and has important implications regarding the intensity at which ambulation is performed in the community setting.
The purposes of the study are to compare oxygen uptake before and after the onset of claudication in subjects with PAD during a 6-minute walk test, and to identify predictors of the change in oxygen uptake following the onset of claudication pain. The hypotheses are that oxygen uptake increases after the onset of claudication, and that severity of PAD and the change in ambulatory cadence and velocity are predictive of the change in oxygen uptake following the onset of claudication.
METHODS
SUBJECTS
Recruitment
Subjects were evaluated in the General Clinical Research Center at the University of Oklahoma Health Sciences Center (HSC). Subjects were recruited by referrals from the HSC vascular clinic, as well as by newspaper advertisements for possible enrollment into a randomized controlled exercise rehabilitation study.(19) The data and analyses for this study were part of the baseline assessments obtained for the exercise study. The procedures used in this study were approved by the Institutional Review Board at the University of Oklahoma HSC. Written informed consent was obtained from each subject prior to investigation.
Screening
Subjects with claudication secondary to vascular insufficiency were included in this study if they met the following criteria: (a) a history of any type of exertional leg pain, (b) ambulation during a graded treadmill test limited by leg pain consistent with claudication,(8) and (c) an ankle-brachial index (ABI) ≤ 0.90 at rest,(1;7) or an ABI ≤ 0.73 after exercise because some PAD patients have normal values at rest which only become abnormal following an exercise test.(20) Subjects were excluded from this study for the following conditions: (a) absence of PAD (ABI > 0.90 at rest and ABI > 0.73 after exercise), (b) inability to obtain an ABI measure due to non-compressible vessels, (c) asymptomatic PAD (Fontaine Stage I) determined from the medical history and verified during the graded treadmill test, (d) use of medications indicated for the treatment of claudication (cilostazol and pentoxifylline) initiated within three months prior to investigation, and (e) exercise tolerance during maximal treadmill exercise limited by factors other than claudication pain (e.g., severe coronary artery disease, dyspnea, poorly controlled blood pressure), and (f) active cancer, end-stage renal disease, or liver disease, (g) discontinuing ambulation for any reason during the 6-minute walk test, and (h) for those experiencing pain during the 6-minute walk test, ambulating for less than one complete minute during pain-free or painful conditions because an insufficient amount of data was available for analyses. A total of 77 subjects were evaluated for this study, and 50 subjects were deemed eligible.
MEASUREMENTS
Primary Outcome Measures: Oxygen Uptake and Ambulatory Cadence Obtained During the 6-Minute Walk Test
Procedures
A trained technician administered the over ground, 6-minute walk test in which two cones were placed 100 feet apart in a marked corridor as previously described.(21) Subjects were instructed to walk as many laps around the cones as possible while wearing a light-weight (0.8 kg), portable oxygen uptake unit (COSMED K4 b2, COSMED USA, Inc, Chicago, IL) which continuously measured oxygen uptake via indirect calorimetry, and while wearing a step activity monitor (Step Watch 3, Cyma Inc., Mountlake Terrace, WA) placed on their right ankle. During the test, subjects indicated if and when they experienced the onset of claudication pain. Subjects who experienced pain during the 6-minute walk test (Pain Group) were subsequently compared to those who completed the test without pain (Pain-Free Group), as described in the results section.
Outcome Measures
This test was the experimental protocol used to obtain the primary outcome measures of oxygen uptake and ambulatory cadence, as well as other measures consisting of stride length, ambulatory velocity, time and distance to onset of pain, and total 6-minute walk distance. Oxygen uptake and ambulatory cadence were obtained each minute during the test, and the technician recorded the time and distance to onset of claudication as well as the total distance walked. The walking distances were subsequently converted from feet to meters. Stride length and ambulatory velocity were then calculated during both pain-free and painful ambulation in the Pain Group. However, these calculations were not possible to determine in the Pain-Free Group for two reasons. First, the subjects did not experience claudication during the test, and therefore ambulation did not occur under painful conditions. Second, time and distance measures were not recorded at specified intervals during the test because of concerns that this additional encumbrance on the technician while the test was proceeding might affect the results of the self-paced 6-minute walk test. Therefore the calculation of stride length and ambulatory velocity at different time points during the test in the Pain-Free Group was not possible.
Oxygen uptake and ambulatory cadence obtained during the first and last minute of exercise in either group were not used for analyses because of the possibility that data was not recorded for a full 60 seconds during these time points. Additionally, oxygen uptake and ambulatory cadence obtained during the minute in which the onset of claudication occurred in the Pain Group was not used for analyses because it was not possible to precisely separate the data before and after the onset of pain within the minute. Change scores for oxygen uptake and gait parameters were calculated as the difference in average values obtained during painful ambulation minus the values obtained during pain-free ambulation in the Pain Group, and the difference between the fifth minute values minus the second minute values during exercise in the Pain-Free group
Secondary Outcome Measures
Medical History, Physical Examination, and Anthropometry
Demographic information, height, weight, body mass index (BMI), waist and hip circumferences,(22) cardiovascular risk factors, co-morbid conditions, claudication history, blood samples, and a list of current medications were obtained from a medical history and physical examination at the beginning of the study.
Walking Impairment Questionnaire (WIQ)
Self-reported ambulatory ability was obtained using a validated questionnaire for PAD subjects that assesses ability to walk at various speeds and distances, and to climb stairs.(23)
Graded Treadmill Test
Claudication Times and Peak Oxygen Uptake
Subjects performed a progressive, graded treadmill protocol to determine study eligibility, as well as to obtain outcome measures related to exercise performance.(8) The claudication onset time (COT), defined as the walking time at which the subjects first experienced pain, and the peak walking time (PWT), defined as the walking time at which ambulation could not continue due to maximal pain, were both recorded to quantify the severity of claudication. Peak oxygen uptake was measured by oxygen uptake obtained during the peak exercise work load with a Medical Graphics VO2000 metabolic system (Medical Graphics Inc, St. Paul, MN). Using these procedures, the test-retest intraclass reliability coefficient is R = 0.89 for COT,(8) R = 0.93 for PWT,(8) and R = 0.88 for peak oxygen uptake.(24)
ABI and Ischemic Window
As previously described, ABI measures were obtained from the more severely diseased lower extremity before and 1, 3, 5, and 7 minutes after the treadmill test.(8;25) The reduction in ankle systolic blood pressure after treadmill exercise from the resting baseline value was quantified by calculating the area under the curve, referred to as the ischemic window.(26) Because the ischemic window is a function of both PAD severity and the amount of exercise performed, the ischemic window was normalized per meter walked.
Ambulatory Activity Monitoring
Daily ambulatory activity was assessed using a step activity monitor as previously described.(27) Ambulatory activity was measured during seven consecutive days in which subjects were instructed to wear the monitor during waking hours and to remove it before retiring to bed. The step activity monitor was attached to the right ankle above the lateral malleolus using elastic Velcro straps, and continuously recorded the number of steps taken on a minute-to-minute basis. The accuracy of the step activity monitor exceeds 99% ± 1% in subjects with claudication.(27)
STATISTICAL ANALYSES
An independent sample t-test was used to compare the means of continuous demographic and clinical measures between the Pain Group and the Pain-Free Group. A Chi-square test, or Fisher’s exact test for small expected cell counts, was used to compare the distribution of categorical demographic and clinical measures between the two groups. To address the first aim, a non-parametric Wilcoxon signed rank test was used to compare the median oxygen uptake measures and the median ambulatory parameters (cadence, speed, and stride length) between pain-free and painful ambulation in the Pain Group, and between the second and fifth time points in the Pain-Free Group. Between-group comparisons of the distribution of changes in oxygen uptake and ambulatory parameters were made using an independent sample Wilcoxon rank sum test. For the pain-free time during the 6-minute walk test, between-group comparisons of mean values to a fixed reference were made using a one-sample t-test. A 2-sided 0.05 alpha level was used to define statistical significance.
To address the second aim, linear regression was used to identify clinical and exercise performance characteristics that were independently associated with oxygen uptake after adjustment for height. Clinical and exercise performance characteristics associated with oxygen uptake univariately at a 0.05 alpha level were entered into a multiple linear regression model. Clinical and exercise performance characteristics were deleted from the multiple regression model until all terms were significant at the 0.05 alpha level. The clinical and exercise performance characteristics that were considered in the modeling were all factors listed in Tables I and II. Height was adjusted for in the regression model to adjust for the association between height and stride rate. Data were analyzed using SAS (SAS System for Windows, ver. 9.1, SAS Institute Inc., Cary, NC) and SPSS (SPSS for Windows, rel. 15.0, SPSS Chicago, IL).
Table I.
Clinical characteristics of subjects.
| Pain Group (n=33) | Pain-Free Group (n=17) | P-value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 65 (9) | 67 (11) | 0.59 |
| Weight (kg) | 89.6 (18.7) | 78.0 (17.4) | 0.038 |
| Height (cm) | 171.0 (9.7) | 169.2 (9.1) | 0.51 |
| Body mass index (kg/m2) | 30.6 (5.8) | 27.6 (7.6) | 0.12 |
| Waist girth (cm) (n=19, n=7) | 105.1 (16.0) | 102.6 (18.2) | 0.74 |
| Ankle/brachial index | 0.72 (0.20) | 0.75 (0.21) | 0.61 |
|
| |||
| Percent | Percent | P-value | |
|
| |||
| Sex (% Men) | 67% | 59% | 0.58 |
| Race (% Caucasian) | 61% | 47% | 0.36 |
| Current smoking (%) | 36% | 35% | 0.94 |
| Diabetes (%) | 36% | 41% | 0.74 |
| Hypertension (%) | 88% | 76% | 0.42 |
| Hyperlipidemia (%) | 76% | 82% | 0.73 |
| Abdominal obesity (%) (n=19, 7) |
63% | 71% | >0.99 |
| Obesity (%) | 48% | 29% | 0.20 |
| Metabolic syndrome (%) | 88% | 65% | 0.070 |
Table II.
Treadmill exercise performance, Walking Impairment Questionnaire (WIQ) measures, and daily ambulatory activity of subjects.
| Pain Group (n=33) | Pain-Free Group (n=17) | P-value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Claudication Onset Time (sec) | 240 | 178 | 265 | 214 | 0.68 |
| Peak Walking Time (sec) | 475 | 194 | 438 | 212 | 0.55 |
| Peak Oxygen Uptake (ml·kg−1·min−1) | 13.5 | 3.5 | 12.6 | 3.1 | 0.40 |
| Ischemic Window (mmHg × min/meter) | 0.52 | 0.45 | 0.47 | 0.62 | 0.74 |
| WIQ Distance Score (%) | 39 | 30 | 45 | 35 | 0.56 |
| WIQ Speed Score (%) | 35 | 20 | 42 | 25 | 0.30 |
| WIQ Climbing Score (%) | 45 | 29 | 42 | 31 | 0.73 |
| Daily Ambulatory Activity (strides/day) | 3719 | 1811 | 3688 | 1920 | 0.96 |
RESULTS
Subjects were grouped according to whether they experienced claudication during the 6-minute walk test (Pain Group; n = 33) or did not experience claudication (Pain-Free Group; n = 17). The groups were similar on all clinical characteristics (p > 0.05), except that the Pain Group had higher body weight (p = 0.038), and a non-significant trend for higher prevalence of metabolic syndrome (p = 0.070) (Table I). The groups were similar on all measures of treadmill exercise performance, WIQ measures, and daily ambulatory activity (p > 0.05) (Table II), but the Pain Group, by definition, had shorter 6-minute walk pain-free time (p < 0.001) and pain-free distance (p < 0.001) than the Pain-Free Group (Table III).
Table III.
6-minute walk measurements of subjects.
| Pain Group (n=33) | Pain-Free Group (n=17) | P-value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| 6-Min Walk Pain-free Time (sec) | 156 | 29 | 360 | 0 | <0.0001* |
| 6-Min Walk Pain-Free Distance (meters) | 179 | 45 | 401 | 76 | <0.0001 |
| 6-Min Walk Distance (meters) | 393 | 74 | 401 | 76 | 0.74 |
Comparison made between 6-minute walk pain-free time in the Pain group and a fixed value of 360 seconds in the Pain-Free group using a one-sample t-test.
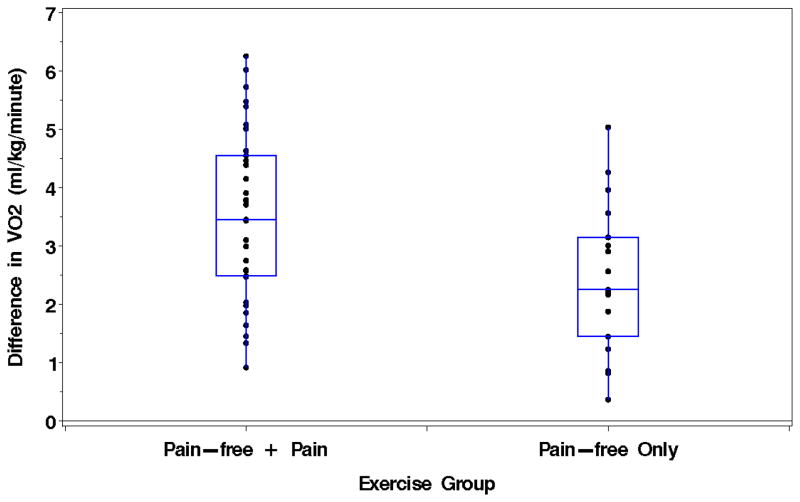
Oxygen uptake during pain-free ambulation increased (p < 0.0001) after the onset of pain in the Pain Group, and this change was greater (p = 0.025) than the increase in oxygen uptake from the second to fifth minute of walking in the Pain-Free Group (Table IV and Figure 1). In contrast, the decrease in ambulatory cadence after pain onset in the Pain Group (p = 0.0003) was similar (p = 0.79) to the decline in cadence from the second to fifth minute of walking in the Pain-Free Group (Table IV). After pain onset, the Pain Group also experienced a shorter stride length (p < 0.0001), a decline in ambulatory velocity (p < 0.0001), and increased oxygen uptake when expressed per stride taken (p < 0.0001) or meter walked (p < 0.0001) than compared to before pain onset (Table IV).
Table IV.
Oxygen uptake and gait measures during a 6-minute walk test in the Pain-Group (n = 33) and Pain-Free Group (n = 17). Data summaries are median (interquartile range: 25th to 75th percentile).
| Variables | Time Point 1 * | Time Point 2 ** | Δ (Time point 2 – Time point 1) | Change Score (P Value) | Difference Between Change Scores (P Value) |
|---|---|---|---|---|---|
| VO2 per minute (ml·kg−1·min−1) | |||||
| Pain Group | 8.4 (6.6 to 9.6) | 11.4 (10.1 to 14.0) | 3.5 (2.5 to 4.6) | <0.0001 | 0.025 |
| Pain-Free Group | 8.4 (7.5 to 10.2) | 11.0 (10.2 to 12.6) | 2.3 (1.5 to 3.2) | <0.0001 | |
| VO2 per meter walked (ml·kg−1·meter−1) | |||||
| Pain Group | 0.10 (0.08 to 0.12) | 0.14 (0.12 to 0.18) | 0.05 (0.02 to 0.07) | <0.0001 | ------- |
| Pain-Free Group | ------- | ------- | ------- | ------- | |
| VO2 per stride taken (ml·kg−1·stride−1) | |||||
| Pain Group | 0.18 (0.15 to 0.20) | 0.21 (0.19 to 0.26) | 0.04 (0.02 to 0.06) | <0.0001 | 0.36 |
| Pain-Free Group | 0.17 (0.13 to 0.20) | 0.21 (0.19 to 0.26) | 0.05 (0.03 to 0.07) | <0.0001 | |
| Velocity (meter/min) | |||||
| Pain Group | 66.6 (61.8 to 75.5) | 65.9 (53.5 to 69.2) | −4.4 (−13.3 to −1.7) | < 0.0001 | ------- |
| Pain-Free Group | ------- | ------- | ------- | ------- | |
| Cadence (strides/min) | |||||
| Pain Group | 55.0 (52.0 to 56.0) | 54.0 (50.5 to 56.0) | −1.0 (−1.5 to 0.0) | 0.0003 | 0.79 |
| Pain-Free Group | 53.0 (50.0 to 56.0) | 52.0 (50.0 to 54.0) | 0.0 (−1.0 to 0.0) | 0.0078 | |
| Stride Length (meter/stride) | |||||
| Pain Group | 1.25 (1.13 to 1.39) | 1.17 (1.09 to 1.33) | −0.05 (−0.14 to −0.02) | < 0.0001 | ------- |
| Pain-Free Group | ------- | ------- | ------- | ------- | |
Values during Time Point 1 were obtained during pain-free ambulation in the Pain Group and during the second minute of ambulation in the Pain-Free Group.
Values for Time Point 2 were obtained during painful ambulation in the Pain Group and during the fifth minute of ambulation in the Pain-Free Group.
Figure 1.
Change in oxygen uptake during a 6-minute walk test in the Pain Group (n = 33) and the Pain-Free Group (n = 17). The change in the Pain Group was defined as the oxygen uptake measured during painful ambulation minus the oxygen uptake during pain-free ambulation. The change in the Pain-Free Group was defined as the oxygen uptake measured during the fifth minute minus the oxygen uptake measured during the second minute.
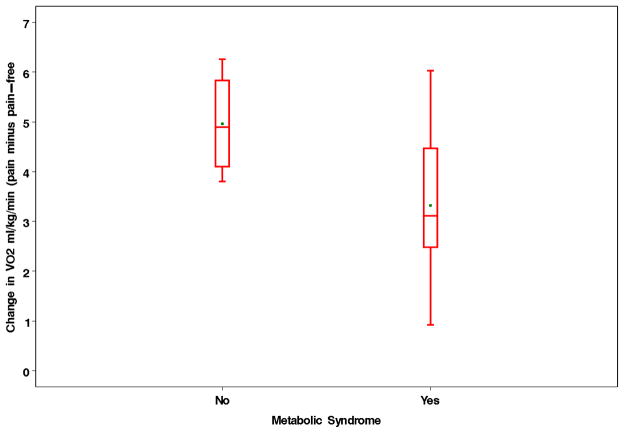
The multiple regression model predicting the change in oxygen uptake following the onset of claudication in patients in the Pain Group is shown in Table V. Metabolic syndrome was negatively associated with the increase in oxygen uptake following pain onset, as subjects with metabolic syndrome had on average a 1.83 ml·kg−1·min−1 lower change in oxygen uptake than those without metabolic syndrome (Figure 2). In contrast, age, 6-minute walk distance, and the oxygen uptake during the second minute of the 6-minute walk test were positively associated with the pain-mediated increase in oxygen uptake.
Table V.
Multiple regression model predicting the change in oxygen uptake following the onset of claudication in subjects in the Pain Group (n = 33).
| Dependent Variables | Independent Variables | Regression Coefficient | 95% Confidence Interval | P-value |
|---|---|---|---|---|
| Δ Oxygen Uptake (ml·kg−1·min−1) | Age (years) | 0.064 | 0.022 to 0.11 | 0.0041 |
| Metabolic syndrome (no syndrome reference) | −1.83 | −2.93 to −0.71 | 0.0023 | |
| 6-minute walk total distance (meters) | 0.0093 | 0.0033 to 0.015 | 0.0037 | |
| Minute 2 VO2 (ml·kg−1·min−1) | 0.20 | 0.049 to 0.36 | 0.012 | |
| Height (cm) | −0.014 | −0.061 to 0.033 | 0.54 |
Figure 2.
Change in oxygen uptake during a 6-minute walk test in subjects with and without metabolic syndrome in the Pain Group. The change in oxygen uptake was defined as the value measured during painful ambulation minus the value obtained during pain-free ambulation.
DISCUSSION
Changes in Oxygen Uptake and Ambulatory Cadence During Painful Ambulation
The primary finding of this study was that oxygen uptake increased by 36% after the transition from pain-free to painful ambulation during the 6-minute walk test. This observation supports a previous report from our laboratory, which found that oxygen uptake was higher while ambulating with pain during a constant-speed, standardized treadmill test.(13) It was not clear whether the observed pain-mediated increase in oxygen uptake during treadmill exercise would occur during self-paced, over-ground ambulation because subjects can attempt to compensate by slowing their pace. We found that ambulatory cadence decreased during painful ambulation, supporting previous reports of changes in gait measures following the onset of claudication, such as reduced ambulatory velocity,(15) shortened step length,(15) and decreased ankle plantar flexor moments(14) at self-selected pace over short distances. However, the reduction in ambulatory cadence during painful ambulation in the present study did not reduce the metabolic cost of walking.
Although this study does not address mechanisms for the increase in oxygen uptake during painful ambulation, it indicates that a given exercise work rate becomes more challenging to perform than pain-free ambulation. Muscle force production decreases with claudication,(28;29) which may explain the decrease in stride length during painful ambulation in the Pain-Group. It is possible that the Pain Group had a greater decrease in their stride length during the test than the Pain-Free Group, and this may be one reason why the increase in oxygen uptake was not different between the two groups when expressed per stride taken. When muscle force production decreases with claudication, the recruitment of more motor units per muscle is needed to generate the required force to ambulate.(18) A change in muscle fiber recruitment patterns would be consistent with more challenging ambulation,(18) suggesting that more motor units, particularly more fast-twitch motor units which are less economical than slow-twitch fibers,(30) are recruited to perform painful ambulation. Furthermore, muscle denervation observed in PAD subjects(31) may impair optimal motor unit recruitment during exercise, becoming more evident during painful ambulation.
It should be noted that those who completed the 6-minute walk test pain-free also had an increase in oxygen uptake during exercise, albeit a smaller one than those who performed painful ambulation. It has been observed that a gradual increase in oxygen uptake occurs during intense exercise compared to light exercise, thus preventing oxygen uptake from reaching a steady-state plateau during exercise at a constant work load.(32) Since the Pain-Free Group ambulated at a relatively high intensity equal to 72% of their peak oxygen uptake during the second minute of exercise, it is not surprising that they experienced a gradual increase in oxygen uptake, referred to as the slow component of the increase in oxygen uptake which we have previously observed in PAD subjects walking on a treadmill at a constant work rate.(33)
Our data shows a lack of agreement between the COT and PWT measures obtained during a standardized treadmill test and the pain-free time and distance obtained during the 6-minute walk test. Although 33 subjects experienced claudication during the 6-minute walk test, and therefore had shorter pain-free walking time and distance than the 17 subjects who did not experience claudication, there were no group differences in COT and PWT values during treadmill exercise. This supports our previous finding that there is no significant correlation between the pain-free distance during the 6-minute walk test and the COT and PWT obtained during a standardized treadmill test.(21) This suggests that the two tests measure different aspects of ambulatory function in subjects with PAD and claudication. Given that the average, freely-chosen walking speed during the 6-minute walk test was higher (approximately 2.5 mph) than the 2.0 mph speed during the treadmill test, the first few minutes of the 6-minute walk test was performed at a higher exercise intensity than the treadmill test. Thus, it is not surprising that two-thirds of the subjects (i.e., Pain Group) experienced claudication sooner during the 6-minute walk test than during the treadmill test. However, it is surprising that one-third of the subjects (i.e., Pain-free Group) did not experience claudication during the 6-minute walk test, even though they walked at a faster pace than during the treadmill test. Although both groups attempted to walk as fast as they could during the 6-minute walk test, it is possible that the Pain-Free Group walked closer to their optimal speed during the 6-minute walk test, and that the walking speed during the treadmill test was relatively too slow for them. When expressed per distance travelled, walking too slow is equally inefficient as walking too fast, thereby decreasing efficiency.(17) Thus, the faster pace during the 6-minute walk test may have been more comfortable for the Pain-Free Group than the slower pace during the treadmill test, even though a 2% incline was added after the first two minutes of the treadmill test.
Predictors of the pain-mediated change in oxygen uptake
Oxygen uptake increased after the onset of claudication in subjects ambulating during a 6-minute walk test. Metabolic syndrome was a predictor of the pain-mediated change in oxygen uptake. This finding suggests that insulin resistance may play a prominent role in interfering with central and peripheral factors affecting the kinetics of oxygen uptake. For example, Type II diabetes slows whole-body oxygen uptake kinetics and heart rate kinetics during exercise,(34) slows oxidative enzyme activity,(35) increases the frequency of mitochondrial DNA deletions in skeletal muscle,(36) impairs endothelial function,(37) reduces leg blood flow during steady-state exercise,(38) and slows microvascular blood flow kinetics.(39) Data from our laboratory also support these findings, as PAD subjects with metabolic syndrome have a blunted increase in reactive hyperemic calf blood flow than those without metabolic syndrome, and they have more limited 6-minute walk performance.(40) Additional factors predictive of the change in pain-mediated oxygen uptake were age, 6-minute walk distance, and the oxygen uptake during the second minute of the test. The increase in oxygen uptake was greater in older subjects, in those who maintained a fast pace during the test even after pain onset to achieve a longer total distance, and in those who had high oxygen uptake values during the early phase of the test.
Limitations
There are several limitations to this study. The regression coefficients calculated between oxygen uptake and clinical characteristics and baseline exercise performance measures from this cross-sectional design do not allow causality to be established. The present findings are also limited by the relatively small sample sizes, particularly in the Pain-Free group. Another limitation is that potential group differences in co-morbid conditions that were not included in this study (e.g., arthritis, chronic obstructive pulmonary disease, congestive heart failure, etc) may partially explain the group difference in the change in oxygen uptake during the 6-minute walk. However, these co-morbid conditions would be of minimal importance unless the prevalence of these conditions was different between groups. Furthermore, the effect of these conditions (e.g., arthritic pain) would typically be present throughout the entire walking test, thereby not eliciting an increase in oxygen uptake at any particular point during the test like we found for the onset of claudication. Additionally, this study is limited to PAD subjects who have claudication, and may not be generalized to subjects with less severe or more severe PAD. However, the subjects in the current study are typical of those with claudication, as there was a good proportion of women and African-Americans, and high prevalence of cardiovascular risk factors for PAD, including smoking, diabetes, hypertension, dyslipidemia, and obesity. Thus, the findings of the present study appear generalizable to subjects with claudication who typically have numerous co-morbid conditions.
Conclusions and Clinical Significance
We conclude that claudication increases oxygen uptake of self-paced, over-ground ambulation despite a decrease in cadence. The pain-mediated increase in oxygen uptake was blunted in subjects with metabolic syndrome, suggesting that they have an impaired ability to increase oxygen uptake during ambulation. The clinical significance is that claudication increases metabolic cost of ambulation, thereby increasing the relative intensity of exercise and reducing the tolerance to sustain ambulation. This information is clinically relevant to exercise professionals who rehabilitate subjects with PAD, as the training intensity should be reduced to compensate for the expected increase in intensity during painful ambulation, thereby providing a safer and more effective exercise prescription. A long-term goal is to determine whether the efficacy of interventions designed to improve claudication translates to improved self-paced, over-ground ambulation, evident by delayed occurrence of pain and by attenuation of the increase in oxygen uptake as subjects transition from pain-free to painful ambulation.
Acknowledgments
This research was supported by grants from the National Institute on Aging (NIA) (R01-AG-24296; AWG), by a Oklahoma Center for the Advancement of Science and Technology grant (HR04-113S; AWG), and by the University of Oklahoma Health Sciences Center General Clinical Research Center grant (M01-RR-14467), sponsored by the National Center for Research Resources from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 2.Brass EP, Hiatt WR. Review of mortality and cardiovascular event rates in patients enrolled in clinical trials for claudication therapies. Vasc Med. 2006;11:141–45. doi: 10.1177/1358863x06069513. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–86. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 4.Dormandy J, Heeck L, Vig S. The natural history of claudication: risk to life and limb. Semin Vasc Surg. 1999;12:123–37. [PubMed] [Google Scholar]
- 5.Muluk SC, Muluk VS, Kelley ME, Whittle JC, Tierney JA, Webster MW, et al. Outcome events in patients with claudication: a 15-year study in 2777 patients. J Vasc Surg. 2001;33:251–57. doi: 10.1067/mva.2001.112210. [DOI] [PubMed] [Google Scholar]
- 6.Vogt MT, Wolfson SK, Kuller LH. Lower extremity arterial disease and the aging process: a review. J Clin Epidemiol. 1992;45:529–42. doi: 10.1016/0895-4356(92)90102-s. [DOI] [PubMed] [Google Scholar]
- 7.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–8. [PubMed] [Google Scholar]
- 9.Gardner AW, Skinner JS, Vaughan NR, Bryant CX, Smith LK. Comparison of three progressive exercise protocols in peripheral vascular occlusive disease. Angiology. 1992;43:661–71. doi: 10.1177/000331979204300806. [DOI] [PubMed] [Google Scholar]
- 10.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 11.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–61. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 12.Sieminski DJ, Gardner AW. The relationship between free-living daily physical activity and the severity of peripheral arterial occlusive disease. Vasc Med. 1997;2:286–91. doi: 10.1177/1358863X9700200402. [DOI] [PubMed] [Google Scholar]
- 13.Gardner AW, Ritti-Dias RM, Stoner JA, Montgomery PS, Scott KJ, Blevins SM. Walking economy before and after the onset of claudication pain in patients with peripheral arterial disease. J Vasc Surg. 2010;51:628–33. doi: 10.1016/j.jvs.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SJ, Pipinos I, Johanning J, Radovic M, Huisinga JM, Myers SA, et al. Bilateral claudication results in alterations in the gait biomechanics at the hip and ankle joints. J Biomech. 2008;41:2506–14. doi: 10.1016/j.jbiomech.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 15.McCully K, Leiper C, Sanders T, Griffin E. The effects of peripheral vascular disease on gait. J Gerontol A Biol Sci Med Sci. 1999;54:B291–B294. doi: 10.1093/gerona/54.7.b291. [DOI] [PubMed] [Google Scholar]
- 16.Gardner AW, Montgomery PS, Ritti-Dias RM, Forrester L. The effect of claudication pain on temporal and spatial gait measures during self-paced ambulation. Vasc Med. 2010;15:21–26. doi: 10.1177/1358863X09106836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larish DD, Martin PE, Mungiole M. Characteristic patterns of gait in the healthy old. Ann N Y Acad Sci. 1988;515:18–32. doi: 10.1111/j.1749-6632.1988.tb32960.x. [DOI] [PubMed] [Google Scholar]
- 18.Martin PE, Rothstein DE, Larish DD. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J Appl Physiol. 1992;73:200–206. doi: 10.1152/jappl.1992.73.1.200. [DOI] [PubMed] [Google Scholar]
- 19.Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123:491–98. doi: 10.1161/CIRCULATIONAHA.110.963066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiatt WR, Marshall JA, Baxter J, Sandoval R, Hildebrandt W, Kahn LR, et al. Diagnostic methods for peripheral arterial disease in the San Luis Valley Diabetes Study. J Clin Epidemiol. 1990;43:597–606. doi: 10.1016/0895-4356(90)90164-k. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc. 1998;46:706–11. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 22.Lohman TC, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. pp. 39–70. [Google Scholar]
- 23.Regensteiner JG, Steiner JF, Panzer RL, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–52. [Google Scholar]
- 24.Gardner AW. Reliability of transcutaneous oximeter electrode heating power during exercise in patients with intermittent claudication. Angiology. 1997;48:229–35. doi: 10.1177/000331979704800305. [DOI] [PubMed] [Google Scholar]
- 25.Gardner AW, Skinner JS, Smith LK. Effects of handrail support on claudication and hemodynamic responses to single-stage and progressive treadmill protocols in peripheral vascular occlusive disease. Am J Cardiol. 1991;68:99–105. doi: 10.1016/0002-9149(91)90719-2. [DOI] [PubMed] [Google Scholar]
- 26.Feinberg RL, Gregory RT, Wheeler JR, Snyder SO, Jr, Gayle RG, Parent FN, III, et al. The ischemic window: a method for the objective quantitation of the training effect in exercise therapy for intermittent claudication. J Vasc Surg. 1992;16:244–50. doi: 10.1067/mva.1992.36947. [DOI] [PubMed] [Google Scholar]
- 27.Gardner AW, Montgomery PS, Scott KJ, Afaq A, Blevins SM. Patterns of ambulatory activity in subjects with and without intermittent claudication. J Vasc Surg. 2007;46:1208–14. doi: 10.1016/j.jvs.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Judge AR, Dodd SL. Oxidative damage to skeletal muscle following an acute bout of contractile claudication. Atherosclerosis. 2003;171:219–24. doi: 10.1016/j.atherosclerosis.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Judge AR, Selsby JT, Dodd SL. Antioxidants attenuate oxidative damage in rat skeletal muscle during mild ischaemia. Exp Physiol. 2008;93:479–85. doi: 10.1113/expphysiol.2007.040972. [DOI] [PubMed] [Google Scholar]
- 30.Wendt IR, Gibbs CL. Energy production of mammalian fast- and slow-twitch muscles during development. Am J Physiol. 1974;226:642–47. doi: 10.1152/ajplegacy.1974.226.3.642. [DOI] [PubMed] [Google Scholar]
- 31.England JD, Regensteiner JG, Ringel SP, Carry MR, Hiatt WR. Muscle denervation in peripheral arterial disease. Neurology. 1992;42:994–99. doi: 10.1212/wnl.42.5.994. [DOI] [PubMed] [Google Scholar]
- 32.Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, et al. Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol. 1991;71:1245–60. doi: 10.1152/jappl.1991.71.4.1245. [DOI] [PubMed] [Google Scholar]
- 33.Womack CJ, Sieminski DJ, Katzel LI, Yataco A, Gardner AW. Oxygen uptake during constant-intensity exercise in patients with peripheral arterial occlusive disease. Vasc Med. 1997;2:174–78. doi: 10.1177/1358863X9700200303. [DOI] [PubMed] [Google Scholar]
- 34.Regensteiner JG, Bauer TA, Reusch JE, Brandenburg SL, Sippel JM, Vogelsong AM, et al. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J Appl Physiol. 1998;85:310–317. doi: 10.1152/jappl.1998.85.1.310. [DOI] [PubMed] [Google Scholar]
- 35.Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol. 1997;83:166–71. doi: 10.1152/jappl.1997.83.1.166. [DOI] [PubMed] [Google Scholar]
- 36.Liang P, Hughes V, Fukagawa NK. Increased prevalence of mitochondrial DNA deletions in skeletal muscle of older individuals with impaired glucose tolerance: possible marker of glycemic stress. Diabetes. 1997;46:920–923. doi: 10.2337/diab.46.5.920. [DOI] [PubMed] [Google Scholar]
- 37.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–74. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 38.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care. 2003;26:899–904. doi: 10.2337/diacare.26.3.899. [DOI] [PubMed] [Google Scholar]
- 39.Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care. 2007;30:2880–2885. doi: 10.2337/dc07-0843. [DOI] [PubMed] [Google Scholar]
- 40.Gardner AW, Montgomery PS, Parker DE. Metabolic syndrome impairs physical function, health-related quality of life, and peripheral circulation in patients with intermittent claudication. J Vasc Surg. 2006;43:1191–96. doi: 10.1016/j.jvs.2006.02.042. [DOI] [PubMed] [Google Scholar]