1. Introduction
The oxygen element is one of the most important components for life on earth because various oxygen containing molecules are present in all levels of biological systems, and oxygen accounts for two thirds of the total human body mass and 90% of the mass of water. However, in vivo oxygen-17 (17O) NMR has received very little attention compared to other in vivo NMR methodologies, such as 1H, 13C and 31P NMR; even though the 17O NMR signal was first observed in 1951 [1] and utilized since then for many chemical and biochemical applications (see a recent review by Gerothanassis [2, 3] and the cited references therein).
It has been demonstrated that in vivo 17O NMR can be used to monitor the uptake or washout of an 17O-labeled exogenous agent (e.g. 17O-labeled water) for studying tissue perfusion [4–7] or for detecting oxygen-containing metabolites in living species [8–10]. Nevertheless, the most valuable and unique capability of in vivo 17O NMR is to non-invasively determine the metabolic rate of oxygen in live animals or humans (see [11–13] and references cited therein).
In this review article, we attempt to provide an overview of the methodology background and the present status of in vivo 17O MR spectroscopy (MRS)/imaging (MRI) approach for imaging the cerebral metabolic rate of oxygen (CMRO2) and studying the central roles of cerebral oxygen metabolism in brain function. The challenges and potentials of this 17O-MR based CMRO2 imaging method will also be discussed.
2. Background
2.1 Importance of oxygen metabolism in brain function and dysfunction
The brain is a highly aerobic organ; it consumes oxygen and glucose extensively in order to generate chemical energy in the form of the adenosine triphosphate (ATP) molecule. A majority of brain energy is used to support the unceasing electrophysiological activities of neurons responsible for inter-neuron transmission and communication throughout the central nervous system. A coupling between neuronal activity and brain energy exists for a wide range of physiological conditions characterized by the highly dynamic change of neuronal activity. This requires a timely efficient balance between ATP demand and supply, which is regulated by a number of crucial biochemical reactions associated with brain metabolisms and neuroenergetics.
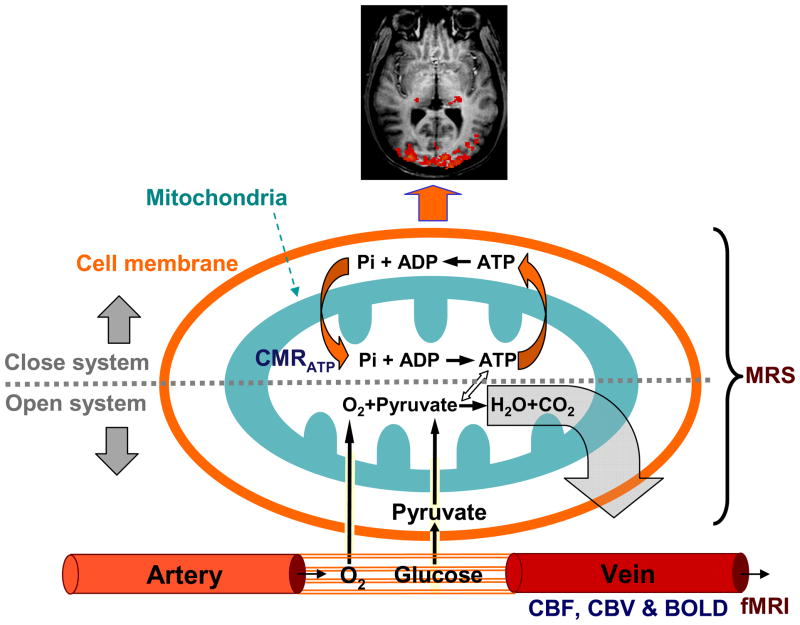
Fig. 1 illustrates the key metabolic processes occurring in various sub-cellular compartments and the associated vascular/hemodynamic events of the brain. Oxygen and glucose as the major fuels for brain metabolism are continuously supplied by the circulating blood flow through the capillary bed. Glucose is transported into brain cells and converted to two pyruvate molecules in the cytosol via glycolysis, which are then converted to acetyl-Co A in the mitochondrion and oxidized via the tricarboxylic acid cycle to generate reducing equivalents of NADH and FADH2. These high energy electron carriers enter the electron transport chain and generate an electrochemical potential gradient across the mitochondrial inner membrane to drive the conversion of adenosine diphosphate (ADP) and inorganic phosphate (Pi) into ATP via oxidative phosphorylation where the electrons are finally transferred to exogenous oxygen and, with the addition of two protons, the final product of water is formed. Under normal physiological conditions with adequate cellular oxygen availability, oxidative phosphorylation comprises ~90% of ATP production [14]. Meanwhile, ATP utilization occurs in the cytosol and results in the reversal conversion of ATP to ADP and Pi with released energy for supporting various neuronal activities.
Fig. 1.
Key metabolic processes occur in various sub-cellular compartments including both mitochondria and cytosol spaces and the associated vascular or hemodynamic events of the brain.
In general, the brain glucose and oxygen consumptions and ATP production are closely coupled in a functional brain as illustrated by Fig. 1. This coupled metabolic reaction chain can be divided into two parts. One is an open system involving the cerebral metabolisms of glucose (from the food chain) and oxygen (from air), resulting in the final products of water and CO2 that are washed out into the blood stream. Another one is a close system involving extremely efficient cycling between the ATP generation in the mitochondria and utilization in the cytosol inside the cells. These two systems are integrated and work together to maintain normal brain function.
In comparison with the non-oxidative glycolysis, oxidative phosphorylation produces at least 15 times more ATP molecules. Therefore, brain function relies on the ATP energy and the ATP generation relies heavily on the oxygen metabolism in mitochondria. Abnormalities in brain metabolisms, in particular, related to oxidative phosphorylation have been linked to numerous brain disorders and neurodegenerative diseases such as: schizophrenia, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, mitochondrial dysfunction and aging problems (e.g., [15–19]). There are evidences indicating that the activity of cytochrome oxidase, a key mitochondrial enzyme that catalyzes the reduction of oxygen to form water, is significantly impaired in schizophrenic [17] and Alzheimer’s patients [18, 19]. Also the studies of diseases caused by mitochondrial DNA mutations suggests that a variety of neurodegenerative processes may be associated with defects in mitochondrial oxidative phosphorylation [20, 21]. Therefore, the cerebral metabolic rate of oxygen may provide an early biomarker of pathophysiology change in various brain disorders.
2.2 Concept of studying oxygen metabolism using 17O NMR
The metabolic processes as illustrated in Fig. 1 can be assessed with various in vivo MR spectroscopy (MRS) techniques. For example, the glucose metabolism has been examined by in vivo 1H and 13C MRS [22, 23], and the ATP metabolism can be studied using in vivo 31P MRS in combination with the magnetization transfer method [24–26]. In principle, in vivo 17O MRS should be able to directly study the oxygen metabolism based on the following simple chemical reaction and the use of 17O-isotope labeled oxygen gas (17O2):
| [1] |
Similar to what has been done with 15O Positron Emission Tomography (PET) [27, 28], when oxygen gas enriched with the MR detectable 17O isotope is introduced into the animal or human body, it binds to the hemoglobin in the blood through lung exchange, and then enters the brain via the arteries and blood circulation. The 17O-labeled oxygen molecules will be metabolized in the brain mitochondrion to produce 17O-labeled water (H217O) molecules. The production rate of the metabolized H217O water reflects the rate of oxygen metabolism in the brain tissue. Thus, using the 17O MRS or imaging technique to monitor the dynamic change of the H217O water content in the brain will provide important information regarding the oxygen metabolism of the brain tissue.
2.3 Early attempts of in vivo 17O NMR studies
The simple concept of utilizing 17O MR to study oxygen metabolism in living species had been recognized many years ago and attempted by several research labs around the world. In the late 1980s and early 1990s, 17O-enriched H217O water was used as a potential T2 (transverse relaxation time) contrast agent for the proton MRI by Hopkins’ group and others for studying tissue perfusion in various animal models [29–33]. Meanwhile, Mateescu et al. demonstrated the in vivo 17O MRS detection of nascent mitochondrial water in larva and mouse breathing air with 17O-enriched oxygen gas [34, 35]; Aria et al. explored the feasibility of in vivo 17O NMR for estimation of cerebral blood flow (CBF) and oxygen consumption in animal models of rat, rabbit and dog [6, 7, 36]; Pekar et al. reported that coarse CMRO2 images (0.8cc nominal resolution) can be obtained in the cat brain using 17O NMR imaging and 17O-enriched oxygen gas [5, 37]; and Fiat et al. examined possible methods for determination of CMRO2 and CBF in animal brain (and potentially in human brain) using in vivo 17O MRS/MRI [38–40]. On the other hand, in the middle and late 1990s, Ronen et al. and Reddy et al. suggested that the 17O-labeled H217O water could be detected by spin-echo proton imaging with 17O decoupling [41–45] or proton T1ρ dispersion imaging [46, 47], respectively, and these indirect 1H-(17O) methods were used to image the H217O distribution in phantoms and animals.
Although these early efforts had demonstrated the important concept and feasibility of 17O NMR for assessing brain oxygen metabolism, the technology advancement had been hampered substantially by the limitations in the sensitivity for detecting the metabolically generated H217O water, as well as the practical applicability for general in vivo studies of the oxygen metabolism in animal or human brain.
3. 17O NMR for studying brain oxygen metabolism
In order to advance the 17O NMR technology for studying brain oxygen metabolism, it is necessary to thoroughly understand the basic aspects of the 17O NMR; for instance, the relaxivity and sensitivity of the 17O signal as well as the factors that influence these properties. In addition, it is important to understand the pros and cons of the different approaches for detecting the H217O signal and find an optimal method for imaging the dynamic changes of the H217O water in vivo. Furthermore, it is essential to develop a reliable method for quantifying the cerebral metabolic rate of oxygen based on 17O MR measurements of the metabolized 17O water signal. Finally, it is crucial to establish a simple and completely non-invasive CMRO2 imaging approach for animal and human applications.
3.1. 17O NMR properties in a biological system
17O is a stable isotope of oxygen existing in nature. Unlike the common, abundant form of oxygen (16O), 17O is the only oxygen nuclei with a magnetic moment that can be detected by NMR. Different from the nuclei of 1H, 31P and 13C used for most in vivo MR applications, 17O has a spin quantum number of greater than ½ (I = 5/2) and possesses an electric quadrupolar moment. The 17O nucleus in water can interact with the two protons, resulting in an 17O signal being a 1:2:1 triplet at extremely low water concentration [48]. However, the 17O triplets collapse to one single and well-defined H217O resonance peak in a biological sample due to rapid proton exchange and quadrupolar relaxation leading to a large effect of line broadening. This feature simplifies the 17O MRS pattern for detecting the 17O water signal in biological systems.
The 17O natural abundance is only 0.037%, which is almost 30 times lower than that of 13C and 2700 times lower than that of 1H and 31P. Moreover, the magnetogyric ratio (γ) of the 17O, which is proportional to the Larmor frequency, is 7.4 times lower than that of 1H. These facts result in the lowest NMR receptivity for 17O spin compared to other spin nuclei commonly used for biomedical research and clinical studies. This low inherent NMR sensitivity has hindered the progress of 17O NMR for in vivo MR studies especially at relatively low magnetic fields, despite its great potential for providing unique and vital biological information.
3.1.1. Relaxation properties of 17O in tissue water
One critical aspect of 17O NMR for in vivo applications is the 17O relaxivity of water in biological samples. Relaxivity can affect the NMR detection sensitivity and ultimately determines the usefulness and applicability of in vivo 17O NMR. The 17O quadrupolar moment can interact with local electric field gradients and the temporal fluctuation in this interaction induced by molecular motion can dominate the 17O relaxation processes and determine both the longitudinal relaxation time (T1) and the transverse relaxation time (T2) [49]. In the case of the water molecule, in which the extreme narrowing limit (i.e., τcω ≪ 1, where τc is the rotational correlation time and ω is the 17O Larmor frequency in radian units) is approximately applicable (except for the very small fraction of bound water), the values of 17O T1 and T2 can be approximated by:
| [2] |
where the term (e2Qq/h) is the 17O quadrupolar coupling constant for bulk water, and η is an asymmetry parameter (0≤ η≤1) [49]. Thus, the 17O T1 and T2 values can be estimated by:
| [3] |
Because the variables in Eq. [3] are independent upon the magnetic field strength (B0), 17O T1, T2 as well as apparent T2 (T2*) are expected to be insensitive to B0. Taken the literature values of η=0.7, e2Qq/h = −8.1 MHz and τc = 2.7×10−12 s for bulk water at 25°C [50], the estimated 17O T2 and T1 according to Eq. [3] should be approximately 5.1 ms. This estimation indicates that the 17O relaxation times of water are extremely short, in the range of few milliseconds.
The prediction of equal 17O T1 and T2 values for the water molecules using Eq. [3] relies on the approximation of neglecting the effect of the 17O-1H scalar coupling. The actual 17O water T2 (or T2*) value in a biological sample or water solution should be smaller than the 17O T1 value because of the combined effects of the 17O-1H scalar coupling and the proton exchange on the 17O transverse relaxation process [51]. The proton exchange rate between H217O and H216O is sensitive to pH. At near-neutral pH, the scalar coupling has a maximal effect for enhancing the apparent 17O transverse relaxation rate.
The 17O longitudinal and transverse relaxation times of the natural abundance water in the rat brain have been explicitly measured and compared at field strengths of 4.7T versus 9.4T [52]. The relaxation times were found to be field independent (T2=3.0 ms, T2*=1.8 ms and T1=4.5 ms at 4.7T versus T2=3.0 ms, T2*=1.8 ms and T1=4.8 ms at 9.4T) [52]. These experimentally measured 17O relaxation times are in line with the predicted values according to Eq. [3] and the reported values in the literature [38, 53]. Recent experimental evidence has indicated that the field-independence of the 17O relaxivity can hold at much higher magnetic fields [54].
3.1.2. Pros and cons of extremely short 17O-water relaxation times
The very short 17O T1 values of the brain H217O (several milliseconds) [38, 52] allow rapid NMR signal acquisitions at a given sampling time, thus, gaining signal-to-noise ratio (SNR) per unit time. The repetition time for acquiring the 17O signal can be pushed to as short as tens of milliseconds. The major constraint is the potential concern of the specific absorption rate (SAR: a measure of the rate of absorption of RF energy in the body) allowed by FDA regulation.
The extremely short 17O T2* of the brain H217O (~2 ms) results in a broadening of the water resonance peak to a line width of 100–200 Hz in vivo and a substantial SNR reduction. It is crucial to minimize the delay (or echo time) between the 17O spin excitation pulse and NMR signal sampling in order to avoid a substantial loss of the 17O signal due to the rapid T2* decay. The linewidth of the 17O resonance peak of H2O is relatively insensitive to the B0 inhomogeneity (hence shimming quality) because of the intrinsically broad linewidth and the much lower 17O magnetogyric ratio (7.4 times lower than that of 1H). This fact implies that the requirement for B0 homogeneity either in the bare magnet or together with room temperature shim compensation is considerably less stringent for in vivo 17O NMR compared to in vivo 1H, 31P or 13C NMR.
3.1.3. Advantage of 17O NMR at high/ultrahigh magnetic field
One of the most important advantages provided by high/ultrahigh magnetic fields is the potential gain in NMR sensitivity. This is particularly crucial for in vivo 17O NMR, where the inherent NMR sensitivity is extremely low. For a magnetic nucleus, the optimal SNR of the NMR signal acquired within a unit time at a given field strength depends on T1, T2*, B0 and the RF coil quality factor (Q) according to [55–58]:
| [4] |
The parameter β was suggested to be approximately 7/4 based on theoretical predictions [56, 58]. Unlike the water proton spins in biological tissues, which are characterized by longer T1 and shorter T2 (or T2*) with increased field strength, the field independence of 17O relaxivity implies that the 17O-water sensitivity gain at higher fields is not compromised by the relaxation times. Although the short T2* (or broad linewidth) of the 17O resonance peak in H217O leads to an effective reduction in the 17O NMR sensitivity, this reduction can be partially compensated by the extremely short 17O T1 (< 5 ms in the brain), allowing rapid signal averaging [38, 52]. Therefore, it is possible to achieve a large sensitivity gain for in vivo 17O NMR at high/ultrahigh fields.
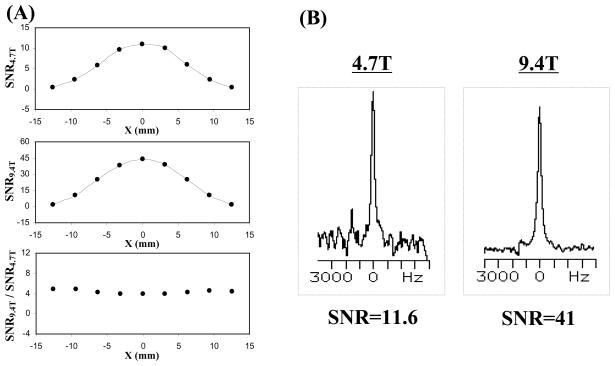
The in vivo 17O NMR sensitivity has been quantitatively compared at field strengths of 4.7T versus 9.4T [52]. The striking finding from this study, as shown in Fig. 2, is the consistent observation of approximately four-fold SNR gain at 9.4T compared to 4.7T indicating an approximated 7/4th power dependence of 17O SNR on B0 as predicted by the NMR theory [52, 56, 58]. These results demonstrate the significant advantage provided by high field strength for the direct detection of 17O NMR signal. The trend for increasing 17O NMR sensitivity is likely to hold much beyond the field strength of 9.4T [54]. Compare to in vivo 1H, 31P or 13C NMR, in vivo 17O NMR is likely to benefit the most from the increasing field strength in terms of the NMR sensitivity gain.
Fig. 2.
(A) One-dimensional SNR profiles of 17O-water signal in the rat brain at 4.7T and 9.4T, and the SNR ratio between 9.4T and 4.7T; (B) Single voxel 17O-MR spectrum of H217O signal obtained from rat brain at 4.7T and 9.4T, respectively (total acquisition time of 15 s, and nominal voxel size of 16 μl. Adapted from Zhu et al. MRM 2001; 45: 543–549.
3.1.4. In vivo NMR invisibility of 17O2
In contrast to the 15O-PET approach which is unable to distinguish the signals emitted by the 15O atoms in the 15O2 molecules from those in the metabolically generated H215O molecules; the 17O resonance peak of the 17O2 molecules, when bound to hemoglobin in the blood, is extremely broad because of very slow rotational motion of the large HbO2 complex, and very difficult to detect with conventional in vivo 17O NMR approaches. Saturation transfer electron paramagnetic resonance studies have shown that the τc value for the rotational motion of the hemoglobin molecule is 2·10−8 s in solution and increases to 8·10−6 s when the hemoglobin molecule is encapsulated within the erythrocyte [59]. This τc value is approximately 106 times slower than that of the free water. Such slow rotational motion leads to extremely fast 17O T2 relaxation according to Eq. [2] and renders the 17O2 molecule bound to hemoglobin invisible for in vivo 17O NMR detection.
The 17O2 molecule while it is in the gas phase or dissolved in water is strongly paramagnetic due to its two unpaired electrons, and hence is undetectable in conventional NMR measurement because of the strong dipolar coupling between the electrons and the 17O nucleus. Thus, the direct in vivo 17O NMR approach will detect only H217O but not 17O2 in the biological sample.
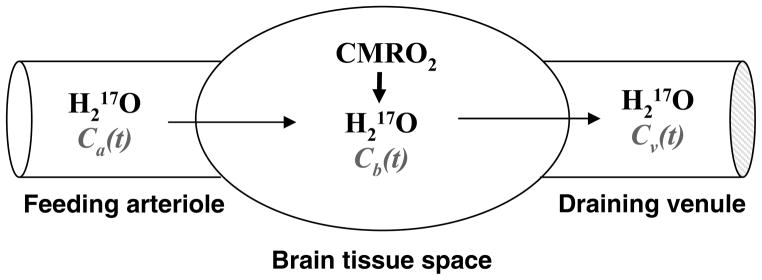
This unique MR specificity for detecting 17O-labeled water significantly simplifies the methodology for measuring and quantifying cerebral metabolic rate of oxygen (CMRO2) because all 17O-labeled components other than the metabolically generated H217O can be ignored, leading to a simple quantification scheme as illustrated in Fig. 3 [60].
Fig. 3.
Schematic illustration of a “complete model” describing three parallel processes of the 17O-labeled metabolic water (H217O) occurring in the brain when the 17O-labeled oxygen gas molecules are introduced via an inhalation. In this model, only the metabolic H217O is considered because the 17O-labeled O2 is invisible by in vivo 17O NMR. Ca(t), Cb(t) and Cv(t) stand for the H217O concentration in arteriole, brain tissue and venule, respectively, as a function of the 17O2 inhalation time.
3.2. Influence of 17O-1H scalar coupling on 1H relaxation of water
The existence of an 17O spin in the tissue water, either in natural abundance or at elevated enrichment levels, will affect the 1H relaxation properties of the tissue. Half a century ago, Meiboom studied the proton chemical exchange in 17O-enriched water solution at pH of neutral range [51]. It was found that the spin-spin coupling (i.e. scalar coupling) between the 1H and the 17O spins could shorten the proton transverse relaxation time or the rotating frame spin-lattice relaxation time (T1ρ) but not the longitudinal relaxation time (T1), and such scalar interaction of 17O-1H was modulated by the fast proton chemical exchange between the H217O and H216O molecules. Meiboom’s finding provided a mechanism for indirect detection of the H217O signal using 1H MR imaging. This indirect 1H-(17O) MR approach was anticipated to better inherent NMR sensitivity than that of the direct 17O MR approach [41].
3.2.1. Effect of 17O-1H scalar coupling on proton T2 relaxation of tissue water
The influence of the 17O-1H scalar coupling on the 1H T2 value (T2,H) of the tissue water can be quantitatively described by [29, 41, 42, 51, 61]:
| [5] |
where a 1H chemical shift difference between H217O and H216O is neglected; and are the proton transverse relaxation times of H217O and H216O, respectively; P is the molar fraction of H217O and is equivalent to the 17O enrichment fraction; τ is the characteristic proton exchange lifetime in H217O and J is the 17O-1H scalar coupling constant. The relation described by Eq. [5] provides the basis for assessing the fractional content of H217O water (i.e., P in Eq. [5]) through the change in proton T2.
It was experimentally verified that the proton transverse relaxation rate indeed linearly correlates with the H217O concentration in biological solutions up to 5% enrichment, whereas the proton longitudinal relaxation time was not significantly affected by the 17O enrichment [29]. Therefore, 17O-labeled H217O can be used as an exogenous and/or endogenous contrast agent, respectively, for cerebral blood flow (CBF) and CMRO2 measurements through measuring the change in the T2-weighted 1H MRI signal using a spin-echo sequence, for instance.
Several early studies observed proton signal reduction in the brain after the introduction of 17O-labeled water [7, 29–32, 62], and its change can be linked to cerebral blood perfusion at different physiological conditions in normal and ischemic brain (e.g., [31]). The time courses of the signal changes were successfully applied to quantify and image CBF for a wide range of CBF values induced by hypercapnia and hypocapnia in animals [7, 32]. Subsequently, this indirect 17O detection approach was applied for imaging the signal change of the H217O metabolized from the 17O-labeled oxygen gas [36, 63]. However, all these early studies were conducted in a qualitative manner and it was difficult to provide an absolute concentration of the 17O-labeled water in the brain. Though this limitation should not be a problem for quantifying CBF in which only the relative concentration of H217O water in the brain is required, it does pose a hurdle for quantifying CMRO2.
One improved method for quantifying the H217O tracer concentration is to introduce another proton spin-echo MRI in the presence of 17O decoupling during the echo time (TE) and/or acquisition time [41–45]. The 17O decoupling can abolish the interaction of the 1H-17O scalar coupling, and ultimately, suppress its effect on the water proton T2 relaxation and increase the T2 value. The difference of the proton spin-echo signals of H217O water in the presence and absence of 17O decoupling provides a simple connection with the H217O content. The absolute H217O concentration can be calibrated by additional paired measurements of natural abundance H217O with a known concentration (i.e., P = 0.037%) before introducing the H217O tracer. This indirect 17O approach with 17O decoupling was tested with phantom solutions and tissue models [41, 43–45]; it was also examined in the rat brain under normal physiological condition [42] and during focal cerebral ischemia [64] by injection of 17O-labeled water tracer into the rat body. The results indicate that when an adequate amount of metabolic H217O is present in the brain, this indirect in vivo 17O approach could potentially be used for quantifying and imaging CMRO2.
3.2.2. Effect of 17O-1H scalar coupling on proton T1ρ relaxation of tissue water
Another alternative indirect 17O approach is to detect H217O by using the proton T1ρ dispersion imaging method [46, 47, 65]. This method applies single proton radiofrequency (RF) channel for acquiring T1ρ-weighted proton images with two different spin locking RF powers. Although the mechanism underlying the T1ρ contrast in biological samples is not fully understood, the major contribution is likely to be from proton exchange [66], which can be potentially linked to the H217O concentration through 17O-1H scalar coupling and the chemical exchange between H217O and H216O. Such an exchange, however, may not be the only process occurring in biological samples, and this complicates the T1ρ method and quantification for determining the H217O concentration. An example of this complication is the observation that T1ρ contrast changes in the animal brain during ischemia even without 17O-labeled water tracer [67, 68]. Another complication is the difficulty of obtaining prior information on the intrinsic T1ρ dispersion of tissue, which is needed for absolute quantification. Nevertheless, this method has been successfully applied to determining CBF where the absolute H217O concentration is not required [69], and to imaging the CBF changes in tumors [70]. Recently, it has also been attempted for estimating the CMRO2 in the animal brain [71–73].
3.2.3. Pros and cons of proton MRI for indirectly detecting 17O-labeled metabolic water
The major advantages of indirect 1H-(17O) approaches for detecting 17O-labeled water in brain tissue is the high sensitivity of the proton signal and the ability to apply conventional MRI acquisition and data processing methods. In addition, the proton T1ρ dispersion imaging approach requires only a single proton RF channel and can be implemented on a clinical MRI scanner.
However, caution should be exercised when absolute quantification of the H217O concentration in a biological sample is required. Both 17O-1H scalar coupling and the chemical exchange between H217O and H216O, the mechanisms responsible for the indirect 17O detection, are all sensitive to many physiological parameters such as pH and temperature. This difficulty is evident from the experimental observations that equal concentrations of H217O tracer do not produce the same magnitude of T2 change (or T2-weigthed proton signal) in different physiological environments [29], and the detected change can completely disappear when pH is shifted away from neutral [64].
Furthermore, it should also be noted that although the intrinsic proton signal of the tissue water offered by indirect 1H-(17O) approach is much higher than the 17O signal measured by the direct 17O approach, the reproducibility or reliability of the 17O-water signal detection in consecutively acquired datasets is far more crucial than the absolute signal (or SNR) acquired in a single dataset. This is because the 17O-MR based CMRO2 imaging approach relies on measuring the small dynamic changes of the metabolically generated H217O and their spatial distribution. In addition, the actual 1H signal that is relevant to the tissue H217O content could be much smaller than the total available 1H signal. For example, it has been shown that a 10% signal increase in the T2-weighted 1H MRI corresponded to about 0.45% of H217O content in the rat brain, which is over twelve times that of the natural abundance H217O level [42]. Therefore, the proton signal changes due to the metabolically generated H217O water in the T2- or T1ρ-weighted MR imaging, which is expected to be in the range of 1%, will likely be compromised by the signal fluctuation caused by the physiological noise (e.g., respiration or pulsation) or scanner instability [13].
Another potential technical limitation posed by both 17O-decoupled and T1ρ-based 1H MRI approaches is the requirement of relatively large RF power either for 17O decoupling or for the proton spin locking, in particular, for human applications at high magnetic fields.
3.3. Theory and quantification of CMRO2 based on in vivo 17O MRS/MRI approach
3.3.1. Theory and quantification model
As illustrated in Fig. 3, the dynamic change of the metabolically generated H217O concentration in the brain during an 17O2 inhalation is affected by three parallel processes: (i) cerebral oxygen utilization for generating the metabolic H217O in the brain tissue; (ii) cerebral blood perfusion resulting in H217O washout from the brain, and (iii) blood recirculation bringing the metabolically generated H217O in the entire body back to the brain. All contributions from these three processes have to be considered for quantifying CMRO2. Based on the Kety - Schmidt theory [74], the mass balance of the 17O-isotope labeled H217O in the brain tissue during an 17O2 gas inhalation can be derived as [5, 39, 60, 75]:
| [6] |
where Ca(t), Cb(t) and Cv(t) are the metabolic H217O concentrations in excess of the natural abundance H217O concentration in the arterial blood, brain tissue and venous blood; respectively, as a function of 17O2 inhalation time (t, unit = minute); α(t) is the 17O enrichment fraction of the oxygen atoms in the inhaled 17O2 gas which could vary with inhalation time; and the factor of 2 accounts for the fact that two H2O molecules are formed from one O2 molecule through the oxidative metabolism according to Eq. [1]. Two unit conversion factors, f1=1.27 and f2=1.05, are used to achieve consistency of units among all parameters used in Eq. [6] [37, 60, 75].
The natural abundance H217O concentration can be used as an internal reference to calibrate the absolute values of Cb(t), Ca(t) and Cv(t) with preferred units of μmol/(g brain water) for Cb(t), μmol/(g blood water) for Ca(t) and Cv(t); and leads to the CMRO2 unit of μmole/min/(g brain tissue).
Therefore, the CMRO2 values can be precisely calculated by solving the linear differential Eq. [6] if the parameters of Cb(t), α(t), CBF, Ca(t) and Cv(t) are known or can be measured.
3.3.2. CMRO2 quantification for small animal models
The methods for quantifying CMRO2 in the small animal case were first examined by Pekar and Fiat et al. [5, 37, 39, 40, 76]. The complete model of CMRO2 quantification in the small animal was clearly demonstrated and established by Zhu et al. for fast imaging of CMRO2 in rat brain within a few minutes of 17O2 inhalation at high field [75]. In this comprehensive study, all parameters involving the oxygen metabolism and perfusion of the brain tissue as shown in Eq. [6] were experimentally determined via independent and concurrent 17O MR measurements in the rat brain at 9.4T [75].
For a small animal such as a rat, due to its fast respiration and high heart rate, the labeled 17O2 gas once introducing into the body, will quickly replace the regular 16O2 gas to produce H217O water. Thus, the 17O enrichment fraction can be approximated as a time independent constant, i.e. α(t) ≈α, and the transition time for 17O2 to replace the 16O2 gas is negligible compare to the total 17O2 inhalation period (≥ few minutes). If one assumes that the water in the brain tissue is in equilibrium with water in the venous blood, then f2Cv(t) = Cb(t)/λ where λ is the brain/blood partition coefficient (≈ 0.90) with the unit of (ml blood)/(g brain tissue) [77]. Substituting this relation and introducing two new correction factors (n and m) into Eq. [6] leads to
| [7] |
The correction factor m accounts for the water permeability restriction across the blood-brain burrier (BBB) [78]; and n accounts for the permeability restriction when the metabolically generated H217O molecules inside the mitochondria crosses the mitochondrial membranes [60, 75]. Both m and n depend on the CBF [60, 75]. The function of Ca(t) (or artery input function) is determined by the total metabolic H217O generated in all aerobic organs of living body. It approximates as a linear function of 17O2 inhalation time (i.e., Ca(t)≈At, where A is a constant) [5, 6, 60, 75]. Thus, the solution for Eq. [7] becomes:
| [8] |
According to this equation, the CMRO2 value at each data point measured at different inhalation time (t) can be precisely calculated using the experimentally measured CBF, A and n values, Cb(t) time courses and other known constants (f1, f2, m, α and λ) [13, 60, 75].
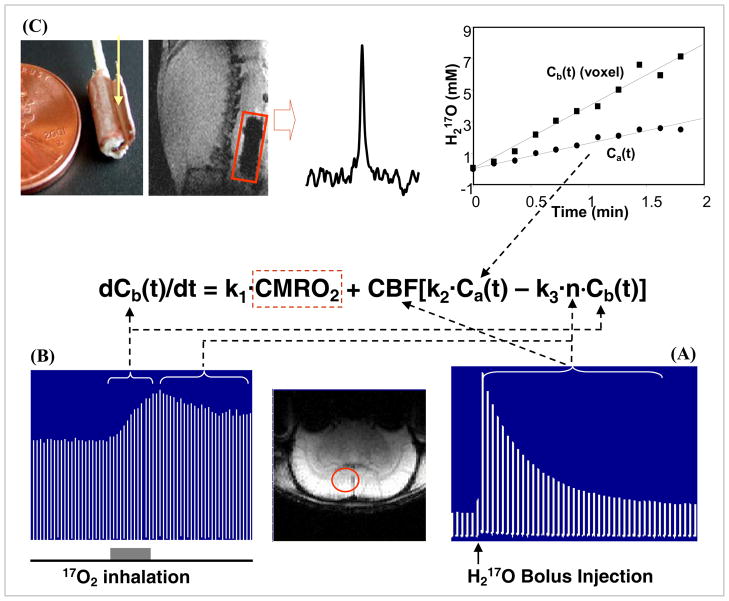
Fig. 4 summaries the multiple in vivo 17O MR measurements performed on anesthetized rat brains for imaging and quantifying CMRO2 [13, 75]. The CBF measurement was performed via bolus injection of a small amount of 17O-enriched H217O into one internal carotid artery and monitoring the washout process of the H217O tracer in the animal brain using 3D 17O chemical shift imaging (CSI) [52]. Fig. 4A demonstrates the stacked plots of H217O spectra acquired from a single voxel of a 3D 17O CSI data set in a representative rat before and after the H217O bolus injection. The peak height of the H217O spectra shows an exponential decay and its decay rate determines the CBF value in the CSI voxel [13, 52, 75]. The crucial step for CMRO2 measurements is to monitor and image the dynamic changes of the metabolic H217O content in the brain (i.e., Cb(t)) during an inhalation of 17O2 gas. Fig. 4B illustrates the stacked plots of 17O spectra of cerebral H217O from one representative CSI voxel acquired before, during and after a 2-minute inhalation of 17O2 [75]. It indicates excellent 17O NMR sensitivity for detecting the cerebral H217O signal and its change during the inhalation; and the approximately linear increase of brain H217O during a short 17O2 inhalation is evident, and the slope is tightly coupled to CMRO2. The arterial input function Ca(t) was measured in vivo by an implanted 17O RF coil [79] wrapped around a carotid artery. Fig. 4C illustrates the implanted 17O RF coil, the natural abundance H217O signal detected only from the rat carotid blood and the Ca(t) time course measured during a two-minute inhalation of 17O2 [75]. The experimental results show an approximately linear relation between the arterial H217O concentration and the 17O2 inhalation time, and the linear regression of Ca(t) gave the value of the constant A required by Eq. [7] and Eq. [8]. Finally, the ratio between the decay rates of H217O signal measured after the cessation of 17O2 inhalation (see Fig. 4B) versus that after a H217O bolus injection (see Fig. 4A) gave the value of the constant n reflecting the H217O permeability restriction across the mitochondrial membranes [60, 75].
Fig. 4.
Schematic diagram showing the multiple in vivo 17O measurements at 9.4T for determining CMRO2 using the complete model according to the mass balance equation of Eq. [7] which links Cb(t), Ca(t), CBF and n with CMRO2. To simplify the equation, three known constants of 2αf1, mf2 and m/λ used in Eq. [7] are replaced by k1, k2 and k3, respectively. (A) Stacked plot of the 17O spectra of cerebral H217O tracer from one representative voxel as indicated by the circle in the anatomical brain image (low center insert). The spectra were acquired before and after a bolus injection of H217O for CBF measurements. (B) Stacked plot of the 17O spectra of the metabolic H217O from the same voxel acquired before (natural abundance), during (as indicated by the gray bar under the stacked plot) and after a 2-minute 17O2 inhalation. (C) Measurement of Ca(t) by using an implanted 17O RF coil (the left insert). The middle insert illustrates an 17O spectrum of natural abundance H217O obtained from the rat carotid artery blood with the implanted coil before 17O2 inhalation. The right insert shows the time course of Ca(t) (circle symbol) and Cb(t) from a representative 3D 17O CSI voxel (square symbol) in the same rat during the 17O2 inhalation. Finally, the ratio between the 17O signal decay detected after a bolus injection of H217O (see Fig. 4A) versus the 17O signal decay detected after the cessation of 17O2 inhalation (see Fig. 4B) gives the constant of n. Adapted from Zhu et. al. PNAS 2002; 99: 13194–13199.
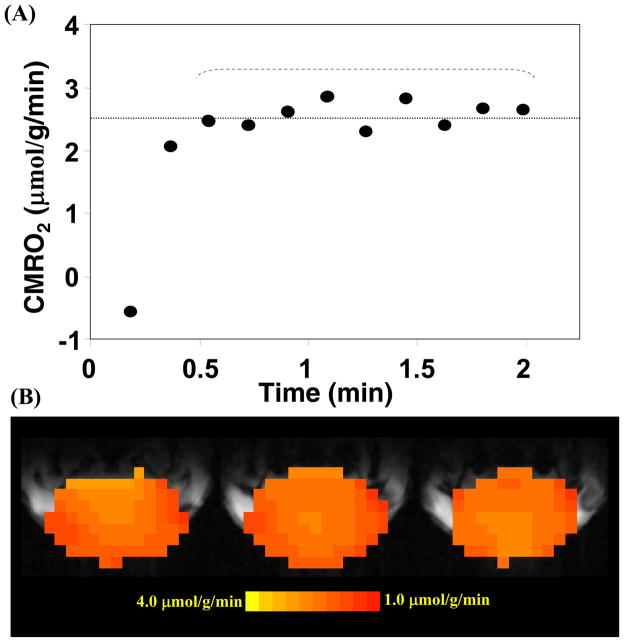
The CBF measurement can be performed concurrently with the CMRO2 measurement on the same animal while Ca(t) (see Fig. 4C) and Cb(t) (see Fig. 4B) measurements can be conducted simultaneously with the configuration of dual 17O RF coils and receivers [79]. The values of Cb(t), CBF and n measured from each 17O MRS imaging (MRSI) voxel and the value of A measured from each 17O inhalation measurement in the same animal as demonstrated in Fig. 4 can be used to calculate the absolute CMRO2 value as a function of inhalation time according to Eq. [8]. Fig. 5A shows one example of CMRO2 time course from a representative 17O-CSI voxel with a temporal imaging resolution of 11 seconds [75]. It is evident that the CMRO2 values are independent of the 17O2 inhalation time if the first two CMRO2 values characterized with relatively large fluctuations are excluded. These CMRO2 values were averaged for improving measurement accuracy. The same procedure and calculation can be applied to all 17O CSI voxels for generating 3D CMRO2 images in the rat brain [12, 75]. Fig. 5B demonstrates three adjacent CMRO2 images in the coronal orientation from a representative rat brain. The averaged CMRO2 and CBF values in the rat brains anesthetized with α-chloralose were found to be 2.19±0.14 μmol/g/min and 0.53±0.07 ml/g/min (n=7), respectively [75]. These results are consistent with the literature reports using other independent techniques under similar physiological condition [80, 81].
Fig. 5.
(A) Plot of the calculated CMRO2 values using the complete model as described by Eq. [8] as a function of 17O2 inhalation time. (B) Three-dimensional coronal CMRO2 images of rat brain measured by in vivo 17O MRS approach during a 2-minute 17O2 inhalation at 9.4 Tesla. Adapted from Zhu et. al. PNAS 2002; 99: 13194–13199.
3.3.3. CMRO2 quantification in humans
Unlike small animals, quantification of CMRO2 in humans faces serious challenges. The human body size, lung capacity and respiration rate as well as blood circulation speed are drastically different from those in the small animal. It is expected that the exchange process between non-labeled and inhaled 17O-labeled oxygen gases in a human lung will be much slower compared to small animals such as a rat or mouse. Moreover, a much longer blood circulation time through the human body could further slow down the binding process of inhaled 17O-labeled oxygen to the hemoglobin in the blood stream. Thus, the 17O fractional enrichment of the oxygen gas in human artery blood (i.e., the α(t) term in Eq. [6]) will take much longer to reach a steady-state level (i.e., the 17O enrichment α of the inhaled 17O2 gas). For this reason, the CMRO2 quantification model that worked well for the rat brain [75] failed to provide acceptable CMRO2 values for a human study in which a short inhalation of 17O2 gas (2–3 minutes) was employed [82].
Recently, Atkinson and Thulborn proposed a three-phase model for quantifying CMRO2 in the human brain based on in vivo 17O and 23Na MR imaging data obtained at 9.4T [83]. In this model, the dynamic change of the H217O water in brain tissue was separated into three-phases: i.e., prior, during and after inhalation of 17O2 gas; two rate constants KL and KG were utilized to represent the loss and gain of the H217O water within the imaging voxel, respectively; and a mass balance equation similar to Eq. [6] was used to describe the amount of the 17O-labeled water in each voxel at each phase where the brain mass of the voxel was computed from co- registered 23Na MRI data. The CMRO2 as well as the rate constants KL and KG values for each imaging voxel were determined by performing a least-square fit of the dynamic H217O data for all three-phases. The key component of this model for applying to the CMRO2 quantification in human is that it considered the transition of the 17O fractional enrichment of the 17O2 gas in arterial blood (i.e. the α(t) term of Eq. [6]). The transitions of α(t) from zero to α during the inhalation phase and from α to zero during washout phase after the inhalation were estimated based on the parameters of the pulmonary arteriovenous difference fraction (FA-V) and the mean blood circulation time (TC), in which their values were approximated from the literature reports [83].
Although there are still many uncertainties in the CMRO2 quantification model for human application, especially for determining and validating the fractional enrichment of the 17O2 gas α(t), the work of Atkinson and Thulborn does provide a forward step towards the quantitative study of the oxygen metabolism in human brain using the in vivo 17O MR imaging approach at high/ultrahigh field.
3.4. Establishing a robust and noninvasive 17O MR method for Imaging CMRO2
3.4.1. Methods for imaging dynamic H217O water content of the brain tissue
The most crucial measurement for determining the CMRO2 value is to monitor the dynamic change of the H217O water content in brain tissue. Therefore, establishing a robust CMRO2 imaging approach relies on the ability to reliably image the dynamic change of the H217O signals in the brain tissue with reasonable spatial and temporal resolution.
For the indirect 1H-(17O) detection approach, conventional T2-weighted spin-echo or T1ρ- weighted MR imaging technique can be used to acquire the proton signal change related to the variation in H217O water content (see detailed discussion in Section 3.2.). For direct 17O-MR detection approach, however, the conventional MR imaging technique is no longer suitable for imaging the H217O signal because the T2 (or T2*) relaxation time of the H217O is extremely short, leading to severe signal loss or even disappearance if the echo time of the MR imaging sequence is relatively long compare to the transverse relaxation time of the 17O-water (in the few millisecond range, see details in Section 3.1.).
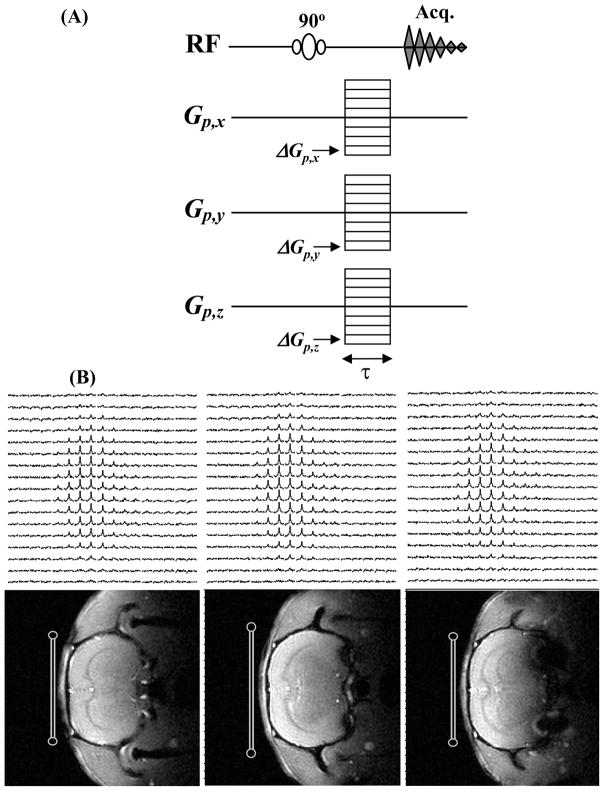
So far, two MR imaging approaches with ultra-short echo time capability have been used for directly imaging the 17O-water signal. One approach applies the flexible twisted projection [84] or a density-adapted 3D radial pulse sequence [85] commonly used for acquiring sodium MRI data; and the other approach images the H217O signals directly using the 3D chemical shift imaging (CSI) technique [86, 87]. Fig. 6 illustrates a typical 3D CSI sequence (Fig. 6A) used for the in vivo 17O MR measurement and an example of the 1H anatomic image as well as 3D 17O-CSI data of the natural abundance H217O signal obtained from a representative rat brain (Fig. 6B). These in vivo 17O-MR image data were acquired with approximately 0.1 ml of spatial resolution (nominal resolution: ~40μl) and total acquisition time of ~11 seconds at 9.4T. Regardless of which imaging sequence is chosen, the sensitivity and reliability of the 17O-water signal obtained with the direct 17O imaging approach depends in large extent on the echo time used for the imaging assuming comparable spatial and temporal resolutions are applied.
Fig. 6.
(A) 3D chemical shift imaging sequence; and (B) 3D 17O CSI data of natural abundance H217O (top row) and corresponding 1H anatomical images (bottom row) of rat brain acquired at 9.4T. The RF 17O surface coil positions and cross sections are indicated in the images.
3.4.2. Feasibility of establishing a noninvasive CMRO2 imaging approach
As described earlier, the major technical limitation of the complete model for noninvasively determining CMRO2 is the requirement of invasive measurements (e.g., CBF, Ca(t), n). This will significantly limit the potential of this in vivo 17O neuroimaging approach for broad biomedical applications, especially in humans. Thus, it is crucial to examine the feasibility of developing a completely noninvasive 17O approach for imaging CMRO2. Attempts have been made to simplify the experimental procedures and the models for determining CMRO2 based on a number of approximations [5, 37, 39, 40, 60, 76].
One of these attempts, in which the invasive measurements could be eliminated completely by using the simplified model based on expanding Cb(t) as a polynomial [60],
| [9] |
In this expansion, the first-order (or linear) coefficient of a1 is directly proportional to CMRO2 according to the following equation |[60]
| [10] |
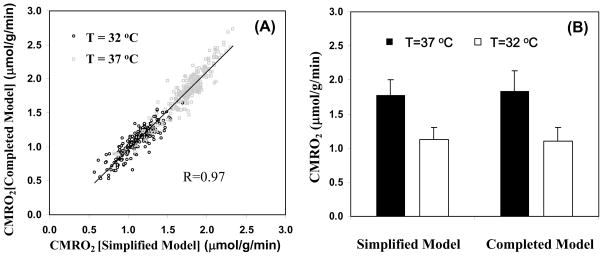
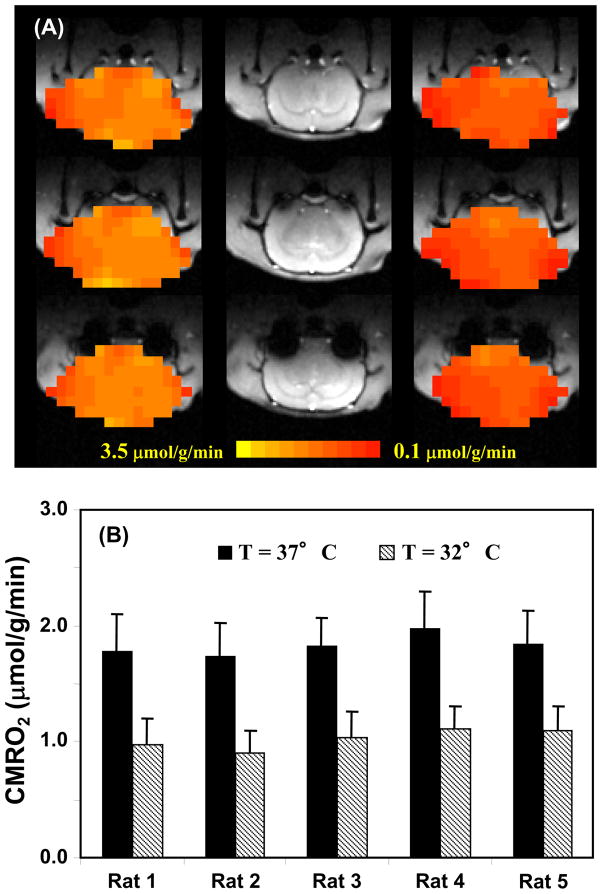
where α and f1 are known constants. Thus, using this simplified model, only the time course of Cb(t) measured noninvasively by in vivo 17O MR imaging is needed and it can be fitted to the polynomial function in Eq. [9] to calculate the linear coefficient of a1, and ultimately determining CMRO2 according to Eq. [10]. For practical applications with adequate SNR, a quadratic polynomial function usually provides a good approximation for fitting the time course of Cb(t) with a moderated fitting error |[60]. It has been demonstrated that the CMRO2 value obtained based on the complete model with invasive procedures has no statistical difference from that based on the simplified model and quadratic function fitting where only a single noninvasive measurement of Cb(t) is required |[60]. Moreover, the results also indicate that the linear fitting of Cb(t) could provide a good approximation for determining CMRO2 in the rat brain when the 17O2 inhalation time is relatively short (e.g., 2 minutes) |[60]. In another study, the simplified model was examined for determining CMRO2 under varied physiological conditions |[88]; and the CMRO2 results obtained with the complete model were compared with the simplified model using linear fitting of Cb(t) under normothermia (37°C) and hypothermia (32°C) condition, which is a well known factor leading to significant suppression of both CBF and CMOR2. Fig. 7 demonstrates an excellent consistency of the CMRO2 results between the complete and simplified models for either the voxel-based comparison (Fig. 7A) or the averaged CMRO2 comparison (Fig. 7B) at both brain temperatures |[88]. The comparison results reveal the validity of the simplified 17O approach for imaging CMRO2 applicable for small animal work across a wide physiological range
Fig. 7.
(A) Voxel based CMRO2 calculation and comparison using the completed and simplified models from a representative rat (total voxel number used was 224 for 32°C and 254 for 37°C, voxel size = 75μl ). (B) Averaged CMRO2 values in the same rat brain at normothermia (37°C) and hypothermia (32°C) condition, calculated with simplified and completed model, respectively. Adapted from Zhu et. al. JCBFM 2007; 27(6): 1225–1234.
The ability to non-invasively image CMRO2 in human brain is even more crucial. However, the above mentioned simplified method by linear or quadratic fitting of the Cb(t) data is no longer appropriate because of the time dependent 17O fractional enrichment of the 17O2 gas (see details in Section 3..3.3) in humans. The quantification method based on the three-phase model has the potential for non-invasively mapping CMRO2 in humans after careful validation and improvement |[83]. It is anticipated that additional technical developments which further advance the in vivo 17O methodology could ultimately provide the simplest and completely non-invasive 17O neuroimaging approach for imaging CMRO2 in both animal and human brains.
3.4.3. Reliability and reproducibility of the CMRO2 measurement
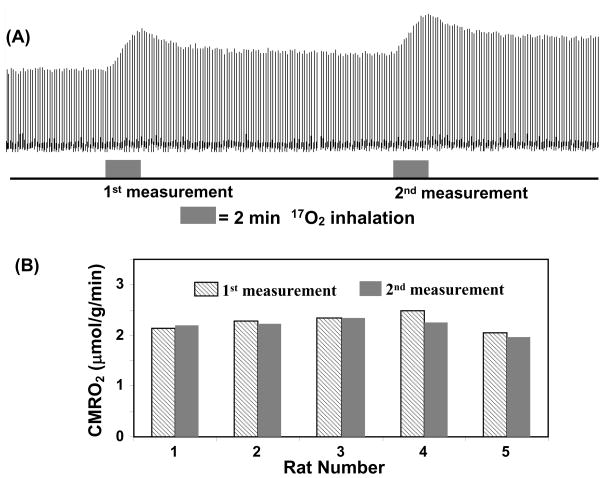
Another merit of the 17O CMRO2 imaging approach is its ability for performing repeated CMRO2 imaging measurements with a short interval between two measurements. This is because the cerebral H217O concentration can quickly reach a new and steady level after the cessation of a brief 17O2 inhalation, which allows repeated CMRO2 measurements in the same subject and experimental session (see Fig. 8A for an example). Fig. 8B shows the excellent reproducibility of repeated CMRO2 measurements in five rats (1st measured CMRO2 = 2.26 ± 0.18; 2nd measured CMRO2 = 2.20 ± 0.14 μmol/g/min; CMRO2 ratio between the 1st and 2nd measurements = 1.03 ± 0.05; n=5) where CMRO2 values were determined solely from the dynamic change of the 17O-water signals |[88]. The results demonstrate the robustness and reliability of the simplified in vivo 17O NMR approach for noninvasively and rapidly imaging CMRO2 repeatedly in the small brain of a rat. This capability is particularly valuable for studies aiming at CMRO2 changes induced by physiological or pathological perturbations in which multiple measurements are required under different conditions (e.g., control versus stimulation for brain function study). Therefore, it is likely that, at least in small animal brains, the combination of the simplified model and ultrahigh-field in vivo 17O MRS may provide an alternative neuroimaging modality for studying the central role of oxidative metabolism in brain function and neurological diseases |[12, 13].
Fig. 8.
(A) Stacked plots of H217O spectra from a representative voxel of 3D 17O MRSI data acquired before, during and after two consecutive 2-min 17O2 inhalations in a rat brain at 9.4T. (B) The comparison results between two repeated CMRO2 measurements in five rat brains. Adapted from Zhu et. al. JCBFM 2007; 27(6): 1225–1234.
3.4.4. Detectability of the CMRO2 change
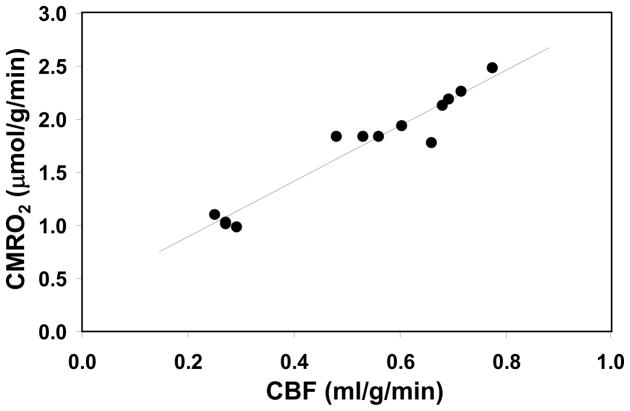
The detectability of the changes in CMRO2 is another important aspect of the high-field 17O-MR based CMRO2 imaging approach that requires careful evaluation for in vivo applications. It is well documented that the basal CMRO2 is sensitive to the brain temperature (see te>|[89, 90] and the references cited therein). Most studies reported in the literature, however, were based on the global CMRO2 measurements of entire brain using the Kety-Schmidt method te>|[74, 91] and were lacking spatial information regarding the regional oxygen consumption rate and/or its change. A CMRO2 imaging study using a 3D in vivo 17O-CSI approach was designed and conducted at 9.4T for quantifying absolute CMRO2 values in the rat brain at normal brain temperature (37°C) (i.e., normothermia) and mild hypothermia (32°C) conditions te>|[88]. Fig. 9A illustrates an example showing three representative slices of 3D CMRO2 images from a rat brain under normothermic and hypothermic conditions. These images clearly show significant reduction of CMRO2 crossing the entire brain induced by lowering brain temperature by several degrees. This metabolic suppression occurring at hypothermia was consistently observed in all five rats studied (Fig. 9B), resulting in an average of 45% CMRO2 reduction as compared to normothermic condition te>|[88]. These findings indicate that the established in vivo 17O MR imaging approach is sufficiently sensitive for determining the dynamic CMRO2 change and its spatial distribution resulting from physiological perturbations. Thus, the measured CMRO2 values can be quantitatively correlated with other associated physiological parameter(s). Fig. 10 illustrates one example showing the quantitative relation between CMRO2 and CBF: both of these were measured by the direct in vivo 17O MR approach in the α-chloralose anesthetized rat under a wide range of physiological conditions from normothermia to hypothermia te>|[88].. It clearly shows a strong correlation between CBF and CMRO2 with a linear correlation coefficient of R = 0.97 indicating a tight vascular-metabolic coupling in the rat brain.
FIG. 9.
(A) Anatomic images (middle column) of a representative rat brain and the corresponding multi-slices CMRO2 maps obtained using 3D 17O-CSI approach at 9.4T under normothermia (left column) and hypothermia (right column) conditions, (B) Summary of CMRO2 results measured at normothermia and hypothermia conditions (n=5). Adapted from Zhu et. al. JCBFM 2007; 27(6): 1225–1234.
Fig. 10.
Correlation of CBF and CMRO2 values in rat brains anesthetized with a-chloralose at brain temperature range of 32–37°C. The linear correlation coefficient (R) is 0.97. Adapted from Zhu et. al. JCBFM 2007; 27(6): 1225–1234.
4. Current status of high-field in vivo 17O MRS/MRI for studying brain bioenergetics
Since early 2000, substantial efforts have been devoted to the development of the high-field 17O MRS/MRI approach for noninvasively imaging CMRO2 with a short 17O2 inhalation, which were mainly carried out using small animal models te>|[12, 54, 60, 75, 79, 88, 92, 93].. These research works have demonstrated not only the feasibility but also the great promise of the high-field 17O MR approach for studying the central roles of oxidative metabolism in a living brain under various physiological conditions. Here, we highlight the major advance made in this regard, which represent the current status of the 17O MR technology for CMRO2 imaging.
4.1. Direct imaging of CMRO2 in animal models
For directly imaging CMRO2 in animal models, several major steps were taken to ensure the quality and reliability of the CMRO2 measurement. Specifically, the development of the high-field in vivo 17O-MR based CMRO2 imaging approach has gone through the following processes: 1) feasibility assessment, where relaxation properties and detection sensitivity of the natural abundance 17O-water of brain tissue were examined at different magnetic field strengths te>|[52, 54, 92].; 2) methodology development, where a comprehensive and quantitative in vivo 3D 17O-CSI approach was established to image CMRO2 in rat brains at 9.4T with only two minutes of 17O2 inhalation te>|[79, 94].; 3) quantification and validation, where a complete CMRO2 quantification model as well as a simplified non-invasive CMRO2 imaging method were established and validated for improving reliability and reproducibility te>|[60, 75, 88]., and 4) applicability assessment, where the ability of the high-field 17O MR approach for imaging CMRO2 and its change in animal brains under various physiological conditions were demonstrated te>|[75, 88, 93].. Accordingly, the in vivo 17O MRS/MRI approach, with a brief introduction of the 17O2 gas, can be readily applied to image the absolute CMRO2 in small animal models at high/ultra-high fields for various physiological, neurological or pathological studies.
4.2. CMRO2 image of the human brain
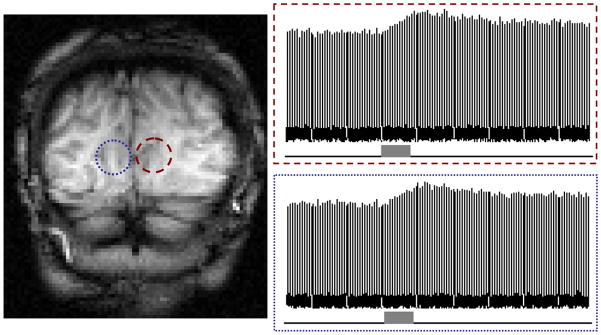
The ultimate goal for developing the CMRO2 imaging approach is to study the oxygen metabolism in healthy and diseased human brains. Several attempts have been made to image CMRO2 in the human brain te>|[38, 82, 83, 95, 96].. Fiat and coworkers examined the natural abundance 17O-water signal of the human brain using 1.5T whole body clinical scanner. However, the extremely short relaxation times (T1≤5ms and T2≈2ms) and the poor sensitivity of the 17O-MR signal available at 1.5T led to very low spatial and/or temporal resolution of the 17O-MR imaging te>|[38, 95].. Zhu and coworkers have investigated the relaxivity and sensitivity of natural abundance 17O-water in the human occipital lobe utilizing a 7T scanner te>|[82].. The results confirmed the advantage of the high magnetic field, which had been observed in animal models, for substantially improving the 17O detection sensitivity, and which makes it possible to image the dynamic change of the H217O signal in human brain during a 2-min 17O2 inhalation with excellent temporal resolution (11 sec) and reasonable spatial resolution (<1.4 cc nominal resolution). Fig. 11 displays the time courses of the H217O signals in two 3D-CSI voxels obtained from an representative subject before, during and after a brief 17O2 gas inhalation (only 2 min), and it clearly demonstrates the feasibility of the 17O MR approach for imaging the human brain CMRO2 at the high field of 7T te>|[82]. Recently, Atkinson and Thulborn applied direct 17O MR detection and ultra-short echo imaging sequence by using a 9.4T scanner for mapping CMRO2 of entire human brain te>|[83].. The spatial (~2.7cc) and temporal (42 sec) resolutions of the 3D 17O image achieved in this study were utilized for mapping the dynamic change of the 17O water contents before, during and after ~15min 17O2 gas inhalation in one human subject. With a three-phase CMRO2 quantification model and co-registered 23Na images for anatomic reference and brain tissue mass computation, 3D CMRO2 maps of the entire human brain were obtained te>|[83] at 9.4T. The CMRO2 values determined in this MR study were in a similar range to those reported from PET studies te>|[27]
Fig. 11.
Stacked plots of H217O signal measured by 3D 17O CSI at 7T before (i.e., natural abundance), during and after an 2 min 17O2 gas inhalation from two representative voxels in the human visual cortex.
Despite these advances, quantitative and noninvasive mapping of CMRO2 in human brain still face many technical and methodological challenges (see more discussion in Section 5). Further research efforts are needed before the 17O-MR based approach can be readily applied for studying oxygen metabolism in human brains.
4.3. Mapping functional CMRO2 changes
One important application of the CMRO2 imaging technique is to determine the CMRO2 changes due to the functional activation of the brain. The BOLD (blood-oxygen-level dependence) contrast based functional MR imaging (fMRI) technique is the most widely used neuroimaging modality for studying brain function and human behavior te>|[97–100].. However, the BOLD-fMRI is unable to directly detect neuronal activity; instead, it relies on a complex interplay among CBF, cerebral blood volume (CBV), and CMRO2 changes induced by altered brain activity te>|[101].. Precise interpretation of fMRI results requires a better understanding of the quantitative relationship between the fMRI BOLD contrast and the underlying neurophysiology—in particular the stimulus-evoked CMRO2 change te>|[102]..
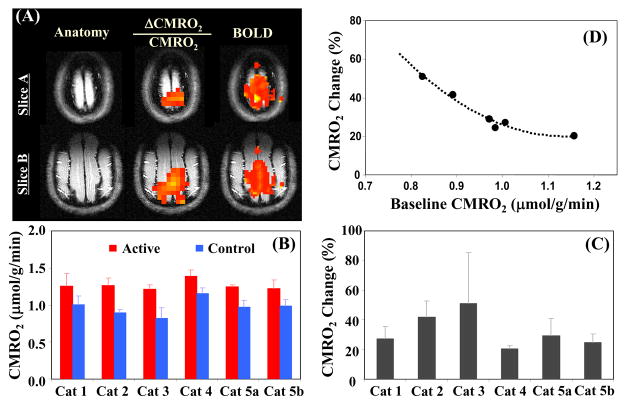
The ability to map the functional CMRO2 changes induced by an external brain stimulus has recently been explored in a lightly anesthetized cat brain. Absolute CMRO2 images with reasonable spatial and temporal resolutions were obtained from each cat during both resting and activation (visual stimulation) states, respectively te>|[93] and functional maps of the relative CMRO2 changes (i.e. ΔCMRO2/CMRO2) were generated accordingly for each animal. Fig. 12 summarizes the findings of this study. In addition to observing significant CMRO2 increases during the visual stimulation, the size and location of the activated brain regions depicted in the functional ΔCMRO2/CMRO2 maps largely coincide with those regions showing positive BOLD-fMRI changes in the same cat brain despite the different spatial resolutions of the CMRO2- and BOLD-based functional images (Fig. 12A). In addition, by directly imaging the absolute CMRO2 values under both resting and activated brain states (Fig. 12B), this functional 17O-CMRO2 study not only provided a quantitative measure of relative CMRO2 change elevated by visual stimulation (Fig. 12C) but also revealed a strong influence of baseline metabolic activity level on the relative CMRO2 change in response to brain stimulation (Fig. 12D). Thus, this crucial finding regarding the quantitative relationship between the absolute CMRO2 change and increased brain activity during activation suggests a tight neural-metabolic coupling and the vital role of oxygen metabolism in supporting the intensified neuronal activity in a working brain te>|[93]..
Fig. 12.
(A) Functional maps of BOLD and CMRO2 changes during visual stimulation from a representative cat brain; (B) averaged control (baseline) and activated CMRO2 values; (C) relative CMRO2 changes; and (D) negative correlation between the percent CMRO2 changes and baseline CMRO2 values (n=6). Adapted from Zhu et. al. JCBFM 2009; 29: 10–18.
4.4. Ultra-fast CMRO2 measurements
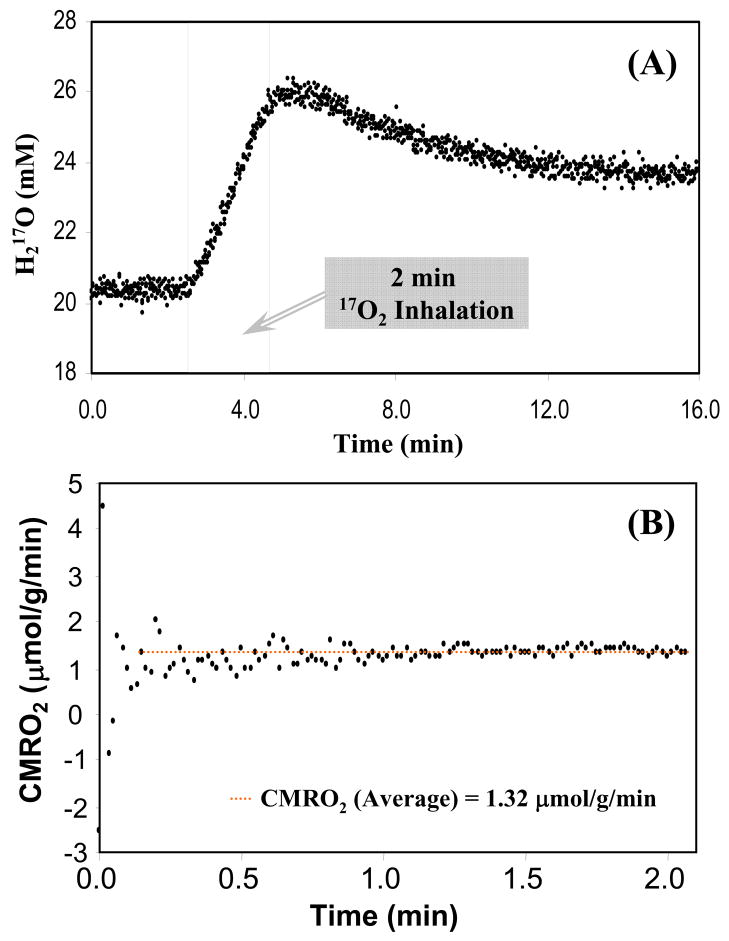
The 17O-MR sensitivity achievable at high magnetic field can be used to image CMRO2 with a much improved temporal resolution while sacrificing the spatial resolution to a certain extent. An ultra-fast CMRO2 measurement strategy has been tested in the rat model at 9.4T. Fig. 13 demonstrates an example of the dynamics of the H217O contents before, during and after a 2 min 17O2 inhalation in a rat brain from measurements acquired with high temporal resolution of 1 s (see Fig. 13A); the averaged CMRO2 value during the inhalation period in this case was found to be 1.32 μmol/g/min using the complete quantification model described in Eq. [8] (see Fig. 13B) and 1.43 μmol/g/min (linear fit) or 1.34 μmol/g/min (quadratic fit) using the simplified model described in Eqs. [9] and [10]. The simplified quantification model provides a reasonable approximation and, most importantly, it allows completely non-invasive determination of the absolute CMRO2 value in vivo.
Fig. 13.
Ultra-fast CMRO2 measurement in rat brain at 9.4T: (A) Dynamics of the 17O-water contents in rat brain tissue before, during and after a 2min 17O2 gas inhalation obtained with 1 sec temporal resolution; and (B) the CMRO2 values obtained during the inhalation period were quantified using the complete model as described by Eq.[8].
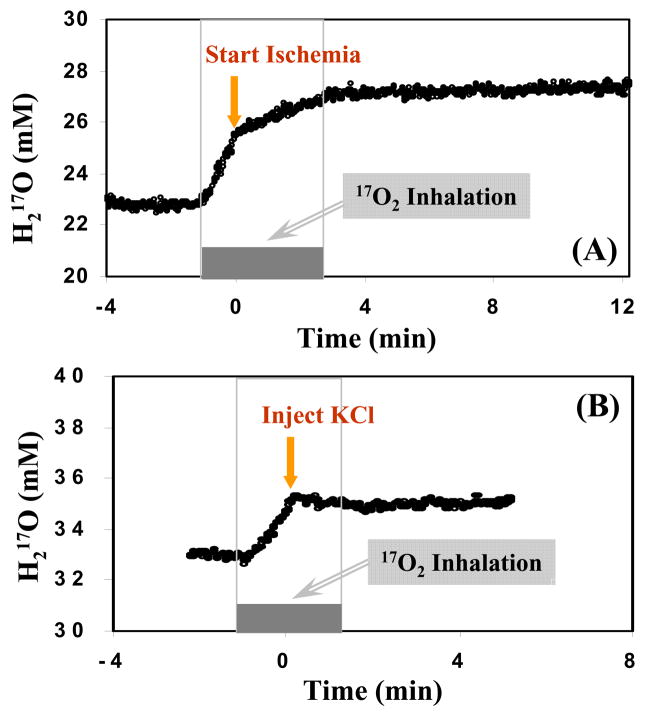
The ultra-fast temporal resolution of the CMRO2 measurement enables the study aiming at rapid temporal changes in the oxygen consumption rate caused by instantaneous physiological or pathological alteration occurs in the animals. Two examples of such a study are shown in Fig. 14. The significant slow down or diminishing of the oxygen metabolism in the rat brain due to global ischemia (Fig. 14A) or heart arrest (Fig. 14B) is reflected in the sudden decrease or halt in the production of the 17O-labeled metabolic water te>|[103].. Thus, through careful experimental design as shown in Fig. 14, the CMRO2 values for the two different conditions (i.e., before and after ischemia or KCl injection) can be determined with only one short 17O2 inhalation of a few minutes te>|[103]..
Fig. 14.
Dynamics of the 17O-water contents in rat brain tissue before, during and after short 17O2 gas inhalation obtained with ultra-fast CMRO2 measurement approach at 9.4T: (A) forebrain ischemia and (B) KCl injection were performed during the inhalation as indicated by the arrows.
4.5. Simultaneous CMRO2 and CBF imaging
As described earlier, the in vivo 17O MR imaging approach at high/ultrahigh field has been established for non-invasively mapping CMRO2 in small animals. However, imaging of CBF using the same 17O MR approach usually requires invasive procedures for introducing the NMR-visible H217O as exogenous tracer. Experimental evidence reveals that the metabolic H217O water generated from a brief 17O2 gas inhalation in the brain tissue had a much slower washout (or decay) rate compared to that of bolus H217O tracer, suggesting possible water permeability restrictions in the mitochondrial and/or cellular membranes te>|[75].. Nevertheless, further investigation found that the decay rate of the metabolic H217O after cessation of the 17O2 gas inhalation was still closely related to the cerebral perfusion and its change; and a linear relationship between CBF and H217O decay rate was determined experimentally from combined CBF and CMRO2 measurements in the rat brains under varied physiological or pathological conditions te>|[104]..
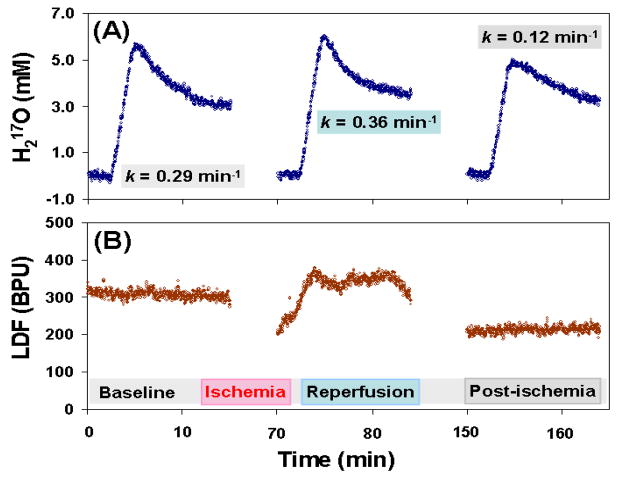
Fig. 15 shows an example of such multiple CMRO2 and CBF measurements where the relative CBF values were also assessed using Laser Doppler Flowmeter (LDF) performed in a representative rat brain after undergoing global forebrain ischemia preparation. The metabolic H217O decay rates obtained in the CMRO2 measurements during baseline, reperfusion and post-ischemia periods are displayed (see Fig. 15A) and their changes correlate well with the relative CBF changes measured by LDF (see Fig. 15B). The linear regression of the experimental data from similar measurements in different animals with different preparations for altering the brain perfusion led to the relation of CBF ≈ 1.86 × k (correlation coefficient R = 0.85), indicating that the measured metabolic 17O-water decay rate k provides a good approximation for estimating CBF in a wide range of physiological (or pathological) conditions te>|[104]..
Fig. 15.
Multiple and simultaneous CMRO2 (A) and CBF (B) measurements using 17O-MR approach at 9.4T and Laser Doppler Flow meter (LDF), respectively, in a representative rat brain with global forebrain ischemia preparation. The H217O decay rates during the baseline, reperfusion and post-ischemia periods were quantified in (A) and they correlate well with the relative CBF changes as shown in (B).
The findings from these researches demonstrate that in vivo 17O MRS/MRI approach is capable of assessing not only CMRO2 but also CBF simultaneously and noninvasively in the rat brain, and thus it provides a new utility for imaging the oxygen extraction fraction (OEF) of the brain tissue, another important physiological parameter, which is proportional to the ratio of CMRO2 and CBF.
5. Challenge and Perspective
As described in this article, the most interesting and important application of in vivo 17O NMR is for quantitatively imaging the rate of cerebral oxygen consumption occurring in mitochondria. Questions related to this rate are encountered frequently in biomedical research when considering either normal brain function or abnormalities related to a variety of brain diseases. Relevant to normal brain function, a question of whether the alterations in CMRO2, cerebral metabolic rate of glucose (CMRglc) and CBF induced by neuronal activity are quantitatively coupled (or matched) is central for understanding the mechanisms underlying most modern neuroimaging techniques including fMRI and PET. On the other hand, the central role of oxidative metabolism and its metabolic rate is also evident in pathologies associated with many brain disorders. Therefore, the ability to quantitatively image CMRO2 in vivo is essential for efforts aimed at investigating and understanding cerebral oxidative metabolism under normal and pathological conditions. The promising in vivo 17O NMR results as demonstrated during the past two decades and reviewed here have provided a crucial step towards the ultimate goal of developing a robust and completely noninvasive 17O NMR approach for imaging CMRO2 in animal brains, and potentially in human brains.
The establishment of high-field in vivo 17O NMR for imaging CMRO2 in small animal brains is quite successful while application of this method to the human brain faces some serious challenges. Firstly, the NMR sensitivity per unit tissue volume is reduced for human applications because the enlarged RF coil size (i.e., reduced reception sensitivity) needed for covering the entire human brain, which is approximately 700 times larger than the rat brain. This disadvantage can be partially compensated for by increasing the 17O imaging voxel size in quantification in humans and using advanced RF array coil technology. Secondly, the CMRO2 human brain is more difficult due to the uncertainty in determining the kinetics of the 17O fractional enrichment of the 17O2 gas and other parameters. The last but not least challenge for routine CMRO2 imaging, especially for human study, is the cost of 17O2 gas. Currently, the cost of the 17O-labeled oxygen gas is high because of the extremely low 17O natural abundance, low production efficiency for achieving high 17O enrichment, and presumably the low demand. Only a few companies are capable of supplying a large amount of 17O2 now. However, it is reasonable to expect that further progress in the technical developments of in vivo 17O approaches should stimulate numerous biomedical applications including clinical diagnosis, and increase the demand, ultimately leading to more efficient 17O2 production and lower retail prices. In addition to reduce the cost of the 17O-CMRO2 measurement, it is also essential to improve the efficiency of the 17O2 gas delivery system and to minimize the lost or waste of the 17O-labeled oxygen gas in the process.
In conclusion, the high/ultrahigh field NMR systems currently available or in development for both animals and humans provide great opportunities for in vivo MRI/MRS applications in medicine, especially for those nuclei with a low magnetogyric ratio. One of the nuclei that benefit the most from ultrahigh field strength is the 17O spin combined with direct in vivo 17O NMR detection, which has shown great promise for imaging CMRO2 noninvasively. Finally, the successful developments of in vivo 17O NMR approaches will lead to an alternative or better CMRO2 neuroimaging tool compared to the established PET method, and could have a profound impact on the study of oxidative metabolism in brains and potentially in other organs such as hearts te>|[84, 105].
Highlights.
This article reviews the developments of in vivo 17O NMR imaging in brain research.
In vivo 17O NMR imaging has improved significantly at high/ultrahigh field.
In vivo 17O NMR can noninvasively image brain oxygen metabolism and perfusion.
In vivo 17O NMR is useful for mapping the functional change in oxygen metabolism.
In vivo 17O NMR imaging could potentially be used for human and clinic applications.
Acknowledgments
We are grateful for the technical assistance, support and participation from Drs. Nanyin Zhang, Xiaoliang Zhang, Hao Lei, Yi Zhang, Run-Xia Tian, Hellmut Merkle, Jae-Hwan Kwag, Pete Thelwall, Peter Andersen, Gregor Adriany and Kamil Ugurbil, and Mr. John Strupp and Hannes Wiesner. We also like to acknowledge Drs. Gheorghe D. Mateescu, Xin Yu, Chris Flask, Gil Navon, Itamar Ronen, Robert G. Shulman, Fahmeed Hyder, Seiji Ogawa, Joseph J.H. Ackerman, Alan C. McLaughlin, Keith Thulborn, Jie Zheng and Steve Blackband for scientific discussion and stimulation.
The work as reviewed in this article was supported partly by NIH grants of NS41262, EB02632, NS39043, EB00329, EB00513, NS057560, NS070839, P41 RR08079 and P30NS057091; and the Keck Foundation.
Abbreviations
- γ
magnetogyric ratio
- T1
longitudinal relaxation time
- T2
transverse relaxation time
- T2*
apparent T2
- τc
rotational correlation time
- B0
magnetic field strength
- SNR
signal-to-noise ratio
- Q
RF coil quality factor
- CSI
chemical shift imaging
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- Pi
inorganic phosphate
- CBF
cerebral blood flow
- LDF
laser Doppler flowmeter
- BBB
brain-blood-barrier
- CMRglc
cerebral metabolic rate of glucose utilization
- α
17O enrichment fraction of inhaled
- CMRO2
cerebral metabolic rate of oxygen utilization
- PET
positron emission tomography
- fMRI
functional magnetic resonance imaging
- Ca(t)
time-dependent H217O concentration in excess of the natural abundance H217O concentration level in the arterial blood
- Cb(t)
time-dependent H217O concentration in excess of the natural abundance H217O concentration level in the brain tissue
- Cv(t)
time-dependent H217O concentration in excess of the natural abundance H217O concentration level in the venous blood
- λ
brain/blood partition coefficient 17O2 gas
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alder F, Yu FC. On the Spin and Magnetic Moment of 17O. Phys Rev. 1951;81:1067–1068. [Google Scholar]
- 2.Gerothanassis IP. Oxygen-17 NMR spectroscopy: Basic principles and applications (Part I) Progr NMR Spectr. 2010;56:95–197. doi: 10.1016/j.pnmrs.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Gerothanassis IP. Oxygen-17 NMR spectroscopy: Basic principles and applications (Part II) Progr NMR Spectr. 2010;57:1–110. doi: 10.1016/j.pnmrs.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Mateescu GD, Yvars GM, LaManna JC, Lust WD, Sudilovsky D. Oxygen-17 MRS: In vivo evaluation of water uptake and residence time in the mouse brain after injection of O-17 labeled water. Proc Inter Soc Magn Reson Med. 1990:1236. [Google Scholar]
- 5.Pekar J, Ligeti L, Ruttner Z, Lyon RC, Sinnwell TM, van Gelderen P, Fiat D, Moonen CT, McLaughlin AC. In vivo measurement of cerebral oxygen consumption and blood flow using 17O magnetic resonance imaging. Magn Reson Med. 1991;21:313–319. doi: 10.1002/mrm.1910210217. [DOI] [PubMed] [Google Scholar]
- 6.Arai T, Mori K, Nakao S, Watanabe K, Kito K, Aoki M, Mori H, Morikawa S, Inubushi T. In vivo oxygen-17 nuclear magnetic resonance for the estimation of cerebral blood flow and oxygen consumption. Biochem Biophys Res Commun. 1991;179:954–961. doi: 10.1016/0006-291x(91)91911-u. [DOI] [PubMed] [Google Scholar]
- 7.Arai T, Nakao S, Morikawa S, Inubushi T, Yokoi T, Shimizu K, Mori K. Measurement of local cerebral blood flow by magnetic resonance imaging: in vivo autoradiographic strategy using 17O-labeled water. Brain Res Bull. 1998;45:451–456. doi: 10.1016/s0361-9230(97)00369-9. [DOI] [PubMed] [Google Scholar]
- 8.Mateescu GD, Yvars GM, Dular T. Oxygen-17 Magnetic Resonance Imaging. Proc Inter Soc Magn Reson Med. 1987:929. [Google Scholar]
- 9.Mateescu GD, Fercu D. Interleave 17O/ 31P MRS: Novel Approach for In Vivo Determination of Defects in Oxidative Phosphorylation. Proc Inter Soc Magn Reson Med. 1993:110. [Google Scholar]
- 10.de Graaf RA, Brown PB, Rothman DL, Behar KL. Natural abundance 17O NMR spectroscopy of rat brain in vivo. J Magn Reson. 2008;193:63–67. doi: 10.1016/j.jmr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mateescu GD. Functional oxygen-17 magnetic resonance imaging and localized spectroscopy. Adv Exp Med Biol. 2003;510:213–218. doi: 10.1007/978-1-4615-0205-0_35. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Zhu XH, Ugurbil K. Imaging Cerebral Metabolic Rate of Oxygen Consumption (CMRO2) using 17O NMR Approach at Ultra-high Field. In: Shulman RG, Rothman DL, editors. Brain Energetics and Neuronal Activity. John Wiley & Sons Ltd; New York: 2004. pp. 125–146. [Google Scholar]
- 13.Zhu XH, Zhang N, Zhang Y, Zhang X, Ugurbil K, Chen W. In vivo 17O NMR approaches for brain study at high field. NMR Biomed. 2005;18:83–103. doi: 10.1002/nbm.930. [DOI] [PubMed] [Google Scholar]
- 14.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 15.Beal MF. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol. 1992;31:119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- 16.Frackowiak RS, Herold S, Petty RK, Morgan-Hughes JA. The cerebral metabolism of glucose and oxygen measured with positron tomography in patients with mitochondrial diseases. Brain. 1988;111:1009–1024. doi: 10.1093/brain/111.5.1009. [DOI] [PubMed] [Google Scholar]
- 17.Maurer I, Zierz S, Moller H. Evidence for a mitochondrial oxidative phosphorylation defect in brains from patients with schizophrenia. Schizophr Res. 2001;48:125–136. doi: 10.1016/s0920-9964(00)00075-x. [DOI] [PubMed] [Google Scholar]
- 18.Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 19.Wong-Riley M, Antuono P, Ho KC, Egan R, Hevner R, Liebl W, Huang Z, Rachel R, Jones J. Cytochrome oxidase in Alzheimer’s disease: biochemical and histochemical, and immunohistochemical analyses of the visual and other systems. Vision Res. 1997;37:3593–3608. doi: 10.1016/S0042-6989(96)00210-6. [DOI] [PubMed] [Google Scholar]
- 20.Wallace DC. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992;256:628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- 21.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Novotny EJ, Zhu XH, Rothman DL, Shulman RG. Localized 1H NMR measurement of glucose consumption in the human brain during visual stimulation. Proc Natl Acad Sci USA. 1993;90:9896–9900. doi: 10.1073/pnas.90.21.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruetter R, Novotny EJ, Boulware SD, Rothman DL, Mason GF, Shulman GI, Shulman RG, Tamborlane WV. Direct measurement of brain glucose concentrations in humans by 13C NMR spectroscopy. Proc Natl Acad Sci USA. 1992;89:1109–1112. doi: 10.1073/pnas.89.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci USA. 2008;105:6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci USA. 2003;100:14409–14414. doi: 10.1073/pnas.2332656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoubridge EA, Briggs RW, Radda GK. 31P NMR saturation transfer measurements of the steady state rates of creatine kinase and ATP synthetase in the rat brain. FEBS Letters. 1982;140:289–292. doi: 10.1016/0014-5793(82)80916-2. [DOI] [PubMed] [Google Scholar]
- 27.Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- 28.Ter-Pogossian MM, Eichling JO, Davis DO, Welch MJ. The measure in vivo of regional cerebral oxygen utilization by means of oxyhemoglobin labeled with radioactive oxygen-15. J Clin Invest. 1970;49:381–391. doi: 10.1172/JCI106247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopkins AL, Barr RG. Oxygen-17 compounds as potential NMR T2 contrast agents: enrichment effects of H217O on protein solutions and living tissues. Magn Reson Med. 1987;4:399–403. doi: 10.1002/mrm.1910040413. [DOI] [PubMed] [Google Scholar]
- 30.Hopkins AL, Haacke EM, Tkach J, Barr RG, Bratton CB. Improved sensitivity of proton MR to oxygen-17 as a contrast agent using fast imaging: detection in brain. Magn Reson Med. 1988;7:222–229. doi: 10.1002/mrm.1910070210. [DOI] [PubMed] [Google Scholar]
- 31.Hopkins AL, Lust WD, Haacke EM, Wielopolski P, Barr RG, Bratton CB. The stability of proton T2 effects of oxygen-17 water in experimental cerebral ischemia. Magn Reson Med. 1991;22:167–174. doi: 10.1002/mrm.1910220118. [DOI] [PubMed] [Google Scholar]
- 32.Kwong KK, Hopkins AL, Belliveau JW, Chesler DA, Porkka LM, McKinstry RC, Finelli DA, Hunter GJ, Moore JB, Barr RG, Rosen BR. Proton NMR imaging of cerebral blood flow using H217O. Magn Reson Med. 1991;22:154–158. doi: 10.1002/mrm.1910220116. [DOI] [PubMed] [Google Scholar]
- 33.Kwong KK, Xiong J, Kuan WP, Cheng HM. Measurement of water movement in the rabbit eye in vivo using H217O. Magn Reson Med. 1991;22:443–450. doi: 10.1002/mrm.1910220252. [DOI] [PubMed] [Google Scholar]
- 34.Mateescu GD, Cabrera ME. In vivo 17O magnetic resonance spectroscopy. Determination of temperature effects on metabolic rates (Q10 factor) Adv Exp Med Biol. 1997;411:585–590. [PubMed] [Google Scholar]
- 35.Mateescu GD, LaManna JC, Lust WD, Mars LM, Tseng J. Oxygen-17 magnetic resonance: in vivo detection of nascent mitochondrial water in animals breathing 17O2 enriched air. Proc Inter Soc Magn Reson Med. 1991:1031. [Google Scholar]
- 36.Arai T, Nakao S, Mori K, Ishimori K, Morishima I, Miyazawa T, Fritz-Zieroth B. Cerebral oxygen utilization analyzed by the use of oxygen-17 and its nuclear magnetic resonance. Biochem Biophys Res Commun. 1990;169:153–158. doi: 10.1016/0006-291x(90)91447-z. [DOI] [PubMed] [Google Scholar]
- 37.Pekar J, Sinnwell T, Ligeti L, Chesnick AS, Frank JA, McLaughlin AC. Simultaneous measurement of cerebral oxygen consumption and blood flow using 17O and 19F magnetic resonance imaging. J Cereb Blood Flow Metab. 1995;15:312–320. doi: 10.1038/jcbfm.1995.36. [DOI] [PubMed] [Google Scholar]
- 38.Fiat D, Dolinsek J, Hankiewicz J, Dujovny M, Ausman J. Determination of regional cerebral oxygen consumption in the human: 17O natural abundance cerebral magnetic resonance imaging and spectroscopy in a whole body system. Neurol Res. 1993;15:237–248. doi: 10.1080/01616412.1993.11740143. [DOI] [PubMed] [Google Scholar]
- 39.Fiat D, Kang S. Determination of the rate of cerebral oxygen consumption and regional cerebral blood flow by non-invasive 17O in vivo NMR spectroscopy and magnetic resonance imaging: Part 1. Theory and data analysis methods. Neurol Res. 1992;14:303–311. doi: 10.1080/01616412.1992.11740074. [DOI] [PubMed] [Google Scholar]
- 40.Fiat D, Kang S. Determination of the rate of cerebral oxygen consumption and regional cerebral blood flow by non-invasive 17O in vivo NMR spectroscopy and magnetic resonance imaging. Part 2. Determination of CMRO2 for the rat by 17O NMR, and CMRO2,rCBF and the partition coefficient for the cat by 17O MRI. Neurol Res. 1993;15:7–22. doi: 10.1080/01616412.1993.11740100. [DOI] [PubMed] [Google Scholar]
- 41.Ronen I, Lee JH, Merkle H, Ugurbil K, Navon G. Imaging H217O distribution in a phantom and measurement of metabolically produced H217O in live mice by proton NMR. NMR Biomed. 1997;10:333–340. doi: 10.1002/(sici)1099-1492(199710)10:7<333::aid-nbm465>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 42.Ronen I, Merkle H, Ugurbil K, Navon G. Imaging of H217O distribution in the brain of a live rat by using proton-detected 17O MRI. Proc Natl Acad Sci USA. 1998;95:12934–12939. doi: 10.1073/pnas.95.22.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronen I, Navon G. A new method for proton detection of H217O with potential applications for functional MRI. Magn Reson Med. 1994;32:789–793. doi: 10.1002/mrm.1910320616. [DOI] [PubMed] [Google Scholar]
- 44.Reddy R, Stolpen AH, Charagundla SR, Insko EK, Leigh JS. 17O-decoupled 1H detection using a double-tuned coil. Magn Reson Imaging. 1996;14:1073–1078. doi: 10.1016/s0730-725x(96)00227-5. [DOI] [PubMed] [Google Scholar]
- 45.Stolpen AH, Reddy R, Leigh JS. 17O-decoupled proton MR spectroscopy and imaging in a tissue model. J Magn Reson. 1997;125:1–7. doi: 10.1006/jmre.1996.1071. [DOI] [PubMed] [Google Scholar]
- 46.Charagundla SR, Stolpen AH, Leigh JS, Reddy R. Off-resonance proton T1rho dispersion imaging of 17O-enriched tissue phantoms. Magn Reson Med. 1998;39:588–595. doi: 10.1002/mrm.1910390412. [DOI] [PubMed] [Google Scholar]
- 47.Reddy R, Stolpen AH, Leigh JS. Detection of 17O by proton T1rho dispersion imaging. J Magn Reson B. 1995;108:276–279. doi: 10.1006/jmrb.1995.1133. [DOI] [PubMed] [Google Scholar]
- 48.Sergeyev NM, Sergeyeva ND, Raynes WT. Isotope Effects on the 17O, 1H Coupling Constant and the 17O-{1H} Nuclear Overhauser Effect in Water. J Magn Reson. 1999;137:311–315. doi: 10.1006/jmre.1998.1682. [DOI] [PubMed] [Google Scholar]
- 49.Abragam A. The Principles of Nuclear Magnetism. Oxford University Press; London: 1961. [Google Scholar]
- 50.Glasel JA. A study ofwater in biological systems of O-17 magnetic resonance spectroscopy. I. Prliminary studies and enon hydrates. Proc Natl Acad Sci USA. 1966;55:479–485. doi: 10.1073/pnas.55.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meiboom S. NMR study of the proton transfer in water. J Chem Phys. 1961;34:375–388. [Google Scholar]
- 52.Zhu XH, Merkle H, Kwag JH, Ugurbil K, Chen W. 17O relaxation time and NMR sensitivity of cerebral water and their field dependence. Magn Reson Med. 2001;45:543–549. doi: 10.1002/mrm.1073. [DOI] [PubMed] [Google Scholar]
- 53.Lauterwein J, Lukacs G, Poupon MF, Schumacher M. Oxygen-17 relaxation times in the blood sera of rats with various cancers. Can a systemic effect be detected? Physiological Chemistry and Physics and Medical NMR. 1986;18:137–140. [PubMed] [Google Scholar]
- 54.Thelwall PE, Blackband SJ, Chen W. Field dependence of 17O T1, T2 and SNR - in vitro and in vivo studies at 4.7, 11 and 17.6 Tesla. Proc Intl Soc Mag Reson Med. 2003:504. [Google Scholar]
- 55.Ernst RR, Bodenhausen G, Wokaun A. Principles of Nuclear Magnetic Resonance in One and Two Dimensions. Oxford University Press; New York: 1987. [Google Scholar]
- 56.Hoult DI, Richards RE. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J Magn Reson. 1976;24:71–85. doi: 10.1016/j.jmr.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Wang DJ, Noyszewski EA, Bogdan AR, Haselgrove JC, Reddy R, Zimmerman RA, Leigh JS. Sensitivity of in vivo MRS of the N-d proton in proximal histidine of deoxymyoglobin. Magn Reson Med. 1992;27:362–367. doi: 10.1002/mrm.1910270217. [DOI] [PubMed] [Google Scholar]
- 58.Wen H, Chesnick AS, Balaban RS. The design and test of a new volume coil for high field imaging. Magn Reson Med. 1994;32:492–498. doi: 10.1002/mrm.1910320411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cassoly R. Interaction of hemoglobin with the red blood cell membrane. A saturation transfer electron paramagnetic resonance study. Biochim Biophys Acta. 1982;689:203–209. doi: 10.1016/0005-2736(82)90252-8. [DOI] [PubMed] [Google Scholar]
- 60.Zhang N, Zhu XH, Lei H, Ugurbil K, Chen W. Simplified methods for calculating cerebral metabolic rate of oxygen based on 17O magnetic resonance spectroscopic imaging measurement during a short 17O2 inhalation. J Cereb Blood Flow Metab. 2004;24:840–848. doi: 10.1097/01.WCB.0000125885.54676.82. [DOI] [PubMed] [Google Scholar]
- 61.Yeung HN, Lent AH. Proton transverse relaxation rate of 17O-enriched water. Magn Reson Med. 1987;5:87–92. doi: 10.1002/mrm.1910050112. [DOI] [PubMed] [Google Scholar]
- 62.Mateescu GD, Yvars GM, Pazara DI, Alldridge NA, LaManna JC, Lust WD, Mattingly M, Kuhn W. Combined oxygen-17/proton magnetic resonance microscopy in plants, animals and materials: present status and potential. In: Bailie TA, Jones JR, editors. Synthesis and applications of isotopically labelled compounds. Elsevier; Amsterdam: 1989. pp. 499–508. [Google Scholar]
- 63.Arai T, Gupte PM, Lasker SE, Del Guercio LR, Mori K. Method for the detection of tissue metabolite (H217O) in brain by proton magnetic resonance imaging. Crit Care Med. 1989;17:1333–1334. doi: 10.1097/00003246-198912000-00018. [DOI] [PubMed] [Google Scholar]
- 64.de Crespigny AJ, D’Arceuil HE, Engelhorn T, Moseley ME. MRI of focal cerebral ischemia using 17O-labeled water. Magn Reson Med. 2000;43:876–883. doi: 10.1002/1522-2594(200006)43:6<876::aid-mrm14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 65.Rizi RR, Charagundla SR, Song HK, Reddy R, Stolpen AH, Schnall MD, Leigh JS. Proton T1rho-dispersion imaging of rodent brain at 1.9 T. J Magn Reson Imaging. 1998;8:1090–1096. doi: 10.1002/jmri.1880080514. [DOI] [PubMed] [Google Scholar]
- 66.Makela HI, Grohn OH, Kettunen MI, Kauppinen RA. Proton exchange as a relaxation mechanism for T1 in the rotating frame in native and immobilized protein solutions. Biochem Biophys Res Commun. 2001;289:813–818. doi: 10.1006/bbrc.2001.6058. [DOI] [PubMed] [Google Scholar]
- 67.Grohn OHJ, Kettunen MI, Makela HI, Penttonen M, Pitkanen A, Lukkarinen JA, Kauppinen RA. Early detection of irreversible cerebral ischemia in the rat using dispersion of the magnetic resonance imaging relaxation time, T1rho. J Cereb Blood Flow Metab. 2000;20:1457–1466. doi: 10.1097/00004647-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 68.Kettunen MI, Grohn OH, Penttonen M, Kauppinen RA. Cerebral T1rho relaxation time increases immediately upon global ischemia in the rat independently of blood glucose and anoxic depolarization. Magn Reson Med. 2001;46:565–572. doi: 10.1002/mrm.1228. [DOI] [PubMed] [Google Scholar]
- 69.Tailor DR, Roy A, Regatte RR, Charagundla SR, McLaughlin AC, Leigh JS, Reddy R. Indirect 17O-magnetic resonance imaging of cerebral blood flow in the rat. Magn Reson Med. 2003;49:479–487. doi: 10.1002/mrm.10403. [DOI] [PubMed] [Google Scholar]
- 70.Tailor DR, Poptani H, Glickson JD, Leigh JS, Reddy R. High-resolution assessment of blood flow in murine RIF-1 tumors by monitoring uptake of H217O with proton T(1rho)-weighted imaging. Magn Reson Med. 2003;49:1–6. doi: 10.1002/mrm.10375. [DOI] [PubMed] [Google Scholar]
- 71.Mellon EA, Beesam RS, Baumgardner JE, Borthakur A, Witschey WR, 2nd, Reddy R. Estimation of the regional cerebral metabolic rate of oxygen consumption with proton detected 17O MRI during precision 17O2 inhalation in swine. J Neurosci Methods. 2009;179:29–39. doi: 10.1016/j.jneumeth.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mellon EA, Beesam RS, Elliott MA, Reddy R. Mapping of cerebral oxidative metabolism with MRI. Proc Natl Acad Sci USA. 2010;107:11787–11792. doi: 10.1073/pnas.1006951107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mellon EA, Beesam RS, Kasam M, Baumgardner JE, Borthakur A, Witschey WR, Jr, Reddy R. Single shot T1rho magnetic resonance imaging of metabolically generated water in vivo. Adv Exp Med Biol. 2009;645:279–286. doi: 10.1007/978-0-387-85998-9_42. [DOI] [PubMed] [Google Scholar]
- 74.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man; theory, rocedure and normal values. J Clin Invest. 1948;27:476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu XH, Zhang Y, Tian RX, Lei H, Zhang N, Zhang X, Merkle H, Ugurbil K, Chen W. Development of 17O NMR approach for fast imaging of cerebral metabolic rate of oxygen in rat brain at high field. Proc Natl Acad Sci USA. 2002;99:13194–13199. doi: 10.1073/pnas.202471399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fiat D, Ligeti L, Lyon RC, Ruttner Z, Pekar J, Moonen CT, McLaughlin AC. In vivo 17O NMR study of rat brain during 17O2 inhalation. Magn Reson Med. 1992;24:370–374. doi: 10.1002/mrm.1910240218. [DOI] [PubMed] [Google Scholar]
- 77.Herscovitch A, Raichle ME. What is the correct value for the brain-blood partition coefficient for water? J Cereb Blood Flow Metab. 1985;5:65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- 78.Herscovitch P, Raichle ME, Kilbourn MR, Welch MJ. Positron emission tomographic measurement of cerebral blood flow and permeability-surface area product of water using [15O]water and [11C]butanol. J Cereb Blood Flow Metab. 1987;7:527–542. doi: 10.1038/jcbfm.1987.102. [DOI] [PubMed] [Google Scholar]
- 79.Zhang X, Zhu XH, Tian R, Zhang Y, Merkle H, Chen W. Measurement of arterial input function of 17O water tracer in rat carotid artery by using a region-defined (REDE) implanted vascular RF coil. Magma. 2003;16:77–85. doi: 10.1007/s10334-003-0013-9. [DOI] [PubMed] [Google Scholar]
- 80.Hyder F, Kennan RP, Kida I, Mason GF, Behar KL, Rothman D. Dependence of oxygen delivery on blood flow in rat brain: a 7 tesla nuclear magnetic resonance study. J Cereb Blood Flow Metab. 2000;20:485–498. doi: 10.1097/00004647-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 81.Nakao Y, Itoh Y, Kuang TY, Cook M, Jehle J, Sokoloff L. Effects of anesthesia on functional activation of cerebral blood flow and metabolism. Proc Natl Acad Sci USA. 2001;98:7593–7598. doi: 10.1073/pnas.121179898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu XH, Zhang X, Zhang N, Zhang Y, Strupp J, Ugurbil K, Chen W. High-field 17O Study of 3D CMRO2 Imaging in human visual cortex. Proc Intl Soc Mag Reson Med. 2006:409. [Google Scholar]
- 83.Atkinson IC, Thulborn KR. Feasibility of mapping the tissue mass corrected bioscale of cerebral metabolic rate of oxygen consumption using 17-oxygen and 23-sodium MR imaging in a human brain at 9.4 T. Neuroimage. 2010;51:723–733. doi: 10.1016/j.neuroimage.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 84.Lu A, Atkinson IC, Claiborne TC, Damen FC, Thulborn KR. Quantitative sodium imaging with a flexible twisted projection pulse sequence. Magn Reson Med. 2010;63:1583–1593. doi: 10.1002/mrm.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagel AM, Laun FB, Weber MA, Matthies C, Semmler W, Schad LR. Sodium MRI using a density-adapted 3D radial acquisition technique. Magn Reson Med. 2009;62:1565–1573. doi: 10.1002/mrm.22157. [DOI] [PubMed] [Google Scholar]
- 86.Brown TR, Kincaid BM, Ugurbil K. NMR chemical shift imaging in three dimensions. Proc Natl Acad Sci USA. 1982;79:3523–3526. doi: 10.1073/pnas.79.11.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu X, Chen W, Patel M, Ugurbil K. Chemical Shift Imaging: An introduction to its theory and practice. In: Bronzino JD, editor. Biomedical Engineering Handbook. CRC; 1995. pp. 1036–1045. [Google Scholar]
- 88.Zhu XH, Zhang Y, Zhang N, Ugurbil K, Chen W. Noninvasive and three-dimensional imaging of CMRO2 in rats at 9.4 T: reproducibility test and normothermia/hypothermia comparison study. J Cereb Blood Flow Metab. 2007;27:1225–1234. doi: 10.1038/sj.jcbfm.9600421. [DOI] [PubMed] [Google Scholar]
- 89.Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab. 2003;23:513–530. doi: 10.1097/01.WCB.0000066287.21705.21. [DOI] [PubMed] [Google Scholar]
- 90.Siesjo BK. Brain energy metabolism. Wiley; New York: 1978. [Google Scholar]
- 91.Kety SS, Schmidt CF. The determination of cerebral blood flow in man by the use of nitrous oxide in low concentrations. Am J Physiol. 1945;143:53–66. [Google Scholar]
- 92.Wiesner HM, Balla DZ, Pohmann R, Chen W, Ugurbil K, Uludag K. 17O T1/T2* tissue-relaxation rates with anatomical contrast in the rat brain at 16.4 T. Proc Intl Soc Mag Reson Med. 2009:353. [Google Scholar]
- 93.Zhu XH, Zhang N, Zhang Y, Ugurbil K, Chen W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. J Cereb Blood Flow Metab. 2009;29:10–18. doi: 10.1038/jcbfm.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu XH, Lei H, Zhang Y, Zhang XL, Zhang N, Ugurbil K, Chen W. Evidence of limited permeation of metabolic water in rat brain observed by 17O magnetic resonance spectroscopic imaging and its implications. Proc Intl Soc Mag Reson Med. 2002:1094. [Google Scholar]
- 95.Fiat D, Hankiewicz J, Liu S, Trbovic S, Brint S. 17O magnetic resonance imaging of the human brain. Neurol Res. 2004;26:803–808. doi: 10.1179/016164104X5156. [DOI] [PubMed] [Google Scholar]
- 96.Hoffmann S, Begovatz P, Nagel A, Umathum R, Bock M. In vivo Oxygen-17 (17O) MRI at 7 Tesla. Proc Intl Soc Mag Reson Med. 2010:724. doi: 10.1002/mrm.22871. [DOI] [PubMed] [Google Scholar]
- 97.Ogawa S, Lee T-M, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- 99.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng HM, Brady TJ, Rosen BR. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buxton RB. Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism. Front Neuroenergetics. :2–8. doi: 10.3389/fnene.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu XH, Zhang Y, Chen W. Simultaneous and Ultrafast Monitoring of CMRO2, CBF and pO2 Changes in Response to Acute Global Ischemia in Rat Brain. Proc Intl Soc Mag Reson Med. 2007:2393. [Google Scholar]
- 104.Zhu XH, Zhang Y, Wiesner H, Ugurbil K, Chen W. Estimation of CBF Based on the Metabolic H217O Decay Rate in CMRO2 Measurement Using In Vivo17O MR Approach. Proc Intl Soc Mag Reson Med. 2010:716. [Google Scholar]
- 105.Zhu XH, Zhang Y, Chen W. In Vivo17O MRS Imaging for Assessing Myocardial Oxygen Metabolism in Rat Heart at 9.4T. Proc Intl Soc Mag Reson Med. 2010:172. [Google Scholar]