Abstract
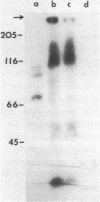
The major constituent of the trypanosomal kinetoplast DNA network are several thousand duplex DNA minicircles whose biological function is still unknown. The coding capacity and expression of these DNA minicircles, was studied in the trypanosomatid Crithidia fasciculata. Kinetoplast DNA minicircle fragments inserted into bacterial plasmid vectors were expressed in the bacterial cell. Sera elicited in rabbits, by immunization with the translational products of kinetoplast DNA minicircles in E. coli, reacted specifically with Crithidia fasciculata cellular antigens. It is inferred that kinetoplast DNA minicircles contain long open reading frames of nucleotides which are expressed in the trypanosomatid cell.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Barrois M., Riou G., Galibert F. Complete nucleotide sequence of minicircle kinetoplast DNA from Trypanosoma equiperdum. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3323–3327. doi: 10.1073/pnas.78.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen K. K., Donelson J. E. Sequences of two kinetoplast DNA minicircles of Tryptanosoma brucei. Proc Natl Acad Sci U S A. 1980 May;77(5):2445–2449. doi: 10.1073/pnas.77.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts D. L., Wolstenholme D. R. Evidence for a partial RNA transcript of the small circular component of kinetoplast DNA of Crithidia acanthocephali. Nucleic Acids Res. 1979 Aug 24;6(12):3785–3804. doi: 10.1093/nar/6.12.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Cunningham I., Gardiner P. R., Taylor A. M., Luckins A. G. Cultivation of infective forms of Trypanosoma congolense from trypanosomes in the proboscis of Glossina morsitans. Parasitology. 1981 Feb;82(1):81–95. doi: 10.1017/s0031182000041883. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P. RNA from the insect trypanosome Crithidia luciliae contains transcripts of the maxi-circle and not of the mini-circle component of kinetoplast DNA. Biochim Biophys Acta. 1978 Nov 21;521(1):407–411. doi: 10.1016/0005-2787(78)90282-4. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Snijders A., Janssen J. W., Borst P. Transcription of kinetoplast DNA in Trypanosoma brucei bloodstream and culture forms. Plasmid. 1981 May;5(3):329–350. doi: 10.1016/0147-619x(81)90009-3. [DOI] [PubMed] [Google Scholar]
- Kidane G. Z., Hughes D., Simpson L. Sequence heterogeneity and anomalous electrophoretic mobility of kinetoplast minicircle DNA from Leishmania tarentolae. Gene. 1984 Mar;27(3):265–277. doi: 10.1016/0378-1119(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Kleisen C. M., Borst P. Sequence heterogeneity of the mini-circles of kinetoplast DNA of Crithidia luciliae and evidence for the presence of a component more complex than mini-circle DNA in the kinetoplast network. Biochim Biophys Acta. 1975 Nov 4;407(4):473–478. doi: 10.1016/0005-2787(75)90301-9. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Riou G., Yot P. Etude de l'Adn kinétoplastique de Trypansoma cruzi à l'aide d'endonucléases de restriction. C R Acad Sci Hebd Seances Acad Sci D. 1975 Jun 16;280(23):2701–2704. [PubMed] [Google Scholar]
- Shlomai J., Zadok A. Reversible decatenation of kinetoplast DNA by a DNA topoisomerase from trypanosomatids. Nucleic Acids Res. 1983 Jun 25;11(12):4019–4034. doi: 10.1093/nar/11.12.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L. Isolation and characterization of kinetoplast DNA networks and minicircles from Crithidia fasciculata. J Protozool. 1974 Nov;21(5):774–781. doi: 10.1111/j.1550-7408.1974.tb03751.x. [DOI] [PubMed] [Google Scholar]
- Simpson L., Simpson A. G. Kinetoplast RNA of Leishmania tarentolae. Cell. 1978 May;14(1):169–178. doi: 10.1016/0092-8674(78)90311-2. [DOI] [PubMed] [Google Scholar]
- Simpson L., Simpson A. M., Kidane G., Livingston L., Spithill T. W. The kinetoplast DNA of the hemoflagellate protozoa. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1053–1063. doi: 10.4269/ajtmh.1980.29.1053. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., ap Rhys C., Berman M. L., Hampar B., Jackson D., Silhavy T. J., Weisemann J., Zweig M. Open reading frame expression vectors: a general method for antigen production in Escherichia coli using protein fusions to beta-galactosidase. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4432–4436. doi: 10.1073/pnas.80.14.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]