Abstract
This study investigated the effects of perinatal dietary omega-3 (n-3) fatty acid depletion and subsequent repletion on the expression of genes that regulate long-chain polyunsaturated fatty acid (PUFA) biosynthesis in rat liver and brain. It was hypothesized that chronic n-3 fatty acid deficiency would increase liver Fads1 and Fads2 mRNA expression/activity, and that n-3 fatty acid repletion would normalize this response. Adult rats fed the n-3-free diet during perinatal development exhibited significantly lower erythrocyte, liver, and frontal cortex LCn-3 fatty acid composition and reciprocal elevations in LCn-6 fatty acid composition compared with controls and repleted rats. Liver Fads2, but not Fads1, Elovl2, or Elovl5, mRNA expression was significantly greater in n-3 deficient rats compared with controls, and was partially normalized in repleted rats. The liver 18:3n-6/18:2n-6 ratio, an index of delta6-desturase activity, was significantly greater in n-3 deficient rats compared with control and repleted rats, and was positively correlated with Fads2 mRNA expression among all rats. The liver 18:3n-6/18:2n-6 ratio, but not Fads2 mRNA expression, was also positively correlated with erythrocyte and frontal cortex LCn-6 fatty acid compositions. Neither Fads1 or Fads2 mRNA expression were altered in brain cortex of n-3 deficient rats. These results confirm previous findings that liver, but not brain, delta6-desaturase expression and activity indices are negatively regulated by dietary n-3 fatty acids.
Keywords: Omega-3 fatty acid; Delta5-desaturase (Fads1); Delta6-desaturase (Fads2); Elongase-2/5 (Elovl2, Elovl5); Erythrocyte; Liver; Frontal cortex; Rat
1. Introduction
Polyunsaturated fatty acids (PUFA), a lipid family comprised of omega-3 (n-3) and omega-6 (n-6) fatty acids, are critical components of cellular membranes and regulate multiple physiological events. A body of preclinical evidence indicates that dietary-induced perturbations in PUFA homeostasis in response to n-3 fatty acid deficiency during development lead to abnormalities in inflammatory [1] and synaptic [2] signaling, alterations in central neurotransmitter systems [3], and neurocognitive deficits [4] in adulthood. Moreover, n-3 fatty acid deficiency has been implicated in the pathophysiology of several diseases, including rheumatoid arthritis [5], coronary heart disease [6], hepatic steatosis [7], and psychiatric disorders including major depression and schizophrenia [8]. Therefore, developing a better understanding of the impact of n-3 fatty acid deficiency on the mechanisms mediating PUFA homeostasis may provide important insight into pathophysiology processes.
The biosynthesis of long-chain (LC) n-3 fatty acids, including docosahexaenoic acid (22:6n-3), and LCn-6 fatty acids, including arachidonic acid (20:4n-6), from short-chain dietary precursors a-linolenic acid (ALA, 18:3n-3) and linoleic acid (LA, 18:2n-6), respectively, are mediated by delta6-desaturase (Fads2), delta5-desaturase (Fads1), and elongases-2/5 (Elovl2, Elovl5) enzymes [9–12]. Extant evidence from pharmacological [13], mutant mouse [14], and human genomic [15] studies support the critical role of delta5- and delta6-desaturase enzymes for maintaining tissue PUFA homeostasis. Furthermore, because delta6-desaturase is a common and rate-limiting step for both LCn-6 and LCn-3 fatty acid biosynthesis, selective dietary LA (18:2n-6) deficiency is associated with reciprocal elevations in peripheral and central membrane LCn-3 fatty acid composition [16], and selective dietary ALA (18:3n-3) deficiency is associated with reciprocal elevations in peripheral and central membrane LCn-6 fatty acid composition [17]. Therefore, tissue PUFA homeostasis is governed by gene-diet interactions.
Although the liver is thought to be the principal organ involved in LCn-3 and LCn-6 PUFA biosynthesis, delta5- and delta6-desaturase enzymes are also expressed in brain [18]. Prior studies have found that LCn-3 fatty acid deficiency is associated with an up-regulation of Fads1 and Fads2 mRNA expression and activity in rat liver but not brain [18,19]. These findings suggest that liver Fads1 and Fads2 mRNA expression are negatively regulated by dietary n-3 fatty acids. The present study sought to replicate these findings using the perinatal n-3 fatty acid deficiency model, which produces robust reductions in peripheral and central LCn-3 fatty acid levels in adulthood, and to additionally investigate whether these effects could be corrected by normalization of n-3 fatty acid status. Based on prior evidence, it was hypothesized that perinatal n-3 fatty acid deficiency would increase liver, but not brain, Fads1 and Fads2 mRNA expression/activity, and that normalization of n-3 fatty acid status would prevent these changes.
2. Methods and materials
2.1. Diets
The compositions of the α-linolenic acid (ALA, 18:3n-3)-fortified (control, ALA+, TD.04285) and ALA- (TD.04286) diets are presented in Table 1 (Harlan-TEKLAD, Madison, WI). Diets were vacuum packaged and stored at 4°C. Both diets provided 3.8 Kcal/g, and were matched for percent kcal from protein (19.2%), carbohydrate (64.4%), and fat (16.5%). The control diet was fortified with flaxseed oil, which contains the short-chain n-3 fatty acid precursor ALA (18:3n-3). Analysis of diet fatty acid composition by gas chromatography confirmed that both diets were closely matched in saturated fatty acids, monounsaturated fatty acids, and the short-chain n-6 fatty acid precursor linoleic acid (LA, 18:2n-6), and that neither diet contained preformed long-chain n-3 or n-6 fatty acids including DHA and AA, respectively (Table 1).
Table 1.
Ingredient and nutrient composition of the experimental diets
| Ingredient1 | ALA+ | ALA− |
|---|---|---|
| Casein, vitamine free | 20 | 20 |
| Carbohydrate | ||
| Cornstarch | 20 | 20 |
| Sucrose | 27 | 27 |
| Dextrose | 9.9 | 9.9 |
| Maltose-dextrin | 6 | 6 |
| Cellulose | 5 | 5 |
| Mineral mix (AIMN-93G-MX) | 3.5 | 3.5 |
| Vitamin mix (AIN-93-VX) | 1 | 1 |
| L-Cystine | 0.3 | 0.3 |
| Choline bitartrate | 0.25 | 0.25 |
| TBHQ | 0.002 | 0.002 |
| Fat | ||
| Hydrogenated coconut oil | 4.5 | 5.1 |
| Safflower | 1.9 | 1.9 |
| Flaxseed | 0.6 | 0 |
| Fatty acid composition2 | ||
| C8:0 | 4.3 | 5.0 |
| C10:0 | 3.8 | 4.2 |
| C12:0 | 29 | 32.8 |
| C14:0 | 11 | 12.5 |
| C16:0 | 8.3 | 8.7 |
| C18:0 | 9.4 | 10.2 |
| 18:1n-9 | 6.7 | 4.7 |
| 18:2n-6 | 22.7 | 21.9 |
| 20:4n-6 | nd | nd |
| 18:3n-3 | 4.6 | nd |
| 22:6n-3 | nd | nd |
g/100 g diet
wt % of total fatty acids
nd = not detected
2.2. Animals
Male offspring bred in-house to nulliparous female Long-Evans hooded rats were used. For perinatal ALA deficiency, dams were fed the ALA- diet for 1 month prior to mating through weaning, and male offspring were maintained on the ALA- diet post-weaning (P21) to adulthood (P100)(n=10). Controls were born to dams maintained on the ALA+ diet, and received the ALA+ diet post-weaning (P21) to adulthood (P100)(n=10). Repleted rats were offspring of dams maintained on the ALA- diet, and switched to ALA+ diet post-weaning (P21) to adulthood (P100)(n=10). Rats were housed 2 per cage with food and water available ad libitum, and maintained under standard vivarium conditions on a 12:12 h light:dark cycle. Food (g/kg/d) and water (ml/kg/d) intake and body weight (kg) were recorded. Rats were sacrificed by decapitation on P100–101 between 9:00–12:00 am in a counter-balanced manner relative to the common removal of food hoppers at 9:00 am. Trunk blood was collected into EDTA-coated tubes, plasma isolated by centrifugation, and erythrocytes washed 3x with 4°C 0.9% NaCl. The brain was dissected on ice to isolate the frontal cortex (olfactory tubercle and residual striatal tissue were removed), and liver samples harvested and flash frozen in liquid nitrogen. All samples were stored at −80°C. All experimental procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee, and adhere to the guidelines set by the NIH.
2.3. Analysis of fatty acid methyl esters
The gas chromatography procedure used to determine erythrocyte, liver, and brain cortex fatty acid composition has been described in detail previously [20]. The methods for saponification and methylation of fatty acids for gas chromatographic analysis follows that originally reported by Mecalfe et al [21]. Total fatty acid composition was determined with a Shimadzu GC-2010 (Shimadzu Scientific Instruments Inc., Columbia MD). The column is a DB-23 (123-2332): 30m (length), I.D. (mm) 0.32 wide bore, film thickness of 0.25 µM (J&W Scientific, Folsom CA). The carrier gas is helium with a column flow rate of 2.5 ml/min. Analysis of fatty acid methyl esters was based on area under the curve calculated with Shimadzu Class VP 7.4 software. Fatty acid identification was based on retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). All analyses were performed by a technician blinded to treatment. For liver, we focused our primary analysis on product/precursor ratios as indices of delta6-desaturase (18:3n-6/18:2n-6), elongase-5 (20:3n-6/18:3n-6), delta5-desaturase (20:4n-6/20:3n-6), and elongase-2 (22:4n-6/20:4n-6) activities. We also determined principal LCn-3 fatty acids (20:5n-3, 22:5n-3, 22:6n-3) and principal LCn-6 fatty acids (20:2n-6, 20:4n-6, 22:4n-6, 22:5n-6). Fatty acid composition data are expressed as weight percent total fatty acid composition.
2.4. Liver and brain mRNA expression
A detailed description of the RT-PCR procedure has been published previously [22]. Frozen liver and frontal cortex were homogenized (BioLogics Model 300 V/T ultrasonic homogenizer, Manassas, VA) in Tri Reagent (MRC Inc., Cincinnati, OH), and total RNA isolated and purified using the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Total RNA was treated to remove potential DNA contamination using RNase-free DNase (Qiagen, Valencia, CA), and RNA quantified using a Nanodrop instrument (Nanodrop Instruments, Wilmington, DE). RNA quality was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). cDNA was prepared from 2 µg total RNA using a high-capacity RT cDNA Archive Kit (Applied Biosystems, Foster City, CA) along with no RT controls to confirm lack of genomic DNA contamination. Liver mRNA levels of delta-6 desaturase (TaqMan Gene Expression Assay ID: Fads2, Rn00580220_m1), delta-5 desaturase (Fads1, Rn00584915_m1), elongase-2 (Elovl2, Rn01450661_m1), elongase-5 (Elovl5, Rn00592812_m1), and sterol regulatory element-binding protein-1c (SREBP1c, Rn01495769_m1) were measured by real-time quantitative PCR using an ABI 7900HT500 Real Time PCR System (Applied Biosystems, Foster City, CA). Frontal cortex mRNA levels of delta-6 desaturase (Fads2, Rn00580220_m1) and delta-5 desaturase (Fads1, Rn00584915_m1) were also determined in a subset (n=5) of rats from each group. The nucleotide sequences of primer/probe sets can be obtained from www.appliedbiosystems.com. Sample were run in triplicate in Microamp Fast 96 well reaction plates using 20 µl reaction volumes consisting of 10 µl of 2X TaqMan Fast Universal PCR Matermix (Applied Biosystems, Foster City, CA), 1 µl of TaqMan gene expression assay, and 9 µl of cDNA. No template controls (NTC) substituting DEPC-treated water for cDNA were run with each plate to verify lack of cross contamination. Thermal cycling conditions were: 95°C for 10 min followed by 95°C for 1 sec denaturing step and 60°C for 20 sec, annealing step for 40 cycles. Data were analyzed by comparing the difference between target gene and endogenous control (GAPDH, Rn99999916_s1) cycle thresholds for each sample using the comparative Ct method [23].
2.5. Statistical analyses
Group (control, deficient, repleted) differences in fatty acid composition and gene expression were evaluated with a one-way ANOVA, and individual group differences were evaluated with unpaired t-tests (2-tail, α=0.05). Parametric (Pearson) correlation analyses were performed to determine relationships between fatty acid and gene expression data (2-tail, α=0.05). Analyses were performed with GB-STAT (V.10, Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Food/water intake and body weight
There were no significant group differences in food intake, F(2,44)=0.6, p=0.58 (CON: 60.8±2.6; DEF: 58±2.5; REP: 60.0±2.1 g/kg/d), water intake, F(2,44)=2.4, p=0.1 (CON: 45.9±5.2; DEF: 45.7±6.2; REP: 42.1±2.9 ml/kg/d), or endpoint (P100) body weight, F(2,29)=1.1, p=0.34 (CON: 461±12; DEF: 431±14; REP: 441±14 kg).
3.2. Peripheral and central long-chain PUFA composition
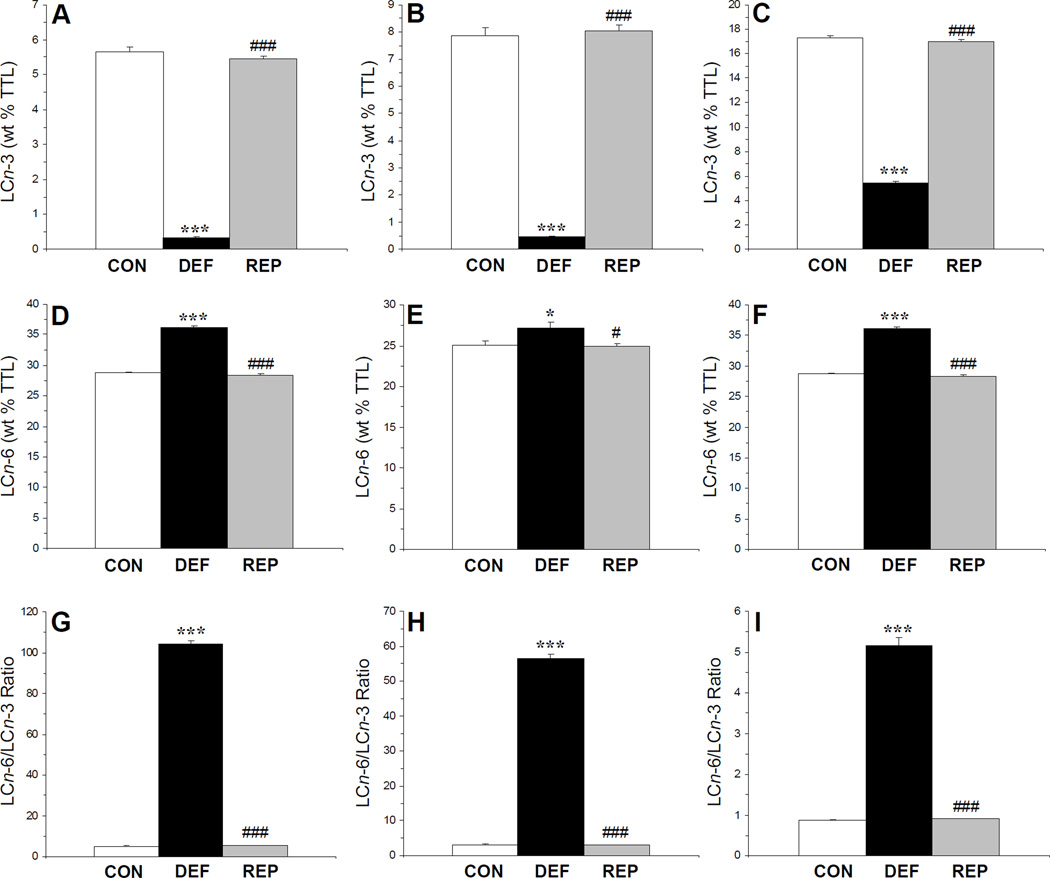
There was a significant main effect of treatment (diet) on LCn-3 fatty acid composition in erythrocytes, F(2,29)=905, p≤0.0001 (Fig. 1A), liver F(2,29)=380, p≤0.0001 (Fig. 1B), and frontal cortex, F(2,29)=1481, p≤0.0001 (Fig. 1C). In all cases, LCn-3 fatty acid composition was significantly lower in rats maintained in the ALA- diet compared with controls and repleted rats, and did not differ between control and repleted rats. There was a significant main effect of treatment on LCn-6 fatty acid composition in erythrocytes, F(2,29)=470, p≤0.0001 (Fig. 1D), liver F(2,29)=2.9, p=0.04 (Fig. 1E), and frontal cortex, F(2,29)=472, p≤0.0001 (Fig. 1F). In all cases, LCn-6 fatty acid composition was significantly greater in rats maintained in the ALA- diet compared with controls and repleted rats, and did not differ between control and repleted rats. Accordingly, the LCn-6/LCn-3 fatty acid ratio was significantly greater erythrocytes, F(2,29)=3447, p≤0.0001 (Fig. 1G), liver F(2,29)=1748, p=0.04 (Fig. 1H), and frontal cortex, F(2,29)=413, p≤0.0001 (Fig. 1I) of rats maintained in the ALA- diet compared with controls and repleted rats, and did not differ between control and repleted rats.
Figure 1.
Effects of dietary ALA depletion and repletion on LCn-3 fatty acid composition in erythrocytes (20:5+22:5+22:6n-3)(A), liver (20:5+22:5+22:6n-3)(B), and frontal cortex (22:6n-3)(C), LCn-6 fatty acid composition in erythrocytes (20:3+20:4+22:4+22:5n-6) (D), liver (20:2+20:3+20:4+22:4+22:5n-6)(E), and frontal cortex (20:4+22:4+22:5n-6)(F), and the LCn-6/LCn-3 ratio in erythrocytes (G), liver (H), and frontal cortex (I) of control (CON, n=10), n-3-deficient (DEF, n=10), and n-3-repleted (REP, n=10) rats. Values are group mean ± S.E.M. *p≤0.05, ***p≤0.0001 vs. controls, #p≤0.05, ###p≤0.0001 vs. DEF rats.
3.3. Liver and brain mRNA expression
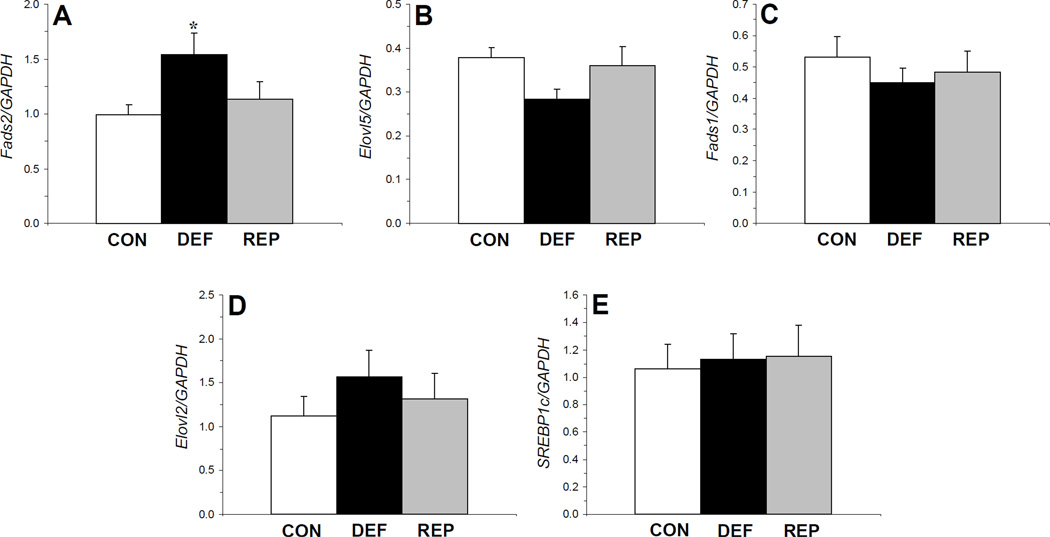
The main effect of treatment was not significant for GAPDH mRNA expression in liver, F(2,29)=2.1, p=0.14, or frontal cortex, F(2,14)=0.19, p=0.83. There was a significant main effect of treatment for liver Fads2/GAPDH mRNA expression, F(2,29)=3.4, p=0.04 (Fig. 2A). Fads2 mRNA expression was significantly greater in rats maintained in the ALA- diet compared with controls (p=0.02) but not repleted rats (p=0.1), and did not differ between control and repleted rats (p=0.47). The main effect of treatment was not significant for liver Elovl5 mRNA expression, F(2,29)=1.2, p=0.33 (Fig. 2B), Fads1 mRNA expression, F(2,29)=0.1, p=0.89 (Fig. 2C), Elovl2 mRNA expression, F(2,29)=0.6, p=0.55 (Fig. 2D), or SREBP1c mRNA expression, F(2,29)=0.06, p=0.94 (Fig. 2E). In frontal cortex, the main effect of treatment was not significant for Fads2 mRNA expression, F(2,14)=0.21, p=0.82, or Fads1 mRNA expression, F(2,14)=0.1, p=0.89 (data not shown).
Figure 2.
Liver expression of Fads2/GAPDH mRNA (A), Elovl5/GAPDH mRNA (B), Fads1/GAPDH mRNA (C), Elovl2/GAPDH mRNA (D), and SREBP1c/GAPDH mRNA (E) in control (CON, n=10), n-3-deficient (DEF, n=10), and n-3-repleted (REP, n=10) rats. Values are group mean ± S.E.M. *p≤0.05 vs. controls.
3.4. Liver product/precursor ratios
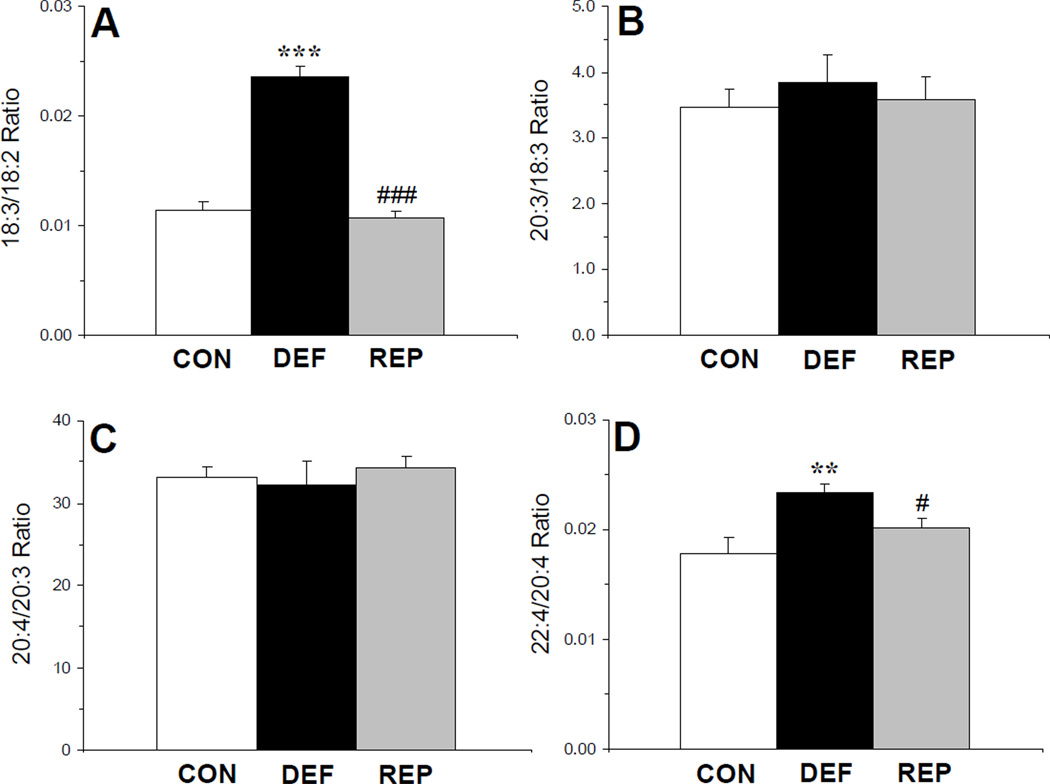
There was a significant main effect of treatment for liver 18:2n-6 composition, F(2,29)=110, p≤0.0001, which was significantly lower in rats maintained in the ALA- diet compared with both controls (p≤0.0001) and repleted rats (p≤0.0001), and did not differ between control and repleted rats (p=0.55). There was a significant main effect of treatment for the liver 18:3n-6/18:2n-6 ratio, an index of delta6-desaturase activity, F(2,29)=81, p≤0.0001 (Fig. 3A). The 18:3/18:2 ratio was significantly greater in rats maintained in the ALA- diet compared with both controls (p≤0.0001) and repleted rats (p≤0.0001), and did not differ between control and repleted rats (p=0.49). The main effect of treatment was not significant for the liver 20:3n-6/18:3n-6 ratio (elongase-5), F(2,29)=0.3, p=0.77 (Fig. 3B) or the 20:4n-6/20:3n-6 ratio (delta5-desaturase), F(2,29)=0.1, p=0.86 (Fig. 3C). There was a significant main effect of treatment for the liver 22:4n-6/20:4n-6 ratio (elongase-2), F(2,29)=5.9, p=0.007 (Fig. 3D). The 22:4n-6/20:4n-6 ratio was significantly greater in rats maintained in the ALA- diet compared with both controls (p=0.005) and repleted rats (p=0.02), and did not differ between control and repleted rats (p=0.22).
Figure 3.
Liver indices of delta6-desaturase (18:3n-6/18:2n-6)(A), elongase-5 (20:3n-6/18:3n-6) (B), delta5-desaturase (20:4n-6/20:3n-6) (C), and elongase-2 (22:4n-6/20:4n-6) (D) activities in control (CON, n=10), n-3-deficient (DEF, n=10), and n-3-repleted (REP, n=10) rats. Values are group mean ± S.E.M. **p≤0.01, ***p≤0.0001 vs. controls, #p≤0.05, ###p≤0.0001 vs. DEF rats.
3.5. Relationships between liver mRNA expression and activity indices
Among all rats (n=30), liver Fads2/GAPDH mRNA expression was positively correlated with liver 18:3n-6/18:2n-6 (r = +0.63, p=0.001), 20:3n-6/18:2n-6 (r = +0.61, p=0.0008), and 20:4n-6/18:2n-6 (r = +0.52, p=0.005) ratios. Liver Fads1/GAPDH mRNA expression was not significantly correlated with the liver 20:4n-6/20:3n-6 ratio (r = +0.12, p=0.56) or the 20:4n-6/18:2n-6 ratio (r = −0.02, p=0.92). Liver Elovl2/GAPDH mRNA expression was not significantly correlated with the liver 22:4n-6/20:4n-6 ratio (r = −0.16, p=0.42) or the 20:4n-6/18:2n-6 ratio (r = +0.07, p=0.71). Liver Elovl5/GAPDH mRNA expression was not significantly correlated with the liver 20:3n-6/18:3n-6 ratio (r = +0.11, p=0.62) or the 20:4 n-6/18:2 n-6 ratio (r = −0.19, p=0.34).
3.6. Relationships between liver mRNA expression and LCn-6 fatty acids
Liver Fads2/GAPDH mRNA expression was not correlated with 20:4n-6 composition in erythrocytes (r = +0.16, p=0.42) or frontal cortex (r = +0.20, p=0.31), 22:4n-6 composition in erythrocytes (r = +0.29, p=0.54) or frontal cortex (r = +0.37, p=0.06), and was moderately correlated with 22:5n-6 composition in erythrocytes (r = +0.45, p=0.02) and frontal cortex (r = +0.34, p=0.09). Liver Fads1/GAPDH, Elovl2/GAPDH, and Elovl5/GAPDH mRNA expressions, and associated activity indices, were not significantly correlated with erythrocyte or frontal cortex 20:4n-6, 22:4n-6, or 22:5n-6 compositions (p>0.05).
4. Discussion
The principal finding of the present study is that chronic dietary ALA (18:3n-3) depletion, resulting in robust elevations in peripheral and central LCn-6/LCn-3 fatty acid ratios, significantly and selectively increased Fads2 mRNA expression and delta6-desaturase activity (18:3n-6/18:2n-6 ratio) in rat liver. Fads1, Elovl2, and Elovl5 mRNA expression were not significantly altered in rat liver, and greater liver Fads2 mRNA expression in n-3-deficient rats was not accompanied by elevations in the transcription factor SREBP1c. Elevations in liver Fads2 mRNA expression and the 18:3n-6/18:2n-6 ratio could not be attributed to group differences in dietary intake of 18:2n-6. Furthermore, elevations in liver Fads2 mRNA expression and the 18:3n-6/18:2n-6 ratio in n-3-deficient rat liver were corrected by prior normalization of n-3 fatty acid status. Neither Fads1 or Fads2 mRNA expression were altered in the frontal cortex n-3 fatty acid deficient rats. These findings demonstrate that rat liver delta6-desaturase expression and activity are selectively up-regulated in rat liver, but not brain, in response to n-3 fatty acid deficiency, and indicate that this response is correctable by normalization of n-3 fatty acid status.
This study has three notable limitations. First, we did not directly evaluate enzyme activity, and used liver product/precursor ratios as estimates. However, the pattern of changes in liver fatty acids observed in n-3 deficient rats, reductions in the short-chain precursor (18:2n-6) and reciprocal elevations in long-chain products, is consistent with elevated delta-6 desaturase activity [13,14]. Moreover, the liver 18:3/18:2 ratio was positively correlated with liver Fads2 mRNA expression. Second, only male rats were investigated, precluding evaluation of gender differences. However, male rats were selected to obviate potential interactions with ovarian hormones previously found to influence primary outcome measures [20,24]. Third, because we investigated one strain of rat, the results may not be generalizable to other rat strains.
In the present study we found that only Fads2 mRNA expression was up-regulated in the liver of n-3-deficient rats, and that Fads1, Elovl2, and Elovl5 mRNA expression were not significantly altered. This finding differs from a prior study finding that dietary ALA depletion initiated post-weaning resulted in a significant elevations in Fads2, Fads1, Elovl2 and Elovl5 mRNA expression in rat liver [18]. We did however observe a significant increase in elongase-2 activity indices, and there was a trend for greater Elovl2 mRNA expression. Potentially relevant differences between these studies include dietary PUFA composition and when n-3 fatty acid deficiency was initiated (perinatal vs. post-weaning). It is notable, however, that both studies observed similar reductions in liver LCn-3 fatty acid levels. It may also be relevant that these studies used different rat strains (Long-Evans hooded vs. Fisher-344), and a prior study found that liver delta-6 and delta-5 desaturase activities differ significantly between rat strains maintained on the same diet [25]. Additional studies will be required to determine whether these methodological differences can account for these discrepant findings.
Consistent with prior studies [17,26], chronic dietary ALA depletion was associated with a reciprocal increase in peripheral and central LCn-6 fatty acid composition. In general, however, peripheral and central LCn-6 fatty acid compositions were not correlated with liver Fads2 mRNA expression. This finding suggests the observed increase in LCn-6 fatty acid composition is mediated predominantly by a reduction in substrate competition for the delta-6 desaturase enzyme and/or reduced competition for incorporation of LCn-6 fatty acids into phospholipids. Furthermore, because n-3 deficiency did not increase Fads2 or Fads1 mRNA expression in frontal cortex, elevations in central membrane LCn-6 fatty acid composition in response to n-3 deficiency may also be mediated by reduced competition for incorporation into phospholipids. Nevertheless, the robust increase in LCn-6 fatty acid biosynthesis and peripheral and central membrane composition in response to n-3 deficiency has implications for cellular functioning [1–4], and may contribute to the pathophysiology of several diseases associated with n-3 fatty acid deficiency [5–8].
In summary, the present data demonstrate that chronic dietary n-3 fatty acid deficiency during perinatal development, resulting in robust deficits in peripheral and central LCn-3 fatty acid composition, is associated with a selective up-regulation in delta6-desaturase expression and activity indices in rat liver. These findings confirm prior reports that n-3-deficiency is associated with elevated delta6-desaturase expression and activity in rat liver [18,19], and provide partial support for our hypothesis that chronic ALA deficiency would increase both Fads1 and Fads2 mRNA expression/activity in rat liver. In support of our hypothesis, the present study additionally demonstrates that elevations in liver delta6-desaturase expression and activity are preventable by prior normalization of n-3 fatty acid status. These data therefore demonstrate that maintaining adequate dietary n-3 fatty acid intake is necessary to prevent dysregulation in liver delta6-desaturase expression/activity and associated perturbations in peripheral and central PUFA homeostasis.
Acknowledgments
This work was supported in part by NIH grants MH083924 (to R.K.M.), DK59630 (to P.T.) and DK056863 (to S.C.B.). The authors thank S. Hofmann and Elizabeth Donelan for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 2.McNamara RK, Ostrander M, Abplanalp W, Richtand NM, Benoit S, Clegg D. Modulation of phosphoinositide-protein kinase C signal transduction by omega-3 fatty acids: Implications for the pathophysiology and treatment of recurrent neuropsychiatric illness. Prostogland Leukotrienes Essential Fatty Acids. 2006;75:237–257. doi: 10.1016/j.plefa.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75:259–269. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Fedorova I, Salem N., Jr Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot Essent Fatty Acids. 2006;75:271–289. doi: 10.1016/j.plefa.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Ruggiero C, Lattanzio F, Lauretani F, Gasperini B, Andres-Lacueva C, Cherubini A. Omega-3 polyunsaturated fatty acids and immune-mediated diseases: inflammatory bowel disease and rheumatoid arthritis. Curr Pharm Des. 2009;15:4135–4148. doi: 10.2174/138161209789909746. [DOI] [PubMed] [Google Scholar]
- 6.Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S–2002S. doi: 10.1093/ajcn/87.6.1997S. [DOI] [PubMed] [Google Scholar]
- 7.Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, et al. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2004;106:635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 8.McNamara RK. Evidence-based evaluation of omega-3 fatty acid deficiency as a risk factor for recurrent neuropsychiatric illness: Current status and future directions. In: Heikkinen EP, editor. Fish Oils and Health. U.S.A.: Nova Science Publishers, Inc.; 2008. pp. 7–67. [Google Scholar]
- 9.Cho HP, Nakamura M, Clarke SD. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J Biol Chem. 1999;274:37335–37339. doi: 10.1074/jbc.274.52.37335. [DOI] [PubMed] [Google Scholar]
- 10.Cho HP, Nakamura MT, Clarke SD. Cloning, expression, and nutritional regulation of the mammalian delta-6 desaturase. J Biol Chem. 1999;274:471–477. doi: 10.1074/jbc.274.1.471. [DOI] [PubMed] [Google Scholar]
- 11.Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Marquardt A, Stöhr H, White K, Weber BH. cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics. 2000;66:175–183. doi: 10.1006/geno.2000.6196. [DOI] [PubMed] [Google Scholar]
- 13.Obukowicz MG, Welsch DJ, Salsgiver WJ, Martin-Berger CL, Chinn KS, Duffin KL, et al. Novel, selective delta6 or delta5 fatty acid desaturase inhibitors as antiinflammatory agents in mice. J Pharmacol Exp Ther. 1998;287:157–166. [PubMed] [Google Scholar]
- 14.Stoffel W, Holz B, Jenke B, Binczek E, Günter RH, Kiss C, et al. Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids. EMBO J. 2008;27:2281–2292. doi: 10.1038/emboj.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaeffer L, Gohlke H, Müller M, Heid IM, Palmer LJ, Kompauer I, et al. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 2006;15:1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi M, Gao F, Kim HW, Ma K, Bell JM, Rapoport SI. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim Biophys Acta. 2009;1791:132–139. doi: 10.1016/j.bbalip.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moriguchi T, Loewke J, Garrison M, Catalan JN, Salem N., Jr Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J Lipid Res. 2001;42:419–427. [PubMed] [Google Scholar]
- 18.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Dinh TK, Bourre JM, Durand G. Effect of age and alpha-linolenic acid deficiency on delta 6 desaturase activity and liver lipids in rats. Lipids. 1993;28:517–523. doi: 10.1007/BF02536083. [DOI] [PubMed] [Google Scholar]
- 20.McNamara RK, Able JA, Liu Y, Jandacek R, Rider T, Tso P. Gender differences in rat erythrocyte and brain docosahexaenoic acid composition: Role of ovarian hormones and dietary omega-3 fatty acid composition. Psychoneuroendocrinology. 2009;34:532–539. doi: 10.1016/j.psyneuen.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- 22.McNamara RK, Levant B, Taylor BC, Ahlbrand R, Liu Y, Sullivan JR, et al. C57BL/6J mice exhibit reduced dopamine D3 receptor-mediated locomotor-inhibition relative to DBA/2J mice. Neuroscience. 2006;143:141–153. doi: 10.1016/j.neuroscience.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. The polyunsaturated fatty acid composition of hepatic and plasma lipids differ by both sex and dietary fat intake in rats. J Nutr. 2010;140:245–250. doi: 10.3945/jn.109.115691. [DOI] [PubMed] [Google Scholar]
- 25.de Antueno RJ, Elliot M, Horrobin DF. Liver delta 5 and delta 6 desaturase activity differs among laboratory rat strains. Lipids. 1994;29:327–331. doi: 10.1007/BF02537185. [DOI] [PubMed] [Google Scholar]
- 26.Xiao Y, Huang Y, Chen ZY. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br J Nutr. 2005;94:544–550. doi: 10.1079/bjn20051539. [DOI] [PubMed] [Google Scholar]