INTRODUCTION
Eugene Braunwald, a world leader in cardiology for more than a half century, considers the coronary care unit (CCU) “the single most important advance in the treatment of acute myocardial infarction (AMI).”1 The first description of the CCU concept published in North America appeared in Circulation in October 1961. Los Angeles cardiologist Morris Wilburne outlined a technology-inspired extension of the intensive care unit (ICU) model that had been developed during the previous decade. There was one crucial difference, however. The ICU was a place to care for acutely ill patients with a broad range of surgical and medical problems. On the other hand, the CCU was conceived as a program of care that targeted a specific group of patients—those at risk of sudden death in the context of an AMI. Vulnerable patients were admitted to a special space staffed by nurses trained to use new electronic technologies for the rapid diagnosis and treatment of life-threatening arrhythmias and to perform cardiopulmonary resuscitation (CPR).
The advent and diffusion of the CCU would transform the care of patients, the careers of cardiologists, and the boundaries of nursing practice in less than a decade. Continuous electrocardiographic (ECG) monitoring alerted the staff to a life-threatening arrhythmia. This innovation was coupled with three new closed-chest treatment technologies: defibrillators, pacemakers, and CPR. The successful treatment of ventricular fibrillation (VF), an arrhythmia that had been invariably fatal, provided compelling evidence that the CCU model saved lives. Cardiac arrest caused irreversible brain damage in less than four minutes so there was no time to wait for a doctor to rush to a patient and discharge a defibrillator. To address this problem, physicians trained specific nurses to deliver a life-saving shock without personal supervision. The first CCUs were opened in 1962. During the next decade, defibrillation was supplemented by new treatment strategies including prophylactic lidocaine to prevent VF and hemodynamic monitoring to help manage patients in shock or congestive heart failure.2,3
THE CARE OF PATIENTS WITH AMI JUST BEFORE THE ADVENT OF THE CCU
The novelty and significance of the CCU concept first published in 1961 is highlighted when it is juxtaposed with contemporary summaries of state-of-the-art care for patients with AMI. A review written the following year by cardiologist William Dock provides perspective. He listed four main complications of AMI: pain, shock, arrhythmia, and congestive heart failure. Dock's first line therapies included morphine for pain, barbiturates and antihistamines for anxiety, norepinephrine for shock, procaine amide for ventricular tachycardia, and digitalis for congestive heart failure or rapid ventricular rates with atrial fibrillation. The portion of this 1962 paper discussing hospitalization reveals the revolutionary nature of the CCU concept: “Many physicians experienced in the care of coronary disease prefer to be at home when they themselves experience the onset of angina or infarction. When adequate care and supervision can be provided, and the patient is intelligent and cooperative, there is much is favor of home care for the patient with normal pulse rate and blood pressure.” 4 Dock's attitude about hospitalization was not uncommon. Six years earlier, New York cardiologist Charles Friedberg wrote in his best-selling textbook, “Most patients with myocardial infarction can be treated satisfactorily at home.”5 In the 1950s, patients with AMI who were hospitalized were placed in wards or standard private or double rooms. Because their heart rhythms were not monitored unwitnessed cardiac arrests were common, resuscitation attempts were very rare, and successful resuscitations were reportable.6
NEW TECHNOLOGIES CATALYZE THE CONCEPTUALIZATION OF THE CCU
The brief interval between Friedberg's 1956 book and Dock's 1962 article was marked by major technological breakthroughs and important transitions in nursing practice and inpatient care.7 Scientific and social factors combined to set the stage for the CCU concept that would be implemented at hundreds of hospitals in less than a decade. Friedberg declared in 1969 that the development of CCUs for treating patients with AMI was “the outstanding therapeutic achievement of the past few years.”8 Four new technologies were critical for conceptualizing the care model: (1) oscilloscopes that displayed a continuous ECG and included a heart rate alarm, (2) transthoracic defibrillators, (3) transthoracic pacemakers, and (4) closed-chest CPR.
Boston cardiologist Paul Zoll was the lead author of two 1956 papers in the New England Journal of Medicine that described treating cardiac arrest with an external defibrillator or pacemaker.9, 10 He had already commercialized the devices. The journal issue that included his paper on the defibrillator contained a full-page ad by an electronics firm. It depicted “The External Defibrillator Developed by Paul M. Zoll, M.D” and referenced his article in the same issue. The company boasted that the device was “a PROVEN instrument for clinical treatment of ventricular fibrillation...through the unopened chest.”11 Meanwhile, electrical engineer William Kouwenhoven led a team of Johns Hopkins researchers in developing modern CPR. They combined closed-chest (rather than open-chest) cardiac massage with mouth-to-mouth respiration. Their 1960 article in JAMA proclaimed, “Anyone, anywhere, can now initiate cardiac resuscitative procedures.”12 This atraumatic approach replaced an emergency surgical technique reported thirteen years earlier that was confined almost exclusively to the operating room. If a patient developed cardiac arrest in that context, some surgeons cut open the chest and squeezed the heart by hand in an attempt to restore the circulation. Defibrillator electrodes were placed directly on the heart if the mechanism was VF. It was unrealistic to move this open-chest cardiac resuscitation technique outside the operating room, however.
THE FORGOTTEN FIRST AMERICAN PUBLICATION OF THE CCU CONCEPT
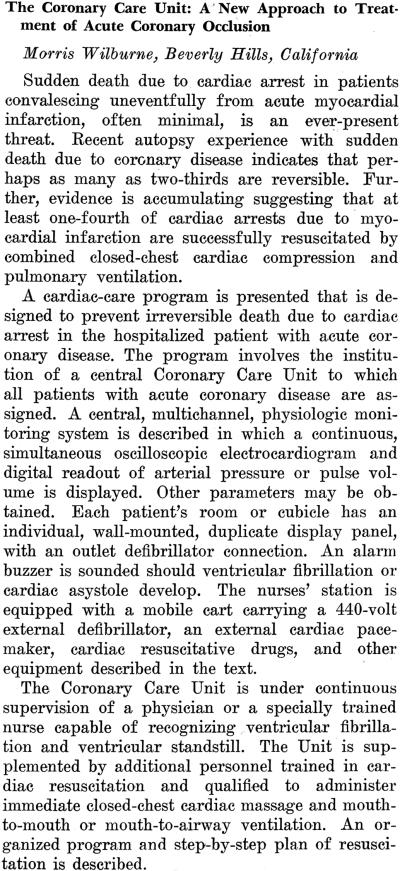
The first publication of the CCU concept in North America has been overlooked despite the fact that it appeared in the world's premier cardiology journal. Part two of the October 1961 issue of Circulation (of which 14,400 copies were printed) included Morris Wilburne's abstract “The Coronary Care Unit: A New Approach to Treatment of Acute Coronary Occlusion.”13 A Los Angeles cardiologist in private practice, Wilburne admitted patients to Cedars of Lebanon Hospital and to Mount Sinai Hospital and had a part-time faculty appointment at the University of Southern California School of Medicine. He hoped that his abstract would be accepted for presentation at the American Heart Association (AHA) meeting in Miami Beach in October, but like three-quarters of the 627 abstracts submitted it did not make the cut. Thanks to a short-lived AHA policy that required publication of all abstracts submitted, not just those presented, Wilburne's concise description of a new patient care model is well documented. The content of the abstract and the worldwide distribution of Circulation justify assigning priority to Wilburne for defining the model and denoting it a “Coronary Care Unit.” (Figure 1)
Figure 1.
The first published description of the CCU concept. Wilburne M. The Coronary Care Unit: A New Approach to Treatment of Acute Coronary Occlusion [Abstract]. Circulation (October) 1981; 24:1071
THE CCU CONCEPT IS DESCRIBED SIMULTANEOUSLY OVERSEAS
On October 14, 1961, almost the same day that Circulation published Wilburne's abstract, the London-based Lancet published Desmond Julian's long article “Treatment of Cardiac Arrest in Acute Myocardial Infarction and Ischæmia.” Julian had received his medical degree in London and trained at the National Heart Hospital and the Peter Bent Brigham Hospital in Boston. He was a senior registrar at the Edinburgh Royal Infirmary when he wrote the Lancet paper in which he called closed-chest massage an “outstanding advance” and recommended combining it with artificial respiration and transthoracic defibrillation. Julian cited a recent article by Los Angeles cardiologists Seymour Cole and Eliot Corday (who, like Wilburne, worked at the Cedars of Lebanon Hospital) that stressed the need to start effective resuscitation less than four minutes after cardiac arrest to avoid severe brain damage. He explained,
There are two ways in which this problem of delay could be reduced. First, all medical, nursing, and auxiliary staff should be trained in the techniques of closed-chest cardiac massage and mouth-to-mouth breathing. Secondly, patients known to be at risk from ventricular fibrillation or cardiac asystole could have their cardiac rhythm constantly monitored. This means that all wards admitting patients with acute myocardial infarction should have a system capable of sounding an alarm at the onset of an important rhythm change and of recording the rhythm automatically on an E.C.G. In most cases, probably, an arrhythmia is present for at least 30 seconds (often for some minutes) before loss of consciousness; if it were diagnosed immediately the chances of resuscitation would be improved and the dangers of brain damage minimized. Such monitoring is particularly necessary during the first 48 hours after infarction, but cardiac arrest may occur at any time in the first 2 weeks. The provision of appropriate apparatus would not be prohibitively expensive if these patients were admitted to special intensive-care units. Such units should be staffed by suitably experienced people throughout the 24 hours, since it is unreasonable to expect good results when the care of the patients is entrusted to inexperienced residents who have many other responsibilities.14
Julian's colleagues in Edinburgh were unenthusiastic about his concept, and he moved to Australia. In November 1962, he launched a program of continuous monitoring of patients who presented to Sydney Hospital with AMI. Not every patient was monitored because of limited availability of the technology, however. Julian's original notion was to place patients with AMI in “special intensive-care units,” but there were none in the institution. Instead, they were admitted “to the recovery, respiratory or clinical research wards of the hospital. These wards have a higher nurse-patient ratio than the general wards, but they are not specifically designed as intensive-care units.”15
Although Wilburne's abstract was rejected for presentation at the 1961 AHA Scientific Sessions, he spoke the following summer at the annual American Medical Association (AMA) meeting in Chicago. Fifteen thousand doctors attended the event, and almost 200,000 received the association's journal. A May 1963 issue included the text of his talk “Cardiac Resuscitation in Coronary Artery Disease: A Central Coronary Care Unit.” Wilburne's paper, coauthored by surgeon Josh Fields who was a member of the Los Angeles County Heart Association's Cardiac Resuscitation Committee, opened with a reference to an unnamed actor's final scene:
Sudden death due to cardiac arrest in patients convalescing from acute myocardial infarction is an ever-present threat. The recent such death of a prominent motion picture performer, whose medical management was of unqualified excellence, precipitated nationwide headlines in the lay press and created a public awareness that, even with optimum care and smooth convalescence, recovery was not assured. Recent developments in cardiac resuscitative technique have brought into clearer focus medicine's potential in preventing such deaths.16
Clark Gable finished filming The Misfits with Marilyn Monroe on November 4, 1961, four days before John Kennedy was elected president. But the fifty-nine-year-old actor had no chance to celebrate the movie's completion or visit a voting booth. Gable was admitted to Hollywood Presbyterian Hospital with a heart attack on the sixth. His cardiologist asked George Griffith, a leading Los Angeles heart specialist, to see him in consultation. The actor's recovery was uneventful until the tenth day when he died suddenly in his hospital bed.17 Gable's death was big news because he was a celebrity, but Americans were reminded regularly that coronary disease could kill without warning. A 1963 Look magazine article described heart disease as the “most critical medical and public health problem facing the nation.” No one was immune: “Good teachers, prominent businessmen, scientists, artists and other useful citizens...[are] cut down in their prime.”18
Wilburne and Fields’ 1963 JAMA paper included the rationale for creating a CCU, an artist's drawing of a unit with a closed-circuit television monitoring system, and a step-by-step guide to CPR. Arguing that the approach could transform the “early stages of death [into] a twoway threshold,” they proclaimed, “The present practice of random assignment of patients with acute coronary disease to various locations in the hospital is, therapeutically, an antiquated procedure.”16 Their concept and paper received favorable reviews. For example, a 1964 report by the American College of Chest Physicians’ Subcommittee on Closed-Chest Cardiac Resuscitation concluded, “Sudden death in some of the patients recovering from acute myocardial infarction could become reversible if these patients were monitored and cardiac resuscitation was promptly performed. Such monitoring would require a central ‘coronary care unit’ as described by Wilburne and Fields, which is fully equipped and manned by specially trained personnel at all times. This subcommittee recommends the establishment of such ‘coronary care units’ in hospitals wherever feasible.”19 Reading the JAMA article, one gets the impression that Wilburne and Fields were describing a CCU that existed either at Cedars of Lebanon Hospital or Mount Sinai Hospital. In fact, neither institution would have such a unit until 1966. The Los Angeles doctors were not trying to deceive readers. They hoped to stimulate the implementation of a brand new care model with the potential to save thousands of lives.
IMPLEMENTING A CONCEPT: THE FIRST CCU OPENS IN KANSAS NOT CALIFORNIA
There is a distinct difference between describing the CCU concept and implementing it. The first special care unit in the United States designed to monitor and treat patients with AMI opened on May 20, 1962 at Bethany Hospital in Kansas City, Kansas.20 Cardiologist Hughes Day got a grant from the John A. Hartford Foundation to create an “Intensive Coronary Care Area” that contained four private rooms adjacent to a seven-bed medical and surgical ICU. Based on his experience with seventeen AMI patients, Day published an article in February 1963 entitled “Preliminary Studies of an Acute Coronary Care Area.” The unit incorporated the key elements that Wilburne outlined in his 1961 Circulation abstract: (1) continuous ECG monitoring with an audible heart rate alarm; (2) a mobile cart with an external defibrillator and pacemaker; and (3) constant attention by specially trained nurses who could activate the hospital's CPR team (an innovation in itself). Importantly, Day shared Wilburne's conviction that all patients with AMI should be admitted to the unit. He concluded that “an area specially designed, equipped, and staffed to provide immediate treatment at the onset of cardiac arrest can be a vital part of every hospital's program of cardiac resuscitation.”21
Very few doctors saw Hughes Day's initial reports of his hospital's experience with a resuscitation team and a CCU because they were published in the Minneapolis-based Journal-Lancet. Day told Cleveland surgeon Claude Beck, a pioneer of open-chest cardiac resuscitation, that his papers appeared in this regional periodical “for the simple reason that I could not get any national journal to publish them.”22 Desmond Julian, who also had trouble getting published, explained, “Our initial report from Sydney Hospital was submitted to the Lancet early in 1963, but it was rejected because the journal had recently accepted the report from Brown et al. in Toronto. The paper was rejected by the British Medical Journal because ‘it was irresponsible to suggest that all patients with myocardial infarction should be admitted to wards in which they could receive intensive care’. It was then submitted to the Medical Journal of Australia where it lay for some months until Graeme Sloman pointed out the importance of the subject and contributed an article on his similar experiences in Melbourne.”23
ELIOT CORDAY ENLISTS ALLIES TO CHAMPION THE CCU MODEL
Los Angeles cardiologist Eliot Corday, who would become Hughes Day's biggest booster, had a long-standing interest in life-threatening arrhythmias. He admitted patients to Cedars of Lebanon Hospital, one of several hospitals where Wilburne practiced. Corday heard Day present his early CCU experience at the Interim Clinical Meeting of the American College of Chest Physicians in Los Angeles in November 1962. The organization's journal Diseases of the Chest was mailed to 10,000 doctors, and the January 1963 issue included an abstract of Day's talk “An Intensive Coronary Care Area in Action.” His Los Angeles audience listened to the Kansas City cardiologist describe his unit and watched “a color motion picture of the area showing the equipment and its use.”24 The college's journal published the text of his talk in October. Recalling the California meeting, Day explained that Corday “saw the possibilities of the idea and became one of the outstanding leaders in the field of training.”25
Corday became president of the American College of Cardiology (ACC) in 1965 and used his position to promote the CCU model. He created and chaired the college's Special Committee for Liaison with Congress, the Surgeon General, and the National Institutes of Health the same year that President Lyndon Johnson signed the Heart Disease, Cancer, and Stroke Amendments of 1965 into law. Known as the Regional Medical Programs Act, the law would accelerate the diffusion of the CCU model.26 It authorized grants to “assist in the establishment of regional cooperative arrangements among medical schools, research institutions, and hospitals for research and training (including continuing education) and for related demonstrations of patient care in the fields of heart disease, cancer, stroke, and related diseases...to afford to the medical profession and the medical institutions...the opportunity of making available to their patients the latest advances in the diagnosis and treatment of these diseases.”27
Corday also created the ACC's Bethesda Conferences, a theme-based meeting model that united a small number of cardiologists considered topic experts with government representatives and other interested parties to discuss a specific subject and publish a report. He invited Day to chair a conference on “Training Technics for the Coronary Care Unit” and suggested a dozen doctors and four nurses who should participate. In addition to Corday and Day, twenty-three individuals attended the December 1965 conference. They included the cardiologist-head nurse teams who launched America's earliest CCUs at the Presbyterian Hospital in Philadelphia (Lawrence Meltzer, Roderick Kitchell, and Rose Pinneo), the New York Hospital in Manhattan (Thomas Killip and Mary Fordham), the Miami Heart Institute (Paul Unger and Adeline Jenkins), and the Peter Bent Brigham Hospital in Boston (Bernard Lown). Their report contained recommendations on CCU design, cardiac resuscitation, and coronary care nursing.28
The following year Corday wrote to Senator Lister Hill, coauthor of the Hill-Burton Act that supported hospital construction and a champion of National Institutes of Health funding. Hill thanked Corday for writing “with respect to the need for coronary care units in hospitals” and said that he would give the Bethesda Conference Report his “careful attention.” [Lister Hill to Eliot Corday, 27 June 1966. ACC archives, Washington, D.C. Quoted with permission.] With one exception, the participants in the Bethesda Conference were pioneers of the CCU movement or in positions of influence at the Public Health Service or the National Heart Institute (as it was then known). Jeremy Swan, an Irish-American physiologist who had been at the Mayo Clinic since 1951, had never worked in a CCU, but he had extensive experience in cardiac catheterization. Most important was the fact that Corday had just recruited him as the first chief of cardiology at Cedars-Sinai Medical Center. This new name reflected the recent affiliation of Cedars of Lebanon Hospital with Mount Sinai Hospital, located six miles away.
When Corday heard Day speak in Los Angeles in 1962, the Kansas City unit was the only CCU in the United States. Not for long! Four years later, Howard Burchell, a Mayo Clinic cardiologist and the editor of Circulation, marveled over the “mushrooming of coronary units” across the country. More than 200 were already in operation.29 The time lag between the first descriptions of the CCU concept in Circulation and the Lancet in 1961 and the widespread implementation of the model in the United States was incredibly short considering its implications for staff, space, equipment, and patient care. This rapid diffusion was the antithesis of the phenomenon of resistance to innovation written about recently by Ernest Hook: “Scientists and historians can cite many cases of scientific and technological claims, hypotheses, and proposals that, viewed in retrospect, have apparently taken a long time to be recognized, endorsed, or integrated into accepted knowledge and practice.”30 Ironically, this was the case with the recognition of AMI. British physician William Heberden published his classic description of angina pectoris in 1772, and Chicago internist James Herrick published the first English-language description of the clinical syndrome of AMI in 1912.31,32
EVIDENCE-BASED MEDICINE AND THE RISE AND FALL OF LIDOCAINE
The life-saving potential of the CCU seemed self evident to doctors, nurses, hospital administrators, and patients in the mid-1960s, but two Harvard Medical School faculty members challenged the model in 1973: “The use of coronary-care units for the treatment of patients with myocardial infarction has increased explosively with little attention to efficacy, need, or cost.” In their opening paragraph they cited papers by Bernard Lown who established a CCU at the Harvard-affiliated Peter Bent Brigham Hospital in 1965, three years after Hughes Day opened his unit in Kansas City. Referring to Lown's papers and two others from Britain, they declared, “Clinicians justify this intensive and expensive therapy by a definitely reduced in-hospital case fatality rate. Research on the units has centered mainly on end results of clinical experience ‘before and after’ institution of a unit within a hospital or on preventive and therapeutic advances.”33
Lown's legacy in terms of the CCU relates to his development of the DC defibrillator and to his role in promoting the prophylactic use of lidocaine as a strategy to reduce the likelihood of cardiac arrest in patients with AMI. In 1981, he published an insider's perspective on the nearly negligible time lag between lidocaine's use in dogs with experimental infarction, it first clinical application, and its diffusion into practice:
We discovered the remarkable efficacy of lidocaine in the animal laboratory in December 1964. When given as a bolus IV, it consistently abolished the ever-present ventricular arrhythmias in dogs recovering from acute myocardial infarction after coronary artery ligation. One month later, the first patient admitted to the CCU at the Peter Bent Brigham Hospital, Boston, received lidocaine IV. The policy was to control ventricular premature beats (VPBs), considered the putative harbingers of ventricular fibrillation. Adhering to this policy, not a single episode of ventricular fibrillation was encountered in 130 consecutive patients with proved acute myocardial infarction. Mortality among patients with myocardial infarction at the Peter Bent Brigham Hospital, which had ranged from 29% to 33% over the preceding five years, declined to 11.5%. These findings led to the promulgation of the view that the proper objective of CCU care was to prevent the need for resuscitation by treating VPBs with lidocaine.34
In a 1968 debate about the CCU with San Francisco cardiologist Arthur Selzer, Lown promoted three “policies that almost completely abolish the need of resuscitation for primary derangements in heart rhythm....1) abolition of ventricular beats during the first 48 to 72 hours after onset of acute myocardial infarction; (2) acceleration of the ventricular rate in the presence of bradyarrhythmias that are associated with ectopic beats or with hemodynamic disturbance; and (3) early detection of left ventricular failure and prompt digitalization. When these policies are adhered to, it is possible to reduce the incidence of ventricular fibrillation to less than 1 per cent.”35, 36 Selzer closed his rebuttal with a warning: “My presentation has shown in detail why statistics mentioned by Dr. Lown are unreliable and should not be accepted at face value. What we need is a properly designed alternate case study with good controls. However, this may be morally impossible, and we may be left permanently with conflicting statistics and no real answer.”37
For a generation, lidocaine was central to the strategy of preventing life-threatening ventricular arrhythmias in patients with AMI. Its meteoric rise and subsequent fall is a compelling example of the impact of controlled clinical trials on cardiovascular practice during the past quarter century. Evidence-based medicine, the organizing principle of what has been termed the “trial-guideline-education process” revolutionized the care of patients with AMI.38, 39 Between 1990 and 1996 the recommendations regarding prophylactic lidocaine in the ACC/AHA guidelines for the management of patients with AMI changed dramatically—from a class I to a class III indication.40, 41 The various factors contributing to this complete reversal are beyond the scope of this historical essay that focuses on the origin of the CCU concept and its rapid diffusion into practice. The introduction of reperfusion therapy with thrombolytic agents or catheter-based interventions and the routine use of beta-blocking drugs transformed the natural history of AMI and reduced its early and late complications.1 Meanwhile, evidence-based medicine also contributed to a dramatic decline in the use of the Swan-Ganz catheter, a technology designed to help manage the most frequent non-arrhythmic causes of death in AMI patients: cardiogenic shock and refractory pulmonary edema.
CEDARS OF LEBANON HOSPITAL AND THE SWAN-GANZ CATHETER
Cedars of Lebanon Hospital, where Wilburne and Corday admitted patients, was the largest private hospital in Los Angeles. It opened a CCU in January 1966, six months after Jeremy Swan arrived from the Mayo Clinic to be chief of cardiology at the new Cedars-Sinai Medical Center. The name implied a physical merger of the two hospitals, but that would not occur for another decade. Swan's office was at the Cedars of Lebanon Hospital, and he later recalled that the institution's CCU was established there “largely through the efforts of Eliot Corday.”42 Wilburne, the private practitioner who had described the concept, was not involved in its implementation or operation.
Swan's sophistication in hemodynamics was critical for his hospital's participation in a new federally funded program. The NHI's Myocardial Infarction Research Program, launched in 1966, was designed to study (among other things) the pathophysiology of AMI and to identify more effective treatments for complications such as shock and heart failure. Nine Myocardial Infarction Research Units (MIRUs) were created including one at Cedars of Lebanon.43 By this time, Swan was an acknowledged world leader in clinical cardiovascular physiology as a result of fifteen years of experience in Mayo's high-volume catheterization laboratory. He now had the resources to assemble a team of clinical investigators who would evaluate hemodynamic responses to various interventions in AMI patients in order to “develop therapeutic guidelines” for their “optimal management.”44 Swan coauthored a 1970 paper describing a technology (the Swan-Ganz catheter) that would be used widely to evaluate intracardiac pressures and cardiac output in patients with AMI and other critical illnesses. The bedside technique provided a wealth of insight into the hemodynamic consequences of AMI and their treatment. As with lidocaine, evidence-based medicine resulted in a much more limited role for the Swan-Ganz catheter by the close of the century.45, 46
NURSE EMPOWERMENT: A CRICIAL COMPONENT OF THE CCU MODEL
The widespread implementation of the CCU model in the mid-1960s triggered a major shift in the traditional relationship between doctors and nurses.47 New monitoring technology alerted staff to the sudden onset of VF and to the possibility of reversing death by prompt defibrillation. There was no time to wait for a doctor to deliver the shock because irreversible brain damage occurred in less than four minutes after cardiac arrest. Physicians, seeking ways to save patients’ lives without having to position themselves minutes away from the bedside at all times, trained and empowered specific nurses to defibrillate patients. The founders of America's second CCU (at Philadelphia's Presbyterian Hospital) championed the concept that specific nurses should be trained to play a very active role in the treatment of cardiac arrest. Lawrence Meltzer and Roderick Kitchell opened their unit in November 1962. Meltzer explained two years later, “It was apparent to us, even before we began, that the entire success of the undertaking would depend on nurses. We envisioned that they would constantly attend the patients, be taught to recognize arrhythmias, know the therapy for each catastrophe and, in effect, be in charge of the unit.”48
Meltzer, Kitchell, and their unit's head nurse Rose Pinneo coauthored Intensive Coronary Care: A Manual for Nurses in 1965. The opening sentence set the tone: “It may seem curious that the first book dedicated to a new concept of treatment for acute myocardial infarction has been directed primarily to nurses rather than physicians.” They emphasized that the new treatment technologies had to be used immediately in order to save lives. To achieve this goal doctors must abandon traditional notions of a nurse's limited role in clinical decision making. The authors declared, “Intensive coronary care is essentially an advanced system of nursing. It is not an advanced system of medical practice based on electronics.” Their prescription for saving lives was explicit: “A CCU nurse must be able to perform...therapeutic measures by herself without specific orders.” This included the definitive treatment for VF: “If the physician has not arrived within two minutes of the onset of this fatal arrhythmia, she defibrillates the patient by herself.”49 Support for giving specially trained nurses authority to defibrillate patients grew quickly in the late-1960s as concerns about the legal implications of the practice declined. The CCU-inspired empowerment of nurses represented a critical first step in the evolution of team-based care that is such a conspicuous part of current-day cardiology practice.
THE CHALLENGE OF ASSIGNING PRIORITY TO INNOVATORS AND PIONEERS
The original descriptions of the CCU concept by Wilburne and Julian in 1961 are a compelling example of simultaneous innovation. Working in very different contexts, they recognized an opportunity to reverse sudden death in patients with AMI by implementing an innovative care model that united vulnerable patients with new technologies and specially trained staff in a specific hospital space. Thomas Kuhn, who has written about simultaneous innovation and the challenge of assigning priority, explains, “To the historian discovery is seldom a unit event attributable to some particular man, time, and place.”50, 51 In the case of the CCU, the new care model proposed by Wilburne and Julian was the result of decades of discoveries, inventions, and innovations that, in turn, represented the contributions of thousands of individuals working in countless contexts.
William Grace, who opened one of the nation's first CCUs at St. Vincent's Hospital in New York City in 1964, wrote six years later, “It is clear that the Coronary Care Unit concept in this country was pioneered by Hughes Day and Lawrence E. Meltzer.”52 In fact, there were two types of CCU pioneer: individuals who proposed the concept and those who implemented and promoted it in presentations and publications. Cardiologists from the U. S. Public Health Service's Heart Disease Control Branch made this point in 1966 when they emphasized that the CCU was “really a concept and not necessarily a specific structure. The service to be provided must be stressed, rather than a rigid pattern of bricks and mortar.”53 The concept that Wilburne and Julian outlined in 1961 would be fleshed out during the next decade as doctors, nurses, and administrators collaborated in establishing special units for AMI patients in hundreds of hospitals. Wilburne, a private practitioner, never directed a CCU and faded from view. Julian actually implemented the model and became a leading academic cardiologist.
British scientist Francis Darwin, Charles Darwin's son, wrote a century ago, “In science the credit goes to the man who convinces the world, not to the man to whom the idea first occurs. Not the man who finds a grain of new and precious quality but to him who sows it, reaps it, grinds it and feeds the world on it.”54 In this sense, Eliot Corday also deserves credit. When he was organizing the ACC-sponsored Bethesda Conference on CCUs in 1965, he invited AHA president Helen Taussig to attend. The Johns Hopkins pediatric cardiologist responded, “I greatly appreciate your advising me of the [conference] and shall be extremely happy if the American Heart Association can work closely with the American College on various problems of mutual interest.” [Helen B. Taussig to Eliot Corday, 12 November 1965. ACC Archives, Washington, D.C. Quoted with permission] Her statement was very significant because it signaled a new chapter in the tense relationship that had existed between the organizations since the college was founded fifteen years earlier.55 Seeking to encourage collaboration to catalyze the diffusion of the CCU model, Corday played a key role in organizing the 1967 National Conference on Coronary Care Units sponsored by the ACC, AHA, and the federal government's Heart Disease Control Program. When that conference was held in June, 650 individuals attended and more than 350 CCUs were operating in the United States.56
THE AMERICAN HEART ASSOCIATION PUBLISHES WILBURNE's ABSTRACT
The content of Wilburne's Circulation abstract proves that he deserves credit along with Julian for describing the CCU concept whereas Day was the first American to implement it. Although Wilburne's abstract was published on paper in 1961, it became invisible because it was not included in Index Medicus or PubMed. The authors of an article on the use of citation data to study discoveries and the evolution of science argue that “the development of citation indexes and elaborate methodologies for the use of citation data make it possible to trace discoveries and to assess the credit publishing scientists assign to individual scientists for particular discoveries.” They acknowledge, however, that “citation counts and the use of cluster maps cannot replace the study of original sources.”57 Today, decades of the true original sources—bound journal volumes—are an endangered species as libraries discard print runs when their contents become available electronically. There is another problem. With the passage of time, credit for discoveries and innovations becomes focused on a few leading actors while the supporting cast and other contributors disappear into the recesses of history. The contemporary emphasis, indeed obsession, on the most recent publications does a disservice to past researchers, clinical investigators, and innovators whose significant (sometimes critical) contributions are devalued. This phenomenon raises the interesting philosophical question, “Where does a review of the literature end and medical history begin?”58, 59
One of the more intriguing aspects of the Wilburne story relates to why his rejected abstract was published at all. This was the result of a short-lived AHA policy in place a half century ago. The association announced in the January 1961 issue of Circulation that application forms were available for those interested in submitting an abstract for the annual fall meeting. The wording suggests why Wilburne's was turned down: “Papers intended for presentation must be based on original investigation in, or related to, the cardiovascular field.....All applications will be screened by the Committee on Scientific Sessions Program.”60 Rather than reporting research results, the theme of the “Scientific Sessions,” Wilburne described a new patient care model. When the ten-man program committee chaired by catheterization pioneer James Warren met that January, they debated the policy of publishing all submitted abstracts in Circulation versus only those accepted for presentation. Open-heart surgeon William Glenn did not support publishing abstracts that were not presented: “He felt that the literature was full of such material which he classified as ‘junk.’” Electrocardiographer Elliot Newman “voiced the opinion that publication of the abstracts served good purposes. It provides a survey of investigation in progress, what people are doing or think they are doing, and encouraged investigators to submit abstracts. He felt that if abstracts were not selected for presentation ended up in the waste basket, fewer and poorer abstracts would be submitted.” After further discussion, the committee agreed to continue publishing all abstracts. That is why Wilburne's abstract exists. [Minutes. AHA Committee on Scientific Sessions Program, 13 January 1961. AHA Archives, Dallas, TX. Quoted with permission.]
James Warren wrote in 1965, four years after his committee rejected Wilburne's abstract describing the CCU concept, “Vigorous therapy following acute myocardial infarction might effect a saving of 40,000 lives a year. Among the features of such therapy are placement of patients in special ‘coronary care units’ in hospitals, institution of measures to combat shock and congestive heart failure, and use of anticoagulants and vasopressor drugs.”61 That year, the AHA published a two-part article on the CCU in Modern Concepts of Cardiovascular Disease, a monthly educational leaflet mailed to more than 100,000 doctors. Based on early reports from Day's unit in Kansas City and Meltzer's in Philadelphia, the authors concluded, “Although the number of treated cases in these two units was small, the clinical data nevertheless suggest that a significant reduction in the mortality rate in acute myocardial infarction can be expected when the patients are observed and treated in a Coronary Care Unit for an initial period of three to seven days.”62 Wilburne's Circulation abstract faded from view, but the model he described in it became a very visible part of most American hospitals and had profound consequences for patient care, cardiology practice, and the nursing profession.
Acknowledgments
None
Funding Sources: This article incorporates material from a chapter on the history of the CCU that will appear in a book I am writing, The Mayo Clinic and Cardiovascular Disease: Specialization in the 20th Century. That ongoing project was partially funded (08/01/2005-7/31/2007) by a National Library of Medicine Grant for Scholarly Works in Biomedicine and Health (1-G13-LM007922-01A1)
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Braunwald E. Evolution of the management of acute myocardial infarction: A 20th century saga. Lancet. 1998;352:1771–1774. doi: 10.1016/S0140-6736(98)03212-7. quote p.1772. [DOI] [PubMed] [Google Scholar]
- 2.Lee TH, Goldman L. The coronary care unit turns 25: Historical trends and future directions. Ann Int Med. 1988;108:887–894. doi: 10.7326/0003-4819-108-6-887. [DOI] [PubMed] [Google Scholar]
- 3.Khush KK, Rapaport E, Waters D. The history of the coronary care unit. Can J Cardiol. 2005;21:1041–1045. [PubMed] [Google Scholar]
- 4.Dock W. Treatment of angina pectoris and myocardial infarction. Med Clin North Am. 1962;46:1599–1612. doi: 10.1016/s0025-7125(16)33643-4. quote p. 1602. [DOI] [PubMed] [Google Scholar]
- 5.Friedberg CK. Diseases of the Heart. 2 ed. W.B. Saunders; Philadelphia: 1956. quote p. 569. [Google Scholar]
- 6.Beck CS, Weckesser EC, Barry FM. Fatal heart attack and successful defibrillation: New concepts in coronary artery disease. JAMA. 1956;161:434–436. doi: 10.1001/jama.1956.62970050001008. [DOI] [PubMed] [Google Scholar]
- 7.Abdellah FG, Strachan EJ. Progressive patient care. Am J Nursing. 1959;59:649–655. [PubMed] [Google Scholar]
- 8.Friedberg CK. Preface. In: Friedberg CK, editor. Acute Myocardial Infarction and Coronary Care Units. Grune & Stratton; New York: 1969. pp. v–viii. quote p. v. [Google Scholar]
- 9.Zoll PM, Linenthal AJ, Gibson W, Paul MH, Norman LR. Termination of ventricular fibrillation in man by externally applied electric countershock. N Engl J Med. 1956;254:727–732. doi: 10.1056/NEJM195604192541601. [DOI] [PubMed] [Google Scholar]
- 10.Zoll PM, Linenthal AJ, Norman LR, Paul MH, Gibson W. Treatment of unexpected cardiac arrest by external electrical stimulation of the heart. N Engl J Med. 1956;254:541–546. doi: 10.1056/NEJM195603222541201. [DOI] [PubMed] [Google Scholar]
- 11.The External Defibrillator Developed by Paul Zoll, M.D. [Electrodyne advertisement]. N Engl J Med. 1956 April 19;254(16):xiii. [Google Scholar]
- 12.Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed-chest cardiac massage. JAMA. 1960;173:1064–1067. doi: 10.1001/jama.1960.03020280004002. quote p. 1064. [DOI] [PubMed] [Google Scholar]
- 13.Wilburne M. The coronary care unit: A new approach to treatment of acute coronary occlusion [Abstract]. Circulation. 1961;24:1071. [Google Scholar]
- 14.Julian DG. Treatment of cardiac arrest in acute myocardial ischaemia and infarction. Lancet. 1961;2:840–844. doi: 10.1016/s0140-6736(61)90738-3. quote p. 843. [DOI] [PubMed] [Google Scholar]
- 15.Julian DG, Valentine PA, Miller GG. Routine electrocardiographic monitoring in acute myocardial infarction. Med J Aust. 1964;1:433–436. doi: 10.5694/j.1326-5377.1964.tb134230.x. quote p. 433. [DOI] [PubMed] [Google Scholar]
- 16.Wilburne M, Fields J. Cardiac resuscitation in coronary artery disease: A central coronary care unit. JAMA. 1963;184:453–457. doi: 10.1001/jama.1963.03700190071008. quotes p. 453, 455. [DOI] [PubMed] [Google Scholar]
- 17.Tornabene L. Long Live the King: A Biography of Clark Gable. G. P. Putnam's Sons; New York: 1976. pp. 391–398. [Google Scholar]
- 18.Morgan TB. The heart. Look. 1963 January 15;:62–69. quote p. 62. [Google Scholar]
- 19.Thompson SA, Carr RE, Kennedy JH, Storey CF. Report of the Subcommittee on Closed-chest Cardiac Resuscitation. Dis Chest. 1964;45:440–442. doi: 10.1378/chest.45.4.440. quote p. 441. [DOI] [PubMed] [Google Scholar]
- 20.Keeling AW. Blurring the boundaries between medicine and nursing: Coronary care nursing, circa the 1960s. Nursing Hist Rev. 2004;12:139–164. [PubMed] [Google Scholar]
- 21.Day HW. Preliminary studies of an acute coronary care area. Journal-Lancet. 1963;83:53–55. quote p. 53. [PubMed] [Google Scholar]
- 22.Beck CS. Reminiscences of cardiac resuscitation. Rev Surg. 1970;27:77–86. quote p. 86. [PubMed] [Google Scholar]
- 23.Julian DG. The evolution of the coronary care unit. Cardiovasc Res. 2001;51:621–624. doi: 10.1016/s0008-6363(01)00365-0. quote p. 622. [DOI] [PubMed] [Google Scholar]
- 24.Day HW. An intensive coronary care area in action. Dis Chest. 1963;43:112. doi: 10.1378/chest.44.4.423. [DOI] [PubMed] [Google Scholar]
- 25.Day HW. History of coronary care units. Am J Cardiol. 1972;30:405–407. doi: 10.1016/0002-9149(72)90572-3. quote p. 405. [DOI] [PubMed] [Google Scholar]
- 26.Fye WB. American Cardiology: The History of a Specialty and its College. The Johns Hopkins University Press; Baltimore: 1996. Washington, medicine, and the American College of Cardiology; pp. 215–248. [Google Scholar]
- 27.Public Law 89-239. 89th Cong. S. 596. An Act to Amend the Public Health Service Act to Assist in Combating Heart Disease, Cancer, Stroke, and Related Diseases; Washington, GPO. October 6, 1965; 1965. [Google Scholar]
- 28.Training technics for the coronary care unit.. Am J Cardiol; Second Bethesda Conference of the American College of Cardiology.1966. pp. 736–747. [Google Scholar]
- 29.Burchell HB. Coronary care units: Panel discussion. Isr J Med Sci. 1967;3:320–325. quote p. 320. [PubMed] [Google Scholar]
- 30.Hook EB. A background to prematurity and resistance to “discovery”. In: Hook EB, editor. Prematurity in Scientific Discovery: One Resistance and Neglect. University of California Press; Berkeley: 2002. pp. 3–21. quote p. 3. [Google Scholar]
- 31.Fye WB. The delayed diagnosis of acute myocardial infarction: it took half a century! Circulation. 1985;72:262–271. doi: 10.1161/01.cir.72.2.262. [DOI] [PubMed] [Google Scholar]
- 32.Fye WB, editor. Classic Papers on Coronary Thrombosis and Myocardial Infarction. Gryphon Editions; Birmingham, AL: 1991. [Google Scholar]
- 33.Bloom BS, Peterson OL. End results, cost and productivity of coronary-care units. N Engl J Med. 1973;288:72–78. doi: 10.1056/NEJM197301112880205. quote 72. [DOI] [PubMed] [Google Scholar]
- 34.Lown B. Lidocaine: Antiarrhythmic panacea or cardiac cosmetic agent. JAMA. 1981;246:2482–2483. [PubMed] [Google Scholar]
- 35.Lown B. Controversies in Cardiology. The Coronary Care Unit. Part I. Am J Cardiol. 1968;22:597–599. doi: 10.1016/0002-9149(68)90167-7. quote p. 598. [DOI] [PubMed] [Google Scholar]
- 36.Kimball JT, Killip T. Aggressive treatment of arrhythmias in acute myocardial infarction: Procedures and results. Prog Cardiovasc Dis. 1968;10:483–504. doi: 10.1016/0033-0620(68)90001-7. [DOI] [PubMed] [Google Scholar]
- 37.Selzer A. Controversies in Cardiology. The Coronary Care Unit. Part II. Am J Cardiol. 1968;22:599–602. quote p. 601. [Google Scholar]
- 38.Fye WB. The power of clinical trials and guidelines, and the challenge of conflicts of interest. J Am Coll Cardiol. 2003;41:1237–1242. doi: 10.1016/s0735-1097(03)00232-8. [DOI] [PubMed] [Google Scholar]
- 39.Braunwald E, Antman E. Evidence-based coronary care. Ann Int Med. 1997;126:551–553. doi: 10.7326/0003-4819-126-7-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 40.Gunnar RM, Bourdillon PDV, Dixon DW, Fuster V, Karp RB, Kennedy JW, Klocke FJ, Passamani ER, Pitt B, Rapaport E, Reeves TJ, Russell RO, Sobel BE, Winters WL., Jr Guidelines for the early management of patients with acute myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures. J Am Coll Cardiol. 1990;16:249–292. doi: 10.1016/0735-1097(90)90575-a. [DOI] [PubMed] [Google Scholar]
- 41.Ryan TJ, Anderson JL, Antman E, Braniff BA, Brooks NH, Califf RM, Hillis LD, Hiratzka LF, Rapaport E, Riegel BJ, Russell RO, Smith EE, III, Weaver WD. ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). J Am Coll Cardiol. 1996;28:1328–1428. doi: 10.1016/s0735-1097(96)00392-0. [DOI] [PubMed] [Google Scholar]
- 42.Swan HJC. Early development of the pulmonary artery catheter: A personal perspective. Proc Baylor Univ Med Cent. 1996;9:3–7. quote p. 5. [Google Scholar]
- 43.Frommer PL. The Myocardial Infarction Research Program of the National Heart Institute. Am J Cardiol. 1968;22:108–110. doi: 10.1016/0002-9149(68)90251-8. [DOI] [PubMed] [Google Scholar]
- 44.Danzig R, Swan HJC. Practical experiences in a coronary care unit. Geriatrics. 1969;24:95–101. [PubMed] [Google Scholar]
- 45.Swan HJC, Ganz W, Forrester JS, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283:447–451. doi: 10.1056/NEJM197008272830902. [DOI] [PubMed] [Google Scholar]
- 46.Chatterjee K. The Swan-Ganz catheters: Past, present, and future. A viewpoint. Circulation. 2009;119:147–152. doi: 10.1161/CIRCULATIONAHA.108.811141. [DOI] [PubMed] [Google Scholar]
- 47.Fairman J, Lynaugh JE. Critical Care Nursing: A History. University of Pennsylvania Press; Philadelphia: 1998. [Google Scholar]
- 48.Meltzer LE. The concept and system for intensive coronary care. Acad Med N J Bull. 1964;10:304–311. quote p. 307. [Google Scholar]
- 49.Meltzer LE, Pinneo R, Kitchell JR. Intensive Coronary Care: A Manual for Nurses. The Presbyterian Hospital; Philadelphia: 1965. quotes pp. i, 86. [Google Scholar]
- 50.Kuhn TS. Historical structure of scientific discovery. Science. 1962;136:760–764. doi: 10.1126/science.136.3518.760. quote p. 760. [DOI] [PubMed] [Google Scholar]
- 51.Merton RK. Priorities in scientific discovery: A chapter in the sociology of science. Am Sociological Rev. 1957;22:635–659. [Google Scholar]
- 52.Grace WJ, Keyloun V. The Coronary Care Unit. Appleton-Century-Crofts; New York: 1970. quote p. viii. [Google Scholar]
- 53.Flynn RL, Fox SMI. Coronary care programs in the United States. Isr J Med Sci. 1967;3:279–286. quote p. 281. [PubMed] [Google Scholar]
- 54.Darwin F. Francis Galton, 1822-1911. Eugenics Review. 1914;6:1–17. quote p. 9. [Google Scholar]
- 55.Fye WB. A history of the American Heart Association's Council on Clinical Cardiology. Circulation. 1993;87:1057–1063. doi: 10.1161/01.cir.87.3.1057. [DOI] [PubMed] [Google Scholar]
- 56.Proceedings of the National Conference on Coronary Care Units. U. S. Department of Health, Education, and Welfare; Washington: 1968. [Google Scholar]
- 57.Lindahl BIB, Elzinga A, Welljams-Dorof A. Credit for discoveries: Citation data as a basis for history of science analyses. Theor Med. 1998;19:609–620. doi: 10.1023/a:1009944903620. quotes p. 616. [DOI] [PubMed] [Google Scholar]
- 58.Fye WB. Medical authorship: Traditions, trends, and tribulations. Ann Int Med. 1990;113:317–325. doi: 10.7326/0003-4819-113-4-317. [DOI] [PubMed] [Google Scholar]
- 59.Fye WB. President's Page. Medical history: A valuable tool to help us frame the past and predict the future. J Am Coll Cardiol. 2003;41:346–349. doi: 10.1016/s0735-1097(02)02828-0. [DOI] [PubMed] [Google Scholar]
- 60.Abstracts of papers due May 15 for 1961 AHA Scientific Sessions. Circulation. 1961;23:159. [Google Scholar]
- 61.Warren JV. Acute myocardial infarction: Can deaths be reduced and, if so, how? Postgraduate Med. 1965;38:101–104. doi: 10.1080/00325481.1965.11695605. quote p. 101. [DOI] [PubMed] [Google Scholar]
- 62.Yu PN, Imboden CA, Fox SM, Killip T. Coronary care unit: A specialized intensive care unit for acute myocardial infarction. Mod Concepts Cardiovasc Dis. 1965;34(5-6):23–30. quote p. 29. [PubMed] [Google Scholar]