Abstract
Thymidine analogs (TAs) are synthetic nucleosides that incorporate into newly synthesized DNA. Halogenated pyrimidines (HPs), such as bromodeoxyuridine (BrdU), are a class of TAs that can be detected with antibodies and are commonly used for birthdating individual cells and for assessing the proliferative index of cell populations. It is well established that HPs can act as radiosensitizers when incorporated into DNA chains, but they are generally believed not to impair normal cell function in the absence of secondary stressors. However, we and others have shown that HP incorporation leads to a sustained suppression of cell cycle progression in mammalian cells, resulting in cellular senescence in somatic cells. In addition, we have shown that HP incorporation results in delayed tumor progression in a syngeneic rat model of glioma. Here we examine ethynyldeoxyuridine (EdU), a newly developed and alkylated TA, for its anti-cancer activity, both in vitro and in vivo. We show that EdU, like HPs, leads to a severe reduction in the proliferation rate of normal and transformed cells in vitro. Unlike HPs, however, EdU incorporation also causes DNA damage resulting in the death of a substantial subset of treated cells. When administered over an extended time as a monotherapy to mice bearing subcutaneous xenografts of human glioblastoma multiforme tumors, EdU significantly reduces tumor volume and increases survival without apparent significant toxicity. These results, combined with the fact that EdU readily crosses the blood–brain barrier, support the continued investigation of EdU as a potential therapy for malignant brain tumors.
Keywords: Glioblastoma multiforme (GBM), Proliferation, Ethynyldeoxyuridine (EdU), Thymidine analog
Introduction
Thymidine analogs (TAs) are synthetic nucleosides that are used ubiquitously to detect DNA synthetic events including cell division, DNA repair and cell cycle re-entry [1–3]. The most frequently used TAs are the halogenated pyrimidines (HPs, bromo-, chloro-, and iodo-deoxyuridine). Introduced as potential mutagens in the 1950s [4, 5], they have since been studied as radiosensitizers in a number of clinical trials [6, 7]. Although the final results failed to demonstrate a significant overall improvement in survival [7–12], the HP BrdU does retain orphan drug status as a radiosensitizer for primary brain tumors.
Mounting evidence suggests significant effects of HP administration in the absence of secondary stressor. We and others have recently reported that in vitro administration of HPs impairs cell cycle progression and causes a progressive reduction in the proliferative capacity of a wide panel of somatic and transformed mammalian cell lines [13–16]. Additionally, we have shown such effects in the syngeneic RG2 rat glioma model, where metronomic (i.e. a steady schedule of lower dose chemotherapy) BrdU monotherapy resulted in delayed tumor progression [15].
In order to expand upon these findings we have examined the anti-cancer activity of a relatively new TA, the alkylated, non-halogenated, pyrimidine 5-ethynyl-2′-deoxyuridine (EdU). Walker and colleagues [17] described initial EdU synthesis, and interest in this TA has recently accelerated due to the commercial introduction of “click” chemistry that utilizes the Azide-Alkyne Huisgen Cycloaddition reaction [18]. This reaction allows for the rapid, one-step detection of EdU in double-strand DNA, and is a major improvement over the BrdU/antibody system that requires harsh DNA denaturation that often precludes other DNA analyses, and can distort or remove antigenic sites from cell membranes. Because of its relative novelty, the literature on EdU is sparse; however, EdU uptake has recently been shown to elicit a variable decline in cell viability, as characterized by G2 cell cycle arrest and induction of cell death in a number of breast cancer cell lines [19, 20].
In the present study we investigate the in vitro effects of EdU incorporation on several cancer lines, and assess EdU as a potential cancer therapeutic using a human xenograft GBM model. Despite aggressive FDA-approved therapy, the median overall survival from GBM remains 14–16 months [21–24]. Therefore, the identification of new therapeutic options is a high priority. We hypothesized that EdU administration—if given during active cycling of GBM cells—would significantly alter GBM tumor progression dynamics. Our results show that all EdU-incorporating cells undergo a DNA damage response that results in the death of a substantial subset of treated cells. Additionally, cells that survive EdU exposure demonstrate a profound and long-term reduction in their rate of proliferation. Finally, we show that long-term in vivo EdU administration in mice bearing subcutaneous xenografts of human GBM effectively accesses the entire tumor bulk, results in both reduced overall tumor load and increased animal survival, and causes minimal overt toxicity (as assessed by body conditioning scoring, weight loss and gross neurological status). Collectively, the dual anti-tumor effects of DNA damage-induced cell death and proliferation suppression, the absence of apparent organismal toxicity during prolonged administration, combined with evidence of rapid crossing of the blood–brain barrier, support continued study on the optimization of EdU as a chemotherapeutic option for GBM.
Materials and methods
Cells and media
Primary human glioblastoma cell lines (hGBM) were derived from surgical biopsies in patients diagnosed with primary glioblastoma [25]. Following surgical debulking, tissue samples were processed in a manner similar to that used to isolate neural stem cells from the adult nervous system [25–29]. Specifically, tissue samples were finely minced and placed in trypsin/EDTA (0.05%) for 7 min at 37°C. An equal volume of Trypsin inhibitor was added, solution mixed well, and centrifuged at 700 rpm. The pellet was triturated and passed through a 40 μm mesh and centrifuged again at 700 rpm. hGBM cells were maintained as neurospheres [25–29] in human Neurocult® media (#05751, Stem Cell Technologies, Vancouver, British Columbia, Canada). After 2–3 passages cells typically exhibit an arithmetic increase in the total number of cells generated, at which point they are considered a primary cell line and designated a line number. Cells were expanded and early passage aliquots cryopreserved for future use. Murine embryonic fibroblasts were a kind gift from Dr. Kyle Roux, and were maintained as adherent monolayers in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS). H9 human lymphoma and MG63 human osteosarcoma cell lines were obtained from American Type Culture Collection (Manassas, Virginia). H9 cells were maintained as suspension cultures in Roswell Park Memorial Institute medium (RPMI-1640) plus 10% FBS. MG63 cells were maintained as adherent monolayers in DMEM plus 10% FBS. Brain-seeking subclones of MDA-MB-231 breast cancer cells were a gift from Dr. Toshiyuki Yoneda and were maintained as adherent cells in DMEM containing 10% FBS [30].
Chemicals and reagents
Bromodeoxyuridine (BrdU, #B5002) and thymidine (#T9250) were purchased from Sigma-Aldrich (St. Louis, Missouri). Temozomide (TMZ, #2706) was purchased from Tocris (Ellisville, Missouri). Ethynyldeoxyuridine (EdU, #E10187) and TO-PRO-3 (T3605) were purchased from Invitrogen (Carlsbad, California). Matrigel™ basement membrane (#356234) was purchased from Becton–Dickinson (Franklin Lakes, New Jersey) and was prepared and handled according to manufacturer’s instructions.
Thymidine analog and TMZ administration
For in vitro experiments, cells receiving TAs were exposed to a single, 24 h pulse of 1, 10 or 50 μM EdU or BrdU. Cells receiving TMZ were exposed to a single, 24 h pulse of 25, 50 or 100 μM TMZ. Vehicle controls received a single, 24 h pulse of an equal volume of dimethyl sulfoxide (DMSO). At the end of the 24 h exposure the media were refreshed, and the cells received no further exposure to thymidine, TA or TMZ.
For in vivo “pre-treatment” experiments, cultured hGBM cells were exposed to 10 μM EdU for 48 h prior to subcutaneous implantation into mouse hosts. The pre-treatment animals did not receive any additional EdU. For in vivo “post-treatment” experiments, host animals were implanted with naïve hGBM cells (that had not been previously exposed to EdU), and were administered EdU via intra-peritoneal (i.p.) or subcutaneous (s.q.) injections once palpable tumors were established. Animals in the post-treatment experiments were randomly assigned to one of several treatment groups: (1) 50 mg thymidine/kg/day (control group); (2) 10 mg EdU/kg/day; (3) 25 mg EdU/kg/day; (4) 50 mg EdU/kg/day EdU; (5) 200 mg EdU/kg/day; or (6) 25 mg EdU/kg/every other day. These doses are based on our previous in vivo TA treatment regimens [15, 16].
Animal and tumor implantation/measurement
Adult female NOD/NCrCrl-Prkdcscid mice (NOD/SCID, 8–10 weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, Maine) and housed at the University of Florida’s Department of Animal Care Services. All animal procedures were conducted in compliance with the regulations of the University of Florida Institutional Animal Care and Use Committee. Mice were lightly anesthetized with halothane and injected subcutaneously over the right flank with 1 × 106 freshly dissociated hGBM cells in a total volume of 300 μl (200 μl Neurocult® growth media mixed with 100 μl Matrigel™). Once tumors were established as a palpable mass, post-treatment animals began receiving EdU or thymidine (as a control) according to the regimen described above. Tumors were measured under light halothane anesthesia using microcalipers to determine width and length. The average of these measures was obtained, and one half of this single value was used as the radius to calculate sphere volume [V = (4/3)πr3]. Clinical endpoint was defined as a measurement of ≥15 mm in either length or width. Toxicity was monitored by assessing changes in body condition scores (less than 2/5, 3 being “normal”), total body weight (weight loss of greater than 15%), and by monitoring for neurological signs (e.g. excessive unilateral turning) and signs of stress (e.g. overgrooming, pathological posturing).
Ex vivo tumor cell analysis
Upon reaching clinical endpoint, some animals were euthanized via cervical dislocation under deep halothane anesthesia. Animals were then doused in 70% ethanol to sterilize the fur and skin, and tumors were excised from their subcutaneous locus. In order to obtain a single-cell slurry, the tumors were minced with a sterile scalpel, incubated for 5 min. at 37°Cin 0.25% trypsin with EDTA (#B81310, Atlanta Biologicals, Lawrenceville, Georgia,), triturated with fire-polished glass Pasteur pipettes, and filtered through sterile gauze. The tumor cells were then either plated as a single cell suspension (50,000 cells/ml) for ex vivo analysis of growth kinetics, or fixed with 90% cold methanol for 15 min for subsequent EdU detection and Annexin V labeling.
Tissue analysis
At clinical endpoint some animals were deeply anesthetized with halothane and perfused through the left ventricle with 4% paraformaldehyde in PBS. The brains and tumor were excised and post-fixed overnight in perfusate. The tissues were then cryprotected for 24 h in PBS containing 30% sucrose. The brain and tumor were then serially sectioned at 40 μM intervals using a freezing microtome. EdU was visualized in a 1 in 6 series of sections from each tissue using the Click-iT™ EdU Alexa Fluor 488 kit (#C35002), Invitrogen, Carlsbad, California) using the manufacturer’s protocol. Sections were counterstained with TO-PRO-3 per manufacturer’s protocol.
In vitro growth curves
MEF, MG63 and H9 cell lines were plated at 50,000 cells and treated with one pulse of 1 or 10 μM BrdU (Fig. 1), EdU or DMSO (vehicle control). Cells were passaged every 7 days and counted for total cell yield using a Coulter Z2 Particle Counter (Beckman Coulter, Inc., Brea, California). For long-term growth curves, H9 cells were replated at 50,000 cells at each passage and re-counted every 7 days for 16 passages. For hGBM, cells were plated at 50,000 cells/ml and propagated as neurospheres, which were passaged every 5–7 days as described [26, 31]. Where indicated, neurosphere forming cells were replated at 50,000 cells/ml for the next time point.
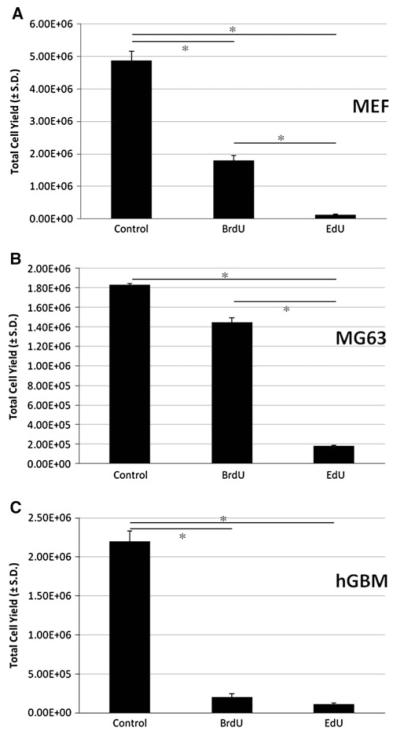
Fig. 1.
EdU exceeds BrdU in suppression of short-term population expansion. Murine embryonic fibroblasts (a), MG63 osteosarcoma cells (b), or human glioblastoma (hGBM) cells (c) were treated with a single 24 h pulse of DMSO (vehicle control), 10 μM BrdU or 10 μM EdU. At 7 days post-pulse, cells treated with 10 μM EdU exhibit a more pronounced decline in cell expansion than that seen with 10 μM BrdU. Data are expressed as total cell yield at day 7 after exposure to thymidine analog ± the standard deviation at day 7, and were subjected to ANOVA (*P < 0.0001). All treated cells are significantly different from controls (P < 0.05). EdU treated cells are significantly different from BrdU treated cells in MEFs (P < 0.05) and in MG63s (P < 0.05)
Flow cytometric analysis of EdU uptake and annexin V-based cell viability
To prepare cells for flow cytometry, cells were fixed in 70% ethanol and stored at 4°C. Annexin V Pacific Blue conjugate kit (#A35122, Invitrogen, Carlsbad, California) was used to assess live, dead and apoptotic cells according to the manufacturer’s protocol. To assess EdU incorporation, ethanol-fixed cells were labeled with the Click-iT™ EdU Alexa Fluor 488 kit (#C35002, Invitrogen, Carlsbad, California) according to the manufacturer’s protocol.
COMET assay
The CometAssay® Kit (#4250-050-K, Trevigen, Inc., Gaithersburg, Maryland) was used to analyze DNA damage under neutral conditions. Fifty microliters of cell suspension (100,000 cells/ml) was spotted onto Comet-Slides™ and allowed to dry for 10 min at 4°C. Cells were lysed for 30 min at 4°C, pre-equilibrated for 30 min at 4°C and electrophoresed at 1 V/cm for 10 min at room temperature. Slides were immersed for 30 min in DNA precipitation solution, followed by 30 min in 70% ethanol, each at room temperature. Finally, the slides were incubated with SYBR® Green and screened for COMET positivity. Five random fields per spot were selected and all cells in each field were counted and rated for the presence of DNA tailing.
Statistical analysis
Results are expressed as the mean ± standard deviation or mean ± standard error, where indicated. Statistical analysis was performed using GraphPad Prism software and consisted of One-way ANOVA with Tukey–Kramer post-hoc analysis for single factor multiple group comparison, Two-way ANOVA with Bonferroni post-hoc analysis for double factor multiple group comparison, or Student’s t-test for two group comparison. The in vivo studies were analyzed using Kaplan–Meier Curves for survival. For tumor growth, two-way ANOVA was used to compare 2 groups, and Kruskal–Wallis with Dunn’s multiple comparison was used to compare multiple groups when multiple doses were used. P-values ≤0.05 are considered statistically significant.
Results
EdU incorporation induces both cytotoxicity and a sustained suppression of expansion
We have previously shown that the HP, BrdU, elicits a sustained reduction in the proliferation rate of all somatic and cancer cell lines tested [15, 16]. In order to discern if EdU similarly perturbs proliferation, we compared the effects of EdU and BrdU on population expansion of a number of cell types. Seven days following administration of a single 24 h, 10 μM pulse of either EdU or BrdU, cells were analyzed for total cell number. As depicted in representative examples, both BrdU and EdU substantially reduce the population expansion of MEFs (Fig. 1a, P < 0.0001), MG63 cells (Fig. 1b, P < 0.005), and hGBM cells (Fig. 1c, P < 0.0001) compared to cultures of sister cells exposed to DMSO vehicle control. While cells treated with BrdU exhibit a large decline in total cell yield after 7 days of culture, those treated with EdU exhibit a near-truncation of proliferation, with EdU exerting a significantly greater decline than BrdU in MEFs (Fig. 1a, P < 0.05) and in MG63s (Fig. 1b, P < 0.05). To determine the longevity of this treatment effect, we then tested a human lymphoma cell line (H9) for long-term growth suppression following a single, short exposure of low-dose EdU (24 h pulse of 1 or 10 μM). A dose-responsive decline extending to ~60 population doublings is observed (Fig. 2, P < 0.005). These results suggest that even a short pulse of EdU exerts a significant anti-proliferative effect in multiple cell lines that is profound, progressive in nature, and sustained over long-term culture.
Fig. 2.
Single-pulse EdU leads to a sustained impairment of population expansion in H9 lymphoma cells. H9 cells (human cutaneous T-cell lymphoma) were maintained as suspension cultures and were treated once with a single 24 h pulse of 1 (red line) or 10 (green line) μM EdU, or DMSO vehicle control (blue line). Weekly growth curves were obtained for 16 weeks. Cells treated with either 1 or 10 μM EdU exhibit a sustained and dose-responsive inhibition of population expansion. Data are expressed as total cumulative cell number ± standard deviation. At all time points beginning with week 2, population expansion of control cultures is significantly higher than the two treated groups (N = 3 independent cultures for each group; P < 0.005). Note: standard deviation bars are not visualized in this logarithmic scale graph
To more closely examine how EdU suppresses population expansion, we assayed treated cells for evidence of apoptosis and cytotoxicity. Annexin V/DAPI labeling of MG63 human osteosarcoma cells reveals that a 24 h pulse of 1 μM EdU results in a steady increase in cell death in the first 3 days following treatment, with a concomitant decrease in live cell counts (Fig. 3a) as compared to control cells. Cells exhibiting an apoptotic profile remained constant at these time-points. These results are coordinate with an initial cytotoxicity and sharp decline in cell numbers that we observed in multiple treated cell lines (data not shown).
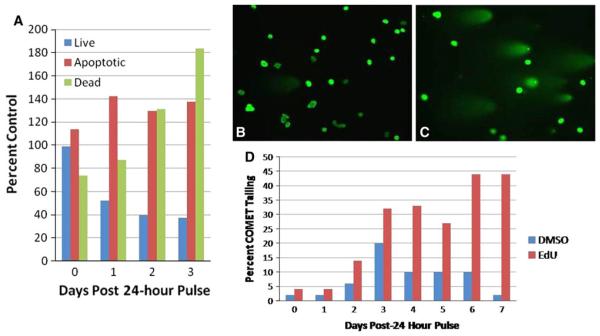
Fig. 3.
Single-pulse EdU induces delayed cell death and DNA strand breaks. MG63 (human osteosarcoma) cells were treated with a single 24 h pulse of 1 μM EdU or DMSO (vehicle control). a At 0, 1, 2, and 3 days post-pulse, MG63 cells were fixed and immunolabeled with antibody against Annexin V in order to distinguish live, dead, and apoptotic cells. EdU administration demonstrates a progressive increase in dead (green series) and apoptotic (red series) cells at the expense of live cells (blue series) compared to controls. Data are expressed as percent of DMSO-treated cells. b–d DNA strand breaks were detected with the COMET assay at 0–7 days post-EdU exposure. MG63 cells exhibit a progressive increase in cells with DNA strand breakage (c and red series in d) as compared to DMSO cells (b and blue series in d). Images are cells labeled with SYBR® Green. Data are expressed as percent of total cells exhibiting COMET-tailing. Scale bar, 50 μm
Finally, to determine if the observed increased cytotoxicity results from EdU-induced DNA damage we conducted COMET assays to identify generalized single- and double-strand DNA breaks. In the 7 days following a 24 h pulse of 1 μM EdU, MG63 cells exhibit a progressive increase in the percentage of cells with COMET tailing (Fig. 3c, d red series), indicating an increase in the number of cells experiencing DNA damage as compared to cells treated with vehicle control (Figure 3b, D blue series).
EdU treatment results in comparable suppression of in vitro cell expansion and greater cell death and DNA damage in a primary human GBM cell line
In order to assess EdU in the context of current therapeutics for GBM [32], we compared the anti-cancer activity of EdU to TMZ, the FDA-approved chemotherapeutic alkylator for GBM [23, 24]. Human GBM cells (hGBM) derived from surgical resection were propagated as neurospheres. In vitro growth suppression was assessed at 5 days following a single 24 h pulse of either EdU (1, 10 or 50 μM) or TMZ (25, 50 100 μM). Both EdU and TMZ treatment results in a substantial decline in population expansion of hGBM (Fig. 4a, b), with EdU eliciting a dose responsive decline. Seven days post-treatment, cells were analyzed for DNA strand breakage and Annexin V/DAPI profiling. Compared to vehicle controls, both TMZ- and EdU-treated cells were characterized by a dose-responsive increase in cell death, with a concomitant decrease in live cells (Fig. 4c, d). These effects were more robust in EdU-treated cells. Again, at this timepoint, no increase in apoptosis was observed. In addition, a substantial increase in COMET positivity was observed following both treatments (Fig. 4e, f), albeit at a higher percentage following EdU as compared to TMZ-treated cells. Similar results were observed in a sub-clone of the MDA-MB-231 breast cancer cell line [30] previously characterized to metastasize exclusively to the brain, and therefore constituting a secondary brain cancer cell model (Supplementary Fig. 1). These results show a dose-responsive effect of EdU treatment, thus suggesting its specificity. We also show the induction of cytotoxicity coupled with DNA strand breakage in highly aggressive hGBM cells. Furthermore, these experiments indicate that EdU is similar in efficacy to the current front-line GBM cancer drug, TMZ, at the doses tested in these in vitro analyses.
Fig. 4.
Single-pulse EdU is similar to TMZ in suppressing expansion and inducing DNA damage/cytotoxicity. Primary adult human GBM cells were propagated as neurospheres and treated with a single 24 h pulse of EdU (1, 10 or 50 μM), TMZ (25, 50 or 100 μM) or DMSO as a vehicle control. Five days after exposure, TMZ-treated cells exhibit a substantial decline in population expansion (a, P < 0.05 vehicle vs all doses) that is coterminous with increases in both cytotoxicity (c) and DNA strand breaks (e). At day 5 post-pulse, EdU-treated cells exhibit a more dramatic, dose-responsive decline in cell proliferation (b, P < 0.05 vehicle vs all doses) that corresponds to a dose-responsive increase in cell death (d) and DNA strand breakage (f). Data are expressed as total cell number (a, b), percent control (c, d) or percent of total cells with COMET-tailing (e, f)
EdU reduces hGBM tumor volume and increases latency to mortality in xenografts
To test the ability of EdU to suppress tumor progression in vivo, we generated hGBM tumor xenografts in mice using either tumor cells that had been exposed to EdU in vitro prior to transplantation, or naïve tumor cells that were exposed to EdU only after establishing bona fide tumors in host mice. Animals (N = 4 per group) were inoculatedsubcutaneously over the right flank with 1 × 106 of either pretreated hGBM cells (50 μM for 48 h immediately prior to implantation) or naïve (non-treated prior to implantation) hGBM cells. Animals receiving EdU pre-treated cells did not receive any additional EdU administration. Following tumor establishment (palpable tumor), animals inoculated with nontreated hGBM cells then began an in vivo EdU treatment regimen (50 mg/kg/day, i.p.). By day 35 post-transplant, tumor volume in control animals was significantly larger than in either treatment group, and this effect was consistent through day 49 post-implantation (P < 0.0001, Supplementary Fig. 2A). There was no apparent difference in tumor growth between pre-treated and post-treated animals. Importantly, post-treatment of animals with EdU was as effective in reducing tumor volume as the direct pre-treatment of tumor cells prior to tumor inoculation. With regard to animal survival, no significant differences were observed in this cohort (Supplementary Fig. 2B), potentially due to small cohort size. However, both pre-treated and post-treated animals exhibited a trend toward an increased latency to mortality due to tumor volume as a cohort. No animals demonstrated overt systemic toxicity, as assessed by monitoring of body conditioning scores, body weight and neurological signs (Table 1).
Table 1.
Toxicity measures during EdU administration
| BCS (1–5)a |
Body weight(%Δ)a |
Neurological signs | |
|---|---|---|---|
| Control | 3 | +16.5 | None noted |
| Pre-treatment | 3 | NA | None noted |
| 10 mg/kg | 3 | NA | None noted |
| 25 mg/kg QOD | 3 | NA | None noted |
| 25 mg/kg QOD | 3 | NA | None noted |
| 50 mg/kg QOD | 3 | NA | Overgrooming (N = l) |
| Unilateral turning (N = 1) |
|||
| Abnormal posture (N = 1) | |||
| 200 mg/kg | 2+ | −3.0 | Unilateral turning(N = 2) |
| QOD | Abnormal posture(N = 2) |
Results indicate mean of available data over all experiments at the indicated doses
NA not available
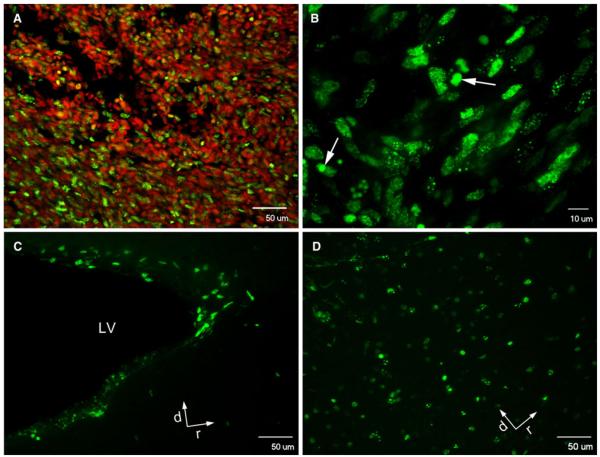
In order to confirm EdU incorporation into the tumor mass using this model, a subset of animals was analyzed when treated with daily EdU injections (50 mg/kg/day for 7 days). Histochemistry for EdU revealed positive labeling throughout (Fig. 5a, b), demonstrating EdU’s ability to perfuse throughout the tumor bulk. Label is also present in the periventricular subependymal zone (Fig. 5c) and olfactory bulb (Fig. 5d), confirming that EdU crosses the blood brain barrier, and that neural stem/progenitors in the SVZ system are labeled but persist in the neurogenic niche. When possible, tumor cells were obtained post-sacrifice, and expanded ex vivo to assess for post-implantation proliferation rates. Compared to cells from untreated animals, cells from both pre-treated cells and post-treated animals exhibited reduced ex vivo proliferative capacity (control cells vs. pre-treated cells, P < 0.001, Supplemental Fig. 3).
Fig. 5.
EdU is incorporated into tumor cells and cells within the neurogenic niche of the adult brain. Subcutaneous xenografted tumors and host brains of animals receiving EdU monotherapy were sectioned and chemically labeled with Alexa Fluor 488 (green) for EdU detection, and TO-PRO-3 for nuclear counterstain. Extensive EdU incorporation is seen in the tumor mass (a, b), as well as in the periventricular subependymal zone (c) and the olfactory bulb (d). LV lateral ventricle, d dorsal, r rostral
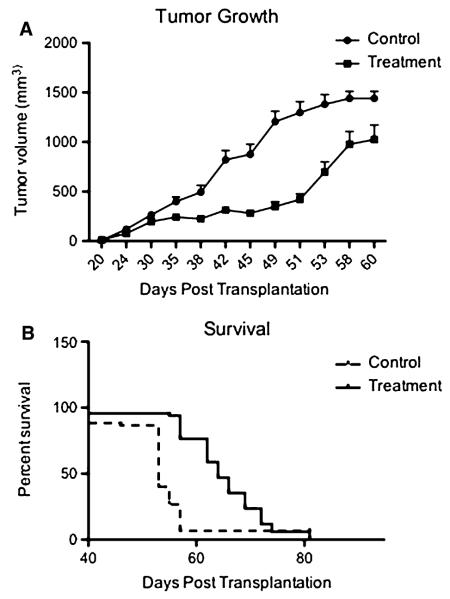
As i.p. delivery of 50 mg/kg/day following tumor seeding proved a dosing regimen that affected both latency and growth of the subcutaneous model in a manner comparable to implanting pre-treated cells, we next assessed this dose for treatment following tumor establishment in a larger cohort of NOD/SCID animals. Animals (N = 15 per group) were implanted with 1 × 106 of hGBM cells and tumor growth was monitored. In animals treated with EdU, tumor volume was significantly decreased over 60 days post-implantation (P < 0.0001, Fig. 6a). With regard to survival, animals treated with EdU had a significant survival benefit, with a median survival of 53 days in control animals and 64 days in treated animals (P < 0.001, Fig. 6b). These results confirmed the observed trends from the previous experiment, suggesting that the chosen EdU treatment regimen results in a reduced tumor growth and an enhanced mean survival time. No animals demonstrated overt systemic toxicity (Table 1).
Fig. 6.
EdU slows tumor progression and increases survival in human GBM xenografts. Host mice (N = 15 for each group) receiving a subcutaneous injection of naive human GBM cells were treated with either thymidine (control) or EdU (50 mg/kg/day via i.p. injection) after palpable tumors were established. a Treated animals demonstrate significantly slower progression of tumor volume over 60 days (P < 0.0001). b A Kaplan–Meier survival curve shows that treated animals also demonstrate a statistically significant delay in time until mortality (control median survival 53 days compared to EdU median survival 64 days; P = 0.001)
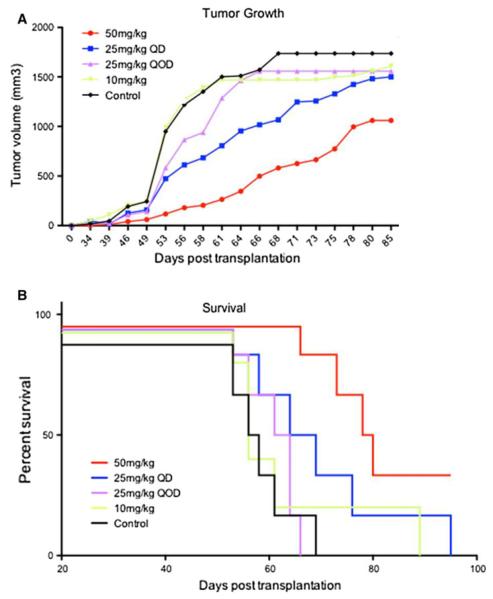
Subsequently, multiple EdU doses were tested to determine if doses lower than 50 mg/kg/day could also prove efficacious in long-term therapy. Host mice (N = 6 per group) were injected with either 50 mg/kg/day thymidine (controls), 10, 25 or 50 mg/kg/day EdU or 25 mg/kg/every other day EdU following tumor inoculation (1 × 106 cells, s.q.) Due to the anticipated length of this dose response study, we chose a subcutaneous (s.q.) delivery method as this method is less stressful in the handling of the animals. Compared to control animals, a significant reduction in tumor volume was observed in the overall cohort (P = 0.003, Fig. 7a), through 85 days post-implantation. Significance in tumor growth between groups was found between 50 mg/kg/day group (median tumor volume 304.5 mm3) vs. both control (median tumor volume 1506 mm3) and 10 mg/kg/day (median tumor volume 1469 mm3) groups (P < 0.05). Survival curves revealed that animals treated with 10 or 25 mg/kg/day and 25 mg/kg/every other day trended toward increased survival, while only those treated with 50 mg/kg/day exhibited a significant increase compared to control (P = 0.002, Fig. 7b). These data confirmed that 50 mg/kg/day (s.q. or i.p. delivery), in long-term paradigms, is an effective therapeutic regimen for EdU administration. Further, these doses were effective for as long as 105 days with minimal signs of overt toxicity in survivors (Table 1).
Fig. 7.
Comparative analysis of tumor progression and survival as a result of different EdU dosing regimens in subcutaneous human GBM xenografts. a A variety of dosing regimens (10, 25, and 50 mg/kg/day) were tested for the ability to suppress progression of human GBM in subcutaneous xenografts (N = 6 for each group). Graphical representation shows a dose-dependent response to EdU treatment with the most benefit from 50 mg/kg/day of EdU. One-way ANOVA followed by Dunn’s multiple comparison test shows a statistically significant difference between the 50 mg/kg group compared to the control and 10 mg/kg groups (P < 0.05). b Kaplan–Meier survival curve demonstrates dose dependence with different EdU doses. The mice treated with 50 mg/kg/d showed the most improved survival (median 79 days) compared to control (mean 57 days) (P = 0.002)
Finally, high dose EdU was tested to determine if this, too, was sustainable as a long term treatment therapy. Adult female (N = 6 per group) NOD/SCID mice were given daily s.q. injections with either EdU or thymidine (200 mg/kg/day) following tumor implantation. Control animals experienced rapid tumor growth until endpoint (as long as 60 days post-implantation). In comparison, animals treated with high dose EdU exhibited tumor growth until approximately 49 days post-implantation, with a subsequent plateau that was sustained through 100 days post-transplantation (Fig. 8a). Further, compared to control animals, high dose-treated animals exhibited a significant increase in survival through 100 days post-transplantation (Fig. 8b). Minimal signs of overt systemic toxicity were observed (Table 1). These results suggest that high dose EdU is a treatment option that is sustainable for long term regimens, and that the maximum biological dose perhaps has not been attained at the presented doses.
Fig. 8.
Analysis of tumor progression and survival comparing high dose EdU to thymidine controls in subcutaneous human GBM xenografts. a High dose EdU (200 mg/kg/day, s.q.) was tested for the ability to suppress progression of human GBM in subcutaneous xenografts (N = 6 for each group). Graphical representation shows a sustained suppression of tumor growth in response to EdU treatment. Treatment animals sustained high dose treatment for ≥100 post-transplant days, as compared to the control group, in its entirety, which reached study endpoint by day 60. b Kaplan–Meier survival curve demonstrates that EdU treatment (200 mg/kg/day), after establishment of tumors, results in a statistically significant survival benefit (P < 0.01). The hazard ratio (HR) for death in the control group was 14.04 compared to the treatment group (95% CI 2.7 to 74.2)
Discussion
This work expands on the characterization of TA monotherapy in the treatment of cancer. Here we report on a relatively new TA, EdU. TAs in general, and BrdU in particular, have an extensive historical record as potential mutagens, radiosensitizers and antiviral agents; additionally, they readily cross the blood–brain barrier, and there is evidence that they can augment the effects of primary cancer therapies by weakening DNA and making it more susceptible to DNA damage [5, 6, 33–36]. Previous clinical trials have used HPs to radiosensitize a variety of cancers (e.g. colorectal, liver, pancreatic, GBM). Although such strategies have not proven beneficial over standard therapy [7–12], preclinical and early clinical efficacy resulted in orphan drug status for BrdU in the 1990s [37]. In assessing EdU as a potential anti-cancer agent, it is important to ask why earlier clinical trials of HP radiosensitization were unable to demonstrate a survival benefit. While the present experiments do not directly address this question we can speculate that, because these earlier trials were formulated on the in vitro evidence of HP radiosensitization [6, 9, 34] they did not account for potential in vivo effects stemming from dosing schedules or drug interaction that would optimize or attenuate HP incorporation. Without antecedent experimentation in animal models, it was not possible to know the optimal dosing paradigms to maximize therapeutic outcome. Furthermore, in these trials HPs were added to existing therapies that included a variety of chemotherapeutics as well as ionizing irradiation. These therapies conceivably eliminated a large portion of actively-cycling cells in their own right, potentially reducing HP uptake and incorporation by dividing tumor cells. Thus, it is unclear the isolated role played by HPs in these relatively short term dosage models. Our current and past work not only investigates monotherapy of thymidine analogs, but also employs chronic administration to add crucial information about long-term administration protocols.
Our present studies focus on EdU. EdU contains an alkyne group (C≡CH) on the 5-position of the pyrimidine, which is a less bulky moiety than a halogen group. This renders EdU suitable for click chemistry [37–40], which can quickly and easily label EdU-containing DNA even in non-denatured, double-strand form [41]. For this reason EdU is becoming increasingly popular in proliferation assays suitable for flow cytometry and immunocytochemistry. Because of its relative novelty, few studies examining the consequences of EdU uptake on cell function have been reported. However, there is evidence that EdU does induce cell cycle arrest and cell death in breast cancer cell lines [19, 20]. Here we present data on normal fibroblasts, lymphoma, osteosarcoma, and a breast-to-brain metastatic cancer line in order to substantiate our techniques and experimental principles, as well as to add to comparative data amongst cancer cell lines presented in this and previous work. in vitro results in these cell lines led us to postulate that EdU could be effective in the treatment of aggressive cancers such as GBM. We therefore follow up with extensive in vitro and in vivo data employing an hGBM cell line. Our data demonstrate that a single in vitro exposure to EdU induces a dramatic reduction in the rate of population expansion by all treated cell types (Figs. 1, 2, 3, 4). In addition to a truncated expansion rate, EdU also induces extensive DNA damage that is associated with the death of a substantial subset of incorporating cells. This dual effect—initial DNA damage followed by sustained suppression of expansion—is in contrast to our previous findings using HPs, and suggests that direct DNA damage might potentiate EdU efficacy as compared to the HPs. Our work is the first to report that EdU monotherapy elicits an initial round of DNA strand breakage and cytotoxicity in cultured lymphoma, osteosarcoma and hGBM cells, and subsequently leads to delayed population expansion by surviving cells. This dual effect of EdU suggests the potential for a novel therapeutic approach that combines shorter-term cytotoxicity with long-term proliferation suppression. By accessing rapidly dividing cells, a subset of the cells that incorporate EdU will be killed outright. The remaining cells that have incorporated EdU would theoretically possess a less aggressive cell cycle profile that slows tumor progression.
To date, the molecular target and therefore the mechanism behind antiproliferation or cytotoxicity associated with TA administration is poorly understood, yet in vitro efficacy has been demonstrated in multiple cell lines from many different types of malignancies [13–16]. The promiscuous effectiveness of EdU suggests therapeutic potential in cancers -such as GBM- that are characterized by a variable genetic makeup, both among different GBMs and between primary and recurrent examples of single GBM cases [42, 43]. In order to address these questions our future studies will continue to pursue the molecular signatures, for example protein expression and phosphorylation profiles, of a wider cast of cellular markers involved in cell signaling, tumor suppression, apoptosis, and DNA damage that may be targets of EdU incorporation. In addition, a variety of additional primary GBM will need to be analyzed before we can reasonably understand how widely we can extrapolate our results to brain cancer as a whole.
Our in vivo studies focused on a systematic initiation of treatment regimens for subcutaneous hGBM xenografts. Moderate dose experiments in pilot groups (Supplementary Fig. 2) initially showed significant efficacy—both for cells pre-treated before tumor implant (50 μM for 24 h) and for grafted naïve cells exposed to EdU in situ via i.p injection (50 mg EdU/kg/day). It is important to note that post-treatment of animals elicited a similar tumor growth profile as directly pre-treating the cells in culture prior to implant—a protocol that is thought to more strongly affect target cells than when they are treated in vivo—speaking to the efficacy of the chosen in vivo protocol. Dosing efficacy was confirmed in a larger cohort tested with 50 mg/kg/day post-implant (Fig. 6). In pursuing efficacy of in vivo post-treatment regimens further, we explored lower dosing schedules (Fig. 7), and found a trend toward a dose-responsive effect of low dose therapy (10–50 mg/kg/day as well as one non-daily regimen, 25 mg/kg/every other day). High dose therapy (200 mg/kg/day) proved even more efficacious, yet was still compatible with long-term administration (Fig. 8). Animals readily tolerated daily treatment for >100 days without exhibiting obvious signs of toxicity as measured by body conditioning scores, signs of CNS dysfunction, weight loss or other signs of distress. All drugs can have negative side-effects that must be taken into account when calculating the risk:benefit ratio of using them in clinical settings. To the extent that EdU proves to be effective at slowing tumor progression in vivo, it will be important to understand its effect on overall organismal health. Because of EdU’s progressive effect over many population doublings it seems likely that stem cells and long-term progenitor cells will be at risk for EdU-mediated death and/or proliferation suppression. It seems paradoxical, then, that our animals show essentially no signs of overt toxicity even after several months of daily EdU administration. While detailed and extensive toxicity analyses will need to be performed in the future, we hypothesize that, since the majority of stem cell pools are typically quiescent at any given time, the likelihood of EdU affecting all of them during a therapeutic regimen is slight. Even if a majority are suppressed, there is the possibility that the unaffected fraction will increase output—either through increased asymmetric divisions or through a series of symmetric divisions- to compensate for loss of activity throughout the entire pool. Therefore, while animals may show an initial decline of rapidly dividing, transit amplifying cells, we predict that this would be a temporary impairment that is overcome in time as the unaffected stem cells begin to repopulate the niche. Alternatively, it may be that the highly proliferative tumor mass in these animals acts as a “sponge” to sequester circulating EdU, thus sparing normal somatic stem cells from exposure to potentially harmful concentrations. The answer to these and other important aspects of EdU’s effect on normal cellular function will ultimately determine therapeutic potential.
Importantly, animals bearing hGBM cell-initiated tumors that were treated with short term EdU (50 mg/kg for 7 days via i.p injection) demonstrated substantial EdU uptake throughout the tumor mass (Fig. 5). Further, EdU readily crossed the blood–brain barrier and reached the neurogenic niches to label diving stem/progenitor cell populations, yet did not eradicate proliferation (Fig. 5). This suggests a potential for high treatment efficacy if dosing regimens are appropriately optimized and assuming that our results can be successfully translated to an intra-cranial tumor locus. Further, such measures build evidence for the ability of optimized treatment courses to preserve stem cell pools during chemotherapy. These results are compelling for CNS tumors, particularly with respect to highly aggressive GBM cells.
These findings indicate the specificity of EdU monotherapy, as well as a potential to adjust doses based on tolerance and/or tumor response in clinical trials. Importantly, our results show that at all doses, EdU monotherapy causes an increased latency to hGBM tumor-related mortality. Together, these results provide substantial evidence that EdU may contribute to current therapeutic strategies in GBM and potentially other aggressive tumors.
Supplementary Material
Acknowledgements
This work is supported by the following grants: NIH/NINDS NS056019 (EDL); STOP! Children’s Cancer, Inc. (EDL); Florida Center for Brain Tumor Research, Hope Heels Run Award (EDL); NICHD Training Grant 5K12HD055929 (HHR); NIH R21 CA141020 (BAR).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11060-011-0621-6) contains supplementary material, which is available to authorized users.
Contributor Information
Heather H. Ross, Department of Physical Therapy, University of Florida, Gainesville, FL, USA
Maryam Rahman, Department of Neurosurgery, University of Florida, Gainesville, FL, USA.
Lindsay H. Levkoff, Department of Anatomy and Cell Biology, College of Medicine, University of Florida, PO Box 100235, Gainesville, FL 32610, USA
Sebastien Millette, Department of Neurosurgery, University of Florida, Gainesville, FL, USA.
Teresa Martin-Carreras, Department of Physical Therapy, University of Florida, Gainesville, FL, USA.
Erin M. Dunbar, Department of Neurosurgery, University of Florida, Gainesville, FL, USA Preston A. Wells, Jr Center for Brain Tumor Therapy, Gainesville, FL, USA; McKnight Brain Institute, University of Florida, Gainesville, FL, USA; UF Shands Cancer Center, University of Florida, Gainesville, FL, USA.
Brent A. Reynolds, Department of Neurosurgery, University of Florida, Gainesville, FL, USA McKnight Brain Institute, University of Florida, Gainesville, FL, USA; UF Shands Cancer Center, University of Florida, Gainesville, FL, USA.
Eric D. Laywell, Department of Biomedical Sciences, Florida State University College of Medicine, 1115 West Call Street, Tallahassee, FL 32306, USA
References
- 1.Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science. 1982;218:474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- 2.Beisker W, Hittelman WN. Measurement of the kinetics of DNA repair synthesis after UV irradiation using immunochemical staining of incorporated 5-bromo-2′-deoxyuridine and flow cytometry. Exp Cell Res. 1998;174:156–167. doi: 10.1016/0014-4827(88)90151-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, McPhie DL, Hirschberg J, Neve RL. The amyloid precursor protein-binding protein APP-BP1 drives the cell cycle through the S-M checkpoint and causes apoptosis in neurons. J Biol Chem. 2000;275:8929–8935. doi: 10.1074/jbc.275.12.8929. [DOI] [PubMed] [Google Scholar]
- 4.Dubbs DR, Kitt S. Effect of halogenated pyrimidines and thymidine on growth of L-cells and a subline lacking thymidine kinase. Exp Cell Res. 1964;33:19–28. doi: 10.1016/s0014-4827(64)81006-5. [DOI] [PubMed] [Google Scholar]
- 5.Hakala MT. Mode of action of 5-bromodeoxyuridine on mammalian cells in culture. J Biol Chem. 1959;234:3072–3076. [PubMed] [Google Scholar]
- 6.Djordevic B, Szybalski W. Genetics of human call lines: III. Incorporation of 5-bromo- and 5-iododeoxyuridine into the deoxyribonucleic acid of human cells and its effect on radiation sensitivity. J Exp Med. 1960;112:509–531. doi: 10.1084/jem.112.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groves MD, Maor MH, Meyers C, Kyritsis AP, Jaeckle KA, Yung WK, Sawaya RE, Hess K, Bruner JM, Peterson P, et al. A phase II trial of high-dose bromodeoxyuridine with accelerated fractionation radiotherapy followed by procarbazine, lomustine and vincristine for glioblastoma multiforme. Clin Invest. 1999;45:127–135. doi: 10.1016/s0360-3016(99)00122-4. [DOI] [PubMed] [Google Scholar]
- 8.Kinsella TJ, Russo A, Mitchell JB, Rowland J, Jenkins J, Schwade J, Myers CE, Collins JM, Speyer J, Kornbluth P, et al. A phase I study of intermittent intravenous bromodeoxyuridine (BUdR) with conventional fractionated irradiation. Int J Radiat Oncol Biol Phys. 1984;10:69–76. doi: 10.1016/0360-3016(84)90414-0. [DOI] [PubMed] [Google Scholar]
- 9.Phillips TL, Levin WA, Ahn DK, Gutin PH, Wilson CB, Prados MD, Wara WM, Flam MS. Evaluation of bromodeoxyuridine in glioblastoma multiforme: a Northern California cancer center phase II study. Int J Radiat Oncol Biol Phys. 1991;21:709–714. doi: 10.1016/0360-3016(91)90690-6. [DOI] [PubMed] [Google Scholar]
- 10.Robertson JM, McGinn CJ, Walker S, Marx MV, Kessler ML, Ensminger WD, Lawrence TS. A phase I trial of hepatic arterial bromodeoxyuridine and conformal radiation therapy for patients with primary hepatobiliary cancers or colorectal liver metasteses. Clin Invest. 1997;39:1087–1092. doi: 10.1016/s0360-3016(97)00550-6. [DOI] [PubMed] [Google Scholar]
- 11.Robertson JM, Ensminger WD, Walker S, Lawrence TS. A phase I trial of intravenous bromodeoxyuridine and radiation therapy for pancreatic cancer. Clin Invest. 1997;37:331–335. doi: 10.1016/s0360-3016(96)00527-5. [DOI] [PubMed] [Google Scholar]
- 12.Prados MD, Seiferheld MS, Sandler HM, Buckner JC, Phillips T, Schultz C, Urtasun R, Davis R, Gutin P, Cascino TL, et al. Phase III randomized study of radiotherapy plus procarbazine, lomustine, and vincristine with or without BUdR for treatment of anaplastic astrocytoma: final report of RTOG 9404. Int J Radiat Oncol Biol Phys. 2004;58:1147–1152. doi: 10.1016/j.ijrobp.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Michishita E, Nakabayashi K, Suzuki T, Kaul SC, Ogino H, Fujii M, Mitsui Y, Ayusawa D. 5-Bromodeoxyuridine induces senescence-like phenomena in mammalian cells regardless of cell type or species. J Biochem. 1999;126:1052–1059. doi: 10.1093/oxfordjournals.jbchem.a022549. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T, Minigawa S, Michishita E, Ogino H, Fujii M, Mitsui Y, Ayusawa D. Induction of senescence-associated genes by 5-bromodeoxyuridine in HeLa cells. Exp Gerontol. 2001;36:465–474. doi: 10.1016/s0531-5565(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 15.Levkoff LH, Marshall GP, II, Calderia M, Reynolds BA, Cakiroglu M, Mariani CL, Streit WJ, Laywell ED. Bromodeoxyuridine inhibits cancer cell proliferation in vitro and in vivo. Neoplasia. 2008;10(8):804–816. doi: 10.1593/neo.08382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross HH, Levkoff LH, Marshall GP, II, Caldiera M, Reynolds BA, Cakiroglu M, Laywell ED. Bromodeoxyuridine induces senescence in neural stem and progenitor cells. Stem Cells. 2008;26(12):3218–3227. doi: 10.1634/stemcells.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker RT, Jones AS, DeClercq E, Descamps J, Allaudeen HS, Kozarich JW. The synthesis and properties of some 5-substituted uracil derivatives. Nucleic Acids Symp Ser. 1980;8:s95–102. [PubMed] [Google Scholar]
- 18.Huisgen R. Centenary lecture—1,3 dipolar cycloadditions. Proc Chem Soc. 1961:357–396. [Google Scholar]
- 19.Meneni S, Ott I, Sergeant CD, Sniady A, Gust R, Dembinski R. 5-alkynyl-2′deoxyuridines: chromatography-free synthesis and cytotoxicity evaluation against human breast cancer cells. Bioorg Med Chem. 2007;15(80):3082–3088. doi: 10.1016/j.bmc.2007.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diermeier-Daucher S, Clarke ST, Hill D, Vollmann-Zwerenz A, Bradford JA, Brockhoff G. Cell type specific applicability of 5-ethynyl-2′-deoxyuridine (EdU) for dynamic proliferation assessment in flow cytometry. Cytometry A. 2009;75(6):535–546. doi: 10.1002/cyto.a.20712. [DOI] [PubMed] [Google Scholar]
- 21.Aoki T, Hashimoto N, Matsutani M. Management of glioblastoma. Expert Opin Pharmacother. 2007;8(18):3133–3146. doi: 10.1517/14656566.8.18.3133. [DOI] [PubMed] [Google Scholar]
- 22.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G, Network GermanGlioma Long-term survival with glioblastoma multiforme. Brain. 2007;130(Pt 10):2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 23.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 24.CBTRUS . Statistical report: primary brain tumors in the United States, 2000–2004. Central Brain Tumor Registry of the United States; Hindsdale: 2008. [Google Scholar]
- 25.Azari H, Rahman M, Sharififar S, Reynolds BA. Isolation and expansion of the adult mouse neural stem cells using the neurospheres assay. J Vis Exp. 2010;45:e2393. doi: 10.3791/2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deleyrolle LP, Reynolds BA. Isolation, expansion, and differentiation of adult mammalian neural stem and progenitor cells using the neurospheres assay. Methods Mol Biol. 2009;549:91–101. doi: 10.1007/978-1-60327-931-4_7. [DOI] [PubMed] [Google Scholar]
- 27.Louis SA, Reynolds BA. Generation and differentiation of neurospheres from murine embryonic day 14 central nervous system tissue. Methods Mol Biol. 2005;290:265–280. doi: 10.1385/1-59259-838-2:265. [DOI] [PubMed] [Google Scholar]
- 28.Rietze RL, Reynolds BA. Nerual stem cell isolation and characterization. Methods Enzymol. 2006;419:3–23. doi: 10.1016/S0076-6879(06)19001-1. [DOI] [PubMed] [Google Scholar]
- 29.Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444(7120):687–688. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 30.Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Min Res. 2001;16:1486–1495. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
- 31.Marshall GP, II, Ross HH, Suslov O, Zheng T, Steindler DA, Laywell ED. Production of neurospheres from CNS tissue. Methods Mol Biol. 2008;438:135–150. doi: 10.1007/978-1-59745-133-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandes AA, Tosoni A, Franceschi E, Reni M, Gatta G, Vecht C. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139–152. doi: 10.1016/j.critrevonc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Hakala MT. Effect of 5-bromodeoxyuridine incorporation on survival of cultured mammalian cells. Biochim Biophys Acta. 1962;61:815–823. doi: 10.1016/0926-6550(62)90064-6. [DOI] [PubMed] [Google Scholar]
- 34.Hsu TC, Somers CE. Effect of 5-bromodeoxyuridine on mammalian chromosomes. Proc Natl Acad Sci USA. 1961;47:396–403. doi: 10.1073/pnas.47.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson RL, Szybalski W. Molecular radiobiology of human cell lines: comparative radiosensitizing properties of 5-halodeoxycytidines and 5-halodeoxypyrimidines. Radiat Res. 1963;20:252–262. [PubMed] [Google Scholar]
- 36.Smee DF, Humphreys DE, Hurst BL, Barnard DL. Antiviral activities and phosphorylation of 5-halo-2′-deoxyuridines and N-methanocarbathymidine in cells infected with vaccinia virus. Antivir Chem Chemother. 2008;19(1):15–24. doi: 10.1177/095632020801900103. [DOI] [PubMed] [Google Scholar]
- 37.Freese A, O’Rourke D, Judy K, O’Connor MJ. The application of 5-bromodeoxyuridine in the management of CNS tumors. J Neurooncol. 1994;20(1):81–95. doi: 10.1007/BF01057964. [DOI] [PubMed] [Google Scholar]
- 38.Breinbauer R, Köhn M. Azide-alkyne coupling: a powerful reaction for bioconjugate chemistry. Chembiochem. 2003;4(11):1147–1149. doi: 10.1002/cbic.200300705. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Huang J, Wulff WD, Rheingold AL. The first example of a meta-benzannulation from the reaction of Fischer carbine complexes with alkynes. J Am Chem Soc. 2003;125(30):8980–8981. doi: 10.1021/ja035428n. [DOI] [PubMed] [Google Scholar]
- 40.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(i)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41(14):2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA. 2008;105(7):2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang Y, Diehn M, Watson N, Bollen AW, Aldape KD, Nicholas MK, Lamborn KR, Berger MS, Botstein D, Brown PO, Israel MA. Gene expression profiling reveals molecularity and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci USA. 2005;102(16):5814–5819. doi: 10.1073/pnas.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez R, Rohde V, Schackert G. Different molecular patterns in glioblastoma multiforme subtypes upon recurrence. J Neurooncol. 2009;96(3):321–329. doi: 10.1007/s11060-009-9967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.