Abstract
The CD-1 mouse strain is known to have early onset of hearing loss that is progressive with aging. We sought to determine whether a disturbance of K+ homeostasis and pathological changes in the cochlear lateral wall were involved in the age-related hearing loss (AHL) of CD-1 as compared to the CBA/CaJ strain which has minimal AHL. In the present study, the endocochlear potential (EP) and endolymphatic K+ concentration ([K+]e) were measured in both strains of mice with double-barrel microelectrodes at “young” (1–2 mo) and “old” (5–9 mo) ages. CBA/CaJ mice displayed no changes with aging in EP and [K+]e of the basal turn. In the apical turn, there was a small positive shift of the EP (10 mV) with aging under both normoxic and acute anoxic conditions (−EP), without any change of [K+]e. Further, there were no obvious pathological changes in the lateral wall of CBA/CaJ mice. By contrast, old CD-1 mice displayed a significantly reduced [K+]e by 30% in both basal and apical turns with no significant changes in normoxic EP. The −EP in the apical turn was significantly reduced in magnitude by 6 mV. A severe loss of cells with aging was observed in the region of type IV fibrocytes of the apical and basal turns and of type II fibrocytes in the basal turn. A complete degeneration of organ of Corti was also observed at the basal turn of old CD-1 mice, as well as a basalward decline of spiral ganglion neuron density. The pathological changes in spiral ligament of CD-1 mice were similar to those of an inbred mouse strain C57BL/6J that expresses an AHL gene (ahl) and might be a primary etiology of AHL of CD-1 mice. These findings have ramifications for our understanding of AHL and for interpretation of genetic mutations in a CD-1 background.
Keywords: stria vascularis, endocochlear potential, cochlea, potassium concentration, histology, age-related hearing loss
INTRODUCTION
Age-related hearing loss (AHL) affects 50% of the population in developed countries by 80 years of age, due partly to genetic factors (Van Laer and Van Camp 2001). The atrophy of the cochlear lateral wall, especially stria vascularis, was regarded early in the 1970s to be an important aspect of pathology of presbycusis in humans (Schuknecht et al. 1974; Schuknecht 1974).
Presbycusis has been studied with several animal models of AHL, including gerbils and several strains of mice (Jimenez et al. 1999; Johnson et al. 2000; Schulte and Schmiedt 1992). AHL has been attributed to pathological and functional degeneration in the cochlear lateral wall, including atrophy of stria vascularis, reduced staining of Na+,K+–ATPase and connexin 26, and the decline of EP in aged gerbils (Gratton et al. 1996; Ichimiya et al. 2000; Schmiedt 1996). Spiral ligament degeneration was observed as a loss of fibrocytes in aged C57BL/6 mice (Hequembourg and Liberman 2001).
The cochlear lateral wall is known to play a critical role in driving transduction currents by generating the endocochlear potential (EP) and by maintaining the high K+ concentration in the endolymph (Dallos 1996; Wangemann 2002). Disturbance with aging of K+ cycling in the lateral wall may therefore be involved in the process of AHL. Thus, it was of interest to seek direct evidence regarding the possible relationship between endolymphatic K+ homeostasis and AHL.
The CD-1 mouse strain was of particular interest due to its use in transgenic mutation studies (Cowan et al. 2000). The CD-1 mouse strain is also a special example of AHL, exhibiting early sensorineural hearing loss (Le Calvez et al. 1998a; Shone et al. 1991) progressing from 3 weeks after birth. Concurrent with the hearing loss is a progressive loss of both inner and outer hair cells (Shone et al. 1991). CD-1 mice older than 4 months consistently had extensive hair cell loss throughout the cochlea: from 80% to 100% loss of outer hair cells (OHCs) and 7–15% loss of inner hair cells (IHCs) (Le Calvez et al. 1998a, 1998b; Shone et al. 1991). In 6-month-old CD-1 mice, no hair cells were present in the basal cochlear turn and the basilar membrane was covered with a simple epithelium. In the apical turn, about 50% of the IHCs and 80% of the OHCs were missing (Shone et al. 1991).
CD-1 mice are outbred, which could lead to some heterogeneity in the characteristics of hearing loss of individual CD-1 mice. However, consistent observations of severe hearing loss and degeneration of hair cells in the previous studies and the changes in endolymph homeostasis reported here suggest that this variation has not presented critical obstacles to the study of this strain.
By contrast, CBA/CaJ is an inbred strain of mouse that has been accepted as a control in studies on AHL since they maintain their hearing well into advanced age (Zheng et al. 1999). However, even the hearing of CBA/CaJ mice is not totally age-invariant. There was a slight elevation of ABR thresholds (10 dB) at low frequencies (<10 kHz) of very old mice (14 months), while the histological appearance of the stria vascularis, the spiral ligament, and the hair cell populations remained normal at 25 months of age (Hequembourg and Liberman 2001).
The goal of the present study was to determine whether there were age-related changes of EP and/or endolymphatic potassium homeostasis in CD-1 and CBA/CaJ mouse strains and possible associated histopathological changes. In this study, EP was measured at both the apical and basal turns, which correspond to low and high best frequencies, respectively. −EP was used as an indicator of hair cell function since −EP represents a fraction (voltage-divider) of the hair cell basolateral membrane voltage in scala media (Dallos 1996, Konishi et al. 1967, 1978; Marcus 1986; Syka et al. 1981).
MATERIALS AND METHODS
Experimental groups
The CD-1 mice (Charles River Laboratory, Wilmington, MA) were divided into two groups: a “young” group aged from 1 to 1.5 months and an “old” group aged from 5 to 7 months. This classification was based on published reports of the CD-1 strain. In the mice aged less than 3 months, 95% IHC and OHC looked normal, while the mice older than 4 months always had >70 dB hearing loss of ABR threshold at all frequencies tested and had extensive hair cell loss throughout the cochlea (Le Calvez et al. 1998a, 1998b). CBA/CaJ mice (Jackson Laboratory, Bar Harbor, ME) were similarly grouped as “young” (1–2 months) and “old” (8–9 months).
Measurement of EP, −EP, and potassium concentration
CD-1 and CBA/CaJ mice were anesthetized by intraperitoneal injection of inactin (thiobutabarbital sodium, 140 mg/kg; #T-133, Sigma Chemical, St. Louis, MO). The methods for recording EP and K+ concentration with double-barrel K+ selective electrodes were described previously (Marcus et al. 2002). Briefly, the bulla of the mouse temporal bone was exposed by a ventrolateral approach with body temperature maintained at 37°C. Lateral wall access to the apical turn of the cochlea and round window access to the basal turn (Fig. 1) were made in different ears to avoid any possible interaction of the measurements.
Figure 1.
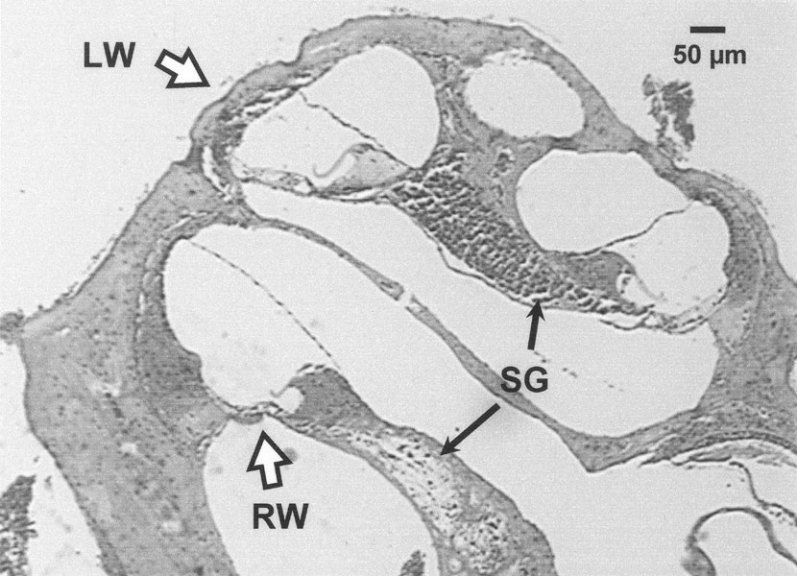
Photomicrograph of the cochlea of 24-week-old CD-1 mouse. The places of electrode penetration into scala media [the lateral wall (LW) at the apical turn and round window (RW) at the basal turn] are indicated with hollow arrows. A basalward loss of density of the spiral ganglion (SG) is indicated with filled arrows. Corresponding to the neuronal loss, the organ or Corti at the basal turn displayed a severe degeneration. In contrast, at the apical turn the organ or Corti appeared normal.
Double-barrel electrodes were constructed as before for measurement of EP and [K+] (Marcus et al. 2002). Briefly, the ion-selective barrel was silanized with dimethyldichlorosilane (Sigma-Aldrich, St. Louis; #440212) vapors and the electrode was baked at 200°C for 2 h. Tips were enlarged to an outer diameter of 2 µm. The silanized barrel was filled with K+ liquid ion exchanger (IE-190, World Precision Instrument, Sarasota, FL) and the reference barrel was filled with 0.5 M NaCl. Each barrel was connected to an input of a dual-channel electrometer with a Ag/AgCl wire. The K+ electrode was calibrated at 37°C before and after the cochlear measurements using solutions of constant [K+] + [Na+].
Recordings of the voltage of each barrel of the electrodes were made with respect to a ground Ag/AgCl electrode inserted into the neck muscles. The entry of the electrode tip into endolymph was characterized by fast changes of the recorded potentials and by a stable recording after penetration. The signals from the two channels of the electrometer were digitized (16-bit) at 480 samples/s and averaged at 0.5 s intervals.
The EP was also measured after about 5 min acute anoxia (termed the −EP) at the apical turn. Anoxia was produced by intramuscular injection of succinylcholine chloride (2 mg/g mouse weight; #85980, Fluka, Milwaukee, WI).
The recording locations (“LW” and “RW” in Fig. 1) were determined with respect to the cochlear base by microdissection and measurement of the component segments of the basilar membrane. The recordings from the basal turn were made at 5% and from the apical turn at 65–74% from the cochlear base. These positions correspond to best frequencies of 67 and 4.9–7.3 kHz based on the relationship determined by Ehret (1983): f (kHz) = 3.109 × (10(100−d) × 0.0142 − 0.7719), where d is the distance from the base in percent.
Histological study
Animals were intracardially perfused with 2.5% glutaraldehyde and 1.5% paraformaldehyde in phosphate buffered saline (PBS): NaCl, 150 mM; KH2PO4, 2 mM; Na2HPO4 2H2O, 8 mM; pH 7.2–7.4. Temporal bones were removed and the cochlea was perfused with the above fixative through the oval and round windows to an outlet in the apex. After 24 h postfixation in the same fixative at 4°C, temporal bones were decalcified for 24 h in 2.4 M formic acid with 1 M formaldehyde (Fisher Scientific, CS511-1D). Inner ears were then rinsed in PBS, dehydrated through a graded series of alcohol and xylene, and embedded in paraffin. Embedded ears were sectioned at 5 µm thickness and stained with eosin and hematoxylin.
All procedures conformed to protocols approved by the Institutional Animal Care and Use Committee of KSU.
Data are expressed as the mean ± SEM (n= number of ears). Increases or decreases were considered significant at a level of p < 0.05. An unpaired t-test was used for comparison of each pair of groups (old and young).
RESULTS
CBA/CaJ strain
The EP of the apical turn in CBA/CaJ mice increased significantly with age by 11 mV (Fig. 2A, Table 1). Concurrently, the −EP in old mice also significantly shifted in the positive direction by 11 mV, compared with young mice (Fig. 2A, Table 1). By contrast, there was no significant age-related change in EP of the basal turn (Fig. 2B, Table 1). There were also no significant differences in the [K+]e of either the apical (Fig. 2A, Table 1) or basal turn (Fig. 2B) between young and old CBA/CaJ mice.
Figure 2.
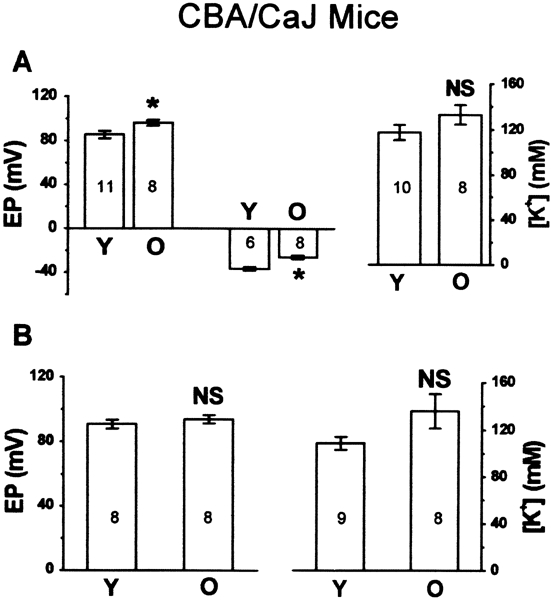
Age-related changes of endocochlear potential (EP) and endolymphatic potassium concentration ([K+]) in CBA/CaJ mice. A. Apical turn: left panel:EP of young (Y) and old (O) groups, including normoxic EP (left two bars) and anoxic −EP (right two bars); right panel: [K+] of young and old groups. B. Basal turn: left panel: normoxic EP of young and old groups; right panel: [K+] of young and old groups. NS, p > 0.05; *, p < 0.05.
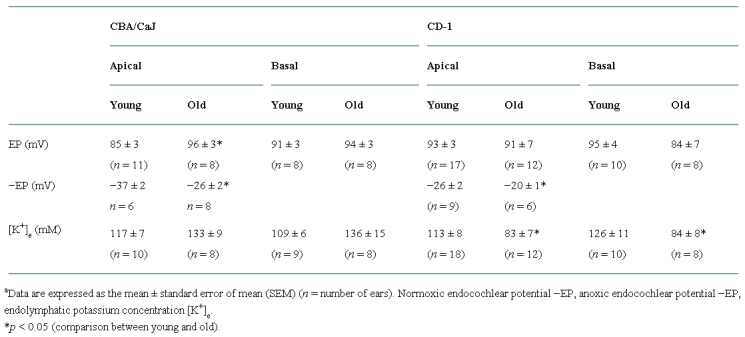
Table 1.
Data summary of normoxic and anoxic endocochlear potential and potassium concentrationsa
Histological sections did not exhibit any obvious pathological change in the organ of Corti, stria vascularis, and spiral ligament of either basal turn (Fig. 3B, D) or apical turn (not shown) of the cochlea of old CBA/CaJ (n = 6) compared with young CBA/CaJ (n = 6) (Fig. 3A, C). This result is consistent with previous findings (Hequembourg and Liberman 2001).
Figure 3.
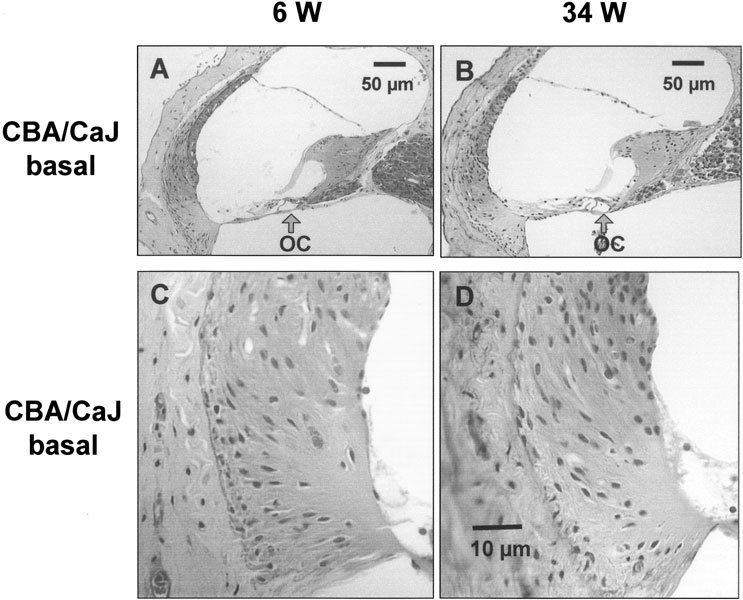
Representative light micrographs of the basal cochlear turn from young and old CBA/CaJ mice. A. The cochlear duct of a 6-week-old mouse (6 W). B. The cochlear duct of a 34-week-old mouse (34 W). Both A and B exhibited almost normal appearance of organ of Corti (OC). C. The basal part of spiral ligament from 6-week-old-mouse (6 W). D. The basal part of spiral ligament from 34-week-old-mouse (34 W). No obvious pathological changes were found in both C and D. The scale bar in D also applies to C.
The pillar cells and the tunnel of Corti were fully developed in both young and old CBA/CaJ mice (Fig. 3A,B), consistent with mature development of the organ of Corti (Lim and Rueda 1992; Pujol and Hilding 1973). The level of observation with the light microscope using paraffin sections in our study does not preclude the presence of more subtle abnormalities.
CD-1 mouse strain
Representative recordings of EP and [K+]e from the apical turn are shown in Figure 4. Our most striking finding was the presence of significant decreases in the [K+]e of both the apical (Fig. 5A, Table 1) and basal turns (Fig. 5B, Table 1) from young to old CD-1 mice. The normoxic EP of both the apical and basal turns, however, was unchanged between young and old CD-1 mice (Fig. 5A, B, Table 1). The −EP (Fig. 5A, Table 1) shifted significantly in the positive direction from young mice to old mice, suggesting compromised function of hair cells in the apical turn. The result was consistent with previous audiological and histological findings in old CD-1 mice at comparable best frequency locations.
Figure 4.
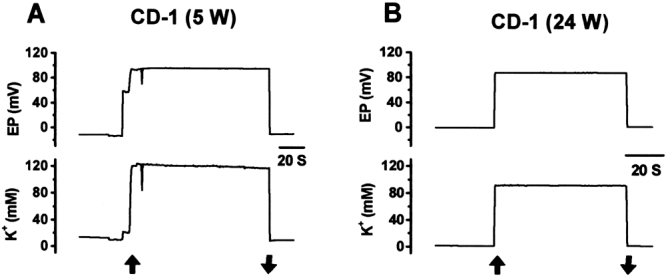
Representative recordings of EP and endolymphatic potassium concentration ([K+]) from apical cochlear turn of young and old CD-1 mice. A. A 5-week-old mouse (5 W). B. A 24-week-old mouse (24 W). Vertical arrows indicate penetration and withdrawal from scala media.
Figure 5.
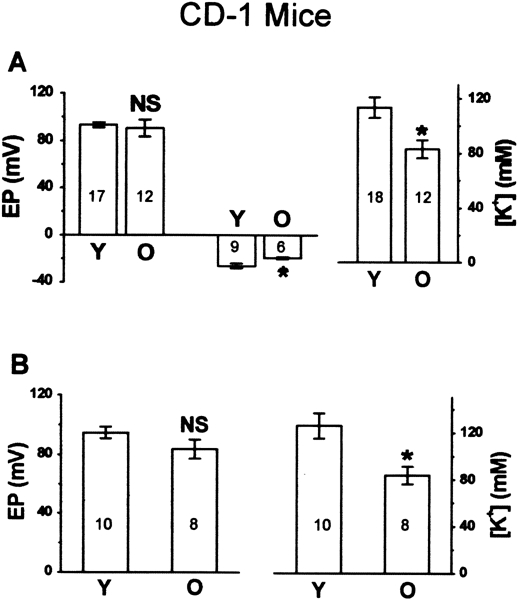
Age-related changes of endocochlear potential (EP) and endolymphatic potassium concentration ([K+]) in CD-1 mice. A. Apical turn: left panel: EP of young (Y) and old (O) groups, including normoxic Ep (left two bars) and anoxic −EP (right two bars); right panel: [K+] of young and old groups. B. Basal turn: left panel: normoxic EP of young and old groups; right panel: [K] of young and old groups. NS, p > 0.05; *, p < 0.05.
The histological findings were consistent in all sections from 6 cochleas of old CD-1 and 4 cochleas of young CD-1 (Fig. 6). The most striking finding was seen in the spiral ligament. A severe loss of cells in old CD-1 mice (n = 6) was observed in the region of the type II fibrocytes (apical turn, Fig. 6B) or the type II and IV fibrocytes (basal turn, Fig. 6D) at the lower part of the spiral ligament compared with young CD-1 mice (n = 4). The location of the different types of fibrocytes is illustrated in Figure 7, based on the classification made by Spicer and Schulte (1991) and Schulte and Schmiedt (1992). The young group of CD-1 mice had a clear but less severe cell loss in the type IV fibrocytes of the basal turn (Fig. 6C) but not in the apical turn (Fig. 6A).
Figure 6.
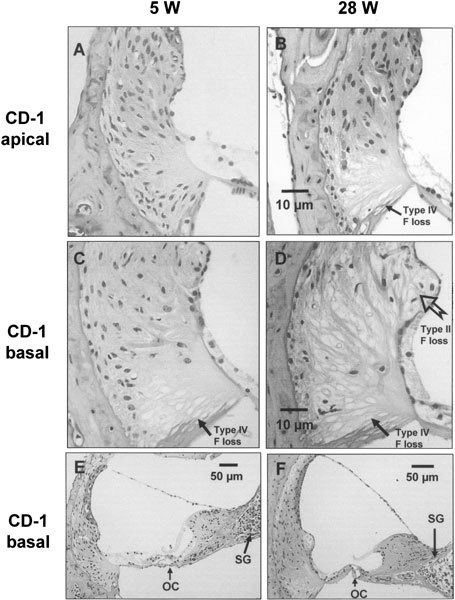
Representative light micrographs from young (5-week-old, 5 W) and old (28-week-old, 28 W) CD-1 cochleas. In the apical turn (A and B at the same scale), there was a loss of type IV fibrocytes (F) at the basal part of the spiral ligament of 28 W (B), compared with that of 5 W (A). In the basal turn (C and D at the same scale), there was a slight loss of type IV fibrocytes at the basal part of spiral ligament of 5 W (C), whereas a more severe and extensive loss of type II and IV fibrocytes in 28 W (D) was observed. In comparison with the normal appearance ofthe organ of Corti (OC) of 5 W basal turn (E), the OC from 28 W basal turn (F) was replaced by simple epithelial cells lacking a distinguishable morphology of the hair cells. The density of the spiral ganglion (SG) in the basal turn of 28 W (F) was clearly lower than that of 5 W (E).
Figure 7.
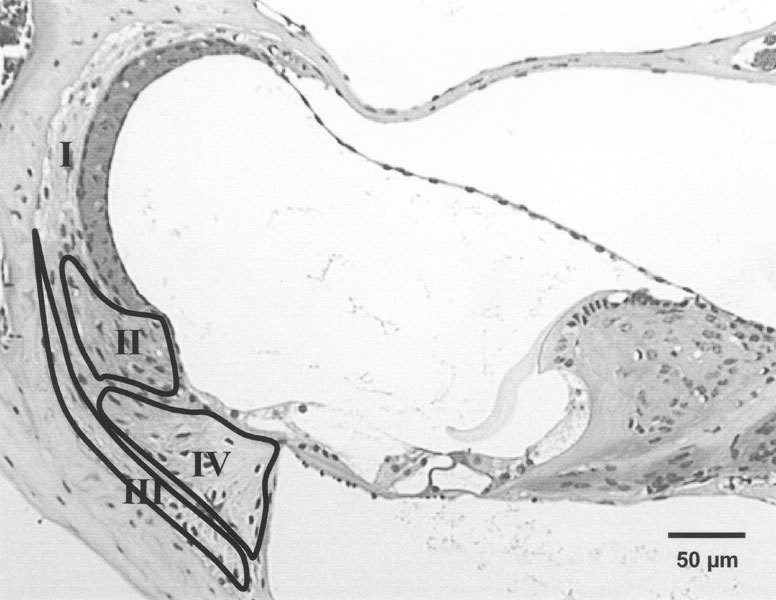
Micrograph of basal cochlear turn of 4-week-old CBA/CaJ mouse demonstrating the locations of type I, II, III, and IV fibrocytes, based on the studies by Spicer and Schulte (1991). The areasof types II, III, and IV fibrocytes are separately represented with labeled outlines and the type I fibrocytes in the rest of the spiral ligament.
The loss of the fibrocytes in old CD-1 was more severe at the basal turn (Fig. 6D) than at the apical turn (Fig. 6B) where there was loss of only type IV fibrocytes. No obvious atrophy of the stria vascularis in old CD-1 mice was observed compared with the young group (not shown). A complete degeneration of the organ of Corti was also observed at the basal turn of old CD-1 mice (Fig. 6F), as reported previously (Shone et al. 1991). The whole organ of Corti was replaced by simple epithelial cells lacking any distinguishable morphology of hair cells.
In contrast to the clear degeneration of organ of Corti observed in the basal turn of old CD-1 mice, the organ of Corti appeared nearly normal in the basal turn of young mice (Fig. 6E) and in the apical turn of both young and old CD-1 mice (not shown). Well-developed pillar cells and an open tunnel of Corti were also observed in the three normal-appearing conditions. In spite of the normal appearance, a small difference in apical hair cell function between young and old CD-1 mice was suggested by changes in the −EP.
The density of the spiral ganglion decreased in both the apical (not shown) and basal turns (Fig. 6E, F) of old CD-1 mice compared with the young group. The neuronal density in old CD-1 presented a basalward gradient of reduction (Fig. 1). The pattern was similar to the pattern of fibrocyte loss and to that of hair cell loss reported previously (Le Calvez et al. 1998a, 1998b; Shone et al. 1991). The neuronal loss of old CD-1 may be secondary to the loss of IHCs as seen in either acoustic injury or by ototoxic drugs (Kiang et al. 1976; Spoendlin 1984).
DISCUSSION
A histopathological classification of human presbycusis has been proposed from the temporal bone studies of Schuknecht and colleagues (Schuknecht et al. 1974; Schuknecht 1974). Four categories are: (1) Sensory, characterized by degeneration of hair cells; (2) Neural, characterized by neuronal loss in the cochlear and central nervous system; (3) Metabolic, characterized by atrophy of the cochlear lateral wall; and (4) Mechanical, characterized by inner ear conductive hearing loss. The first three categories were confirmed and expanded in several species of experimental animals (Adams and Schulte 1997; Hequembourg and Liberman 2001), including gerbils and mice; all three categories are usually present in each animal. A genetic basis of AHL was suggested when an important gene affecting AHL (ahl) was discovered in C57BL/6J, an extensively studied strain, and in nine other inbred mouse strains (Johnson et al. 2000). The mouse provides an excellent animal model for studying genetically based deafness due to the well-established homology between the mouse and human genomes, the similarity of the cochlear structure, the short gestation time, and the similarity of the hereditary pathologic manifestations in the inner ear of both humans and mice (Brown and Steel 1994; Steel and Brown 1996).
AHL in the CD-1 strain has not been studied as extensively as in C57BL/6, although both mouse strains are known to have early sensorineural hearing loss that is probably genetically determined (Le Calvez et al. 1998a, Shone et al. 1991; Walton et al. 1995; Willott 1986). The genetic basis of AHL in the CD-1 strain is not yet known, partly because this strain was outbred and therefore without a strictly identical genetic background. Nonetheless, some data in this study are strongly consistent with those of previous studies, suggesting a close genetic similarity of the CD-1 mice used in both the present and previous studies. Consistent similarities include the apical to basal gradient of organ of Corti and spiral ganglion degeneration with age and their correlation with the gradient of hearing loss from low to high frequencies.
The most striking histopathological changes in CD-1 mice occurred in the lateral cochlear wall: type II and IV fibrocyte loss in the lower part of spiral ligament. The degeneration of fibrocytes in CD-1 mice was similar to the observations in C57BL/6 mice (Hequembourg and Liberman 2001), but displayed a greater severity and extent. More intriguingly, the type IV fibrocyte loss even occurred in the basal turn of young CD-1 mice, which had an apparently normal appearance of organ of Corti. This finding suggested that the pathology in the spiral ligament may be part of the etiology of age-related hearing loss in the CD-1 mouse strain.
The most important finding in CD-1 mice was the decline in [K+]e by about 30% in the older animals. This decrease occurred in the absence of any significant change in normoxic EP, although there was a small decrease in the magnitude of the anoxic EP. The underlying cellular mechanism(s) is unknown. Some possible explanations are suggested by the histologic changes.
Firstly, it is known that the high K+ in endolymph originates from secretion by the stria vascularis, normally associated with an EP of +80 mV (Marcus et al. 2002; Wangemann 2002). It has been proposed that K+ efflux from scala media at the organ of Corti cycles back to the stria vascularis by diffusion through extracellular and/or intracellular routes to the spiral ligament and then moves through the fibrocytes to the stria (Spicer and Schulte 1991; Weber et al. 2001). Since the type II and IV fibrocytes occupy the space in the lateral wall between the stria vascularis and the organ of Corti (Spicer and Schulte 1991), the loss of these fibrocytes in the spiral ligament could reduce the flux of K+ supplying the stria vascularis and thereby the secretory flux into scala media. The pattern of fibrocyte loss in old CD-1 mice corresponded with that of the hair cell degeneration found in present and previous studies, i.e., the hair cell loss started from the basal turn and advanced to the apical turn with aging (Le Calvez et al. 1998a; Shone et al. 1991).
Secondly, the unchanged EP in old CD-1 mice is not surprising since the EP is thought to be the result of a large positive potential generated in the stria vascularis (Marcus et al. 2002) and a smaller negative component generated in the hair cells (Konishi et al. 1967). The hypothesis has been supported by numerous experiments (Konishi et al. 1979; Salt and Konishi 1979; Syka et al. 1981). A decreased rate of K+ secretion by the strial marginal cells would be expected to cause a reduction in the positive component of the EP (Wangemann 2002). This apparently occurred in CD-1 mice since the −EP was significantly reduced and the normoxic EP remained unchanged.
Thirdly, another likely explanation for the unchanged EP with reduced [K+]e in old CD-1 mice is that there may have been an increase of the impedance of scala media resulting from degeneration of hair cells. There would then need to be less K+ influx current from stria to sustain a relatively normal EP. It must be recalled, however, that the rate of K flux by the strial marginal cells is coupled to the voltage generated within the stria by the basal/intermediate cell syncytium (Marcus et al. 2002). These three hypotheses are not mutually exclusive.
An unchanged EP with aging was also recently reported in preliminary form for three inbred mouse strains (C57BL, BALB, 129S6) that express the ahl gene (Ohlemiller et al. 2002). However, in a few studies of old gerbils, unchanged [K+]e (Schmiedt 1996) and decreased EP accompanied by loss of immunostaining of Na+,K+–ATPase in the cochlear lateral wall were reported (Gratton et al. 1995, 1997; Schmiedt 1996; Schulte and Schmiedt 1992). There were no data on audiology and histology of hair cell loss or degeneration in any of these studies. It is therefore difficult to interpret and compare their results, since the magnitude of the EP is determined by the functional state of both the lateral wall and the hair cells. The variability among rodent species and strains points to a multitude of origins of AHL.
Since the blood osmolarity of old CD-1 was 314 ± 2.5 mOsm (n = 3, unpublished observations), the reduction of [K+]e to around 83 mM without any sign of shrinkage of the endolymphatic space (scala media) in old CD-1 suggests that some other ions or solutes might contribute to the maintenance of endolymphatic isotonicity with serum. The most likely candidate is the other predominant cation, Na+, which is known to increase during other conditions that lead to reduction of [K+]e such as anoxia (Sellick and Johnstone 1972).
In the present study, the endolymphatic K+ in both mouse strains was lower than the mean commonly taken to represent mammalian endolymph, e.g., 150–160 mM (Wangemann and Schacht 1996). We also previously found relatively low [K+]e (113 mM) in wild-type mice of a mixed 129Sv and C57BL/6 genetic background in another study from our lab (Marcus et al. 2002). We believe that the difference of [K+]e is related to differences in the strains and species rather than a systematic error in methodology. Two points support this contention. First, our electrodes had a sensitive response of 60 mV per decade [K+] change at 37°C that yielded stable recordings (Fig. 4) and consistent [K+]e during three technically distinct recording situations in the apical turn (via lateral wall), the basal turn (via round window), and the utricle (unpublished data). Second, the reduction of [K+]e could not be generated by systematic hypo-osmolarity due to the normal range of osmolarity found in the blood of old CD-1 mice.
The only other study regarding [K+]e measurement in mice yielded an unusually high [K+]e in adult ICR strain mice: 200 mM (Yamasaki et al. 2000). The basis for the difference from our results is not presently known. There was also substantial variability reported for values of [K+]e in other rodents: guinea pigs, 114 mM (Konishi and Salt 1983), and gerbils, 178 mM (Schmiedt 1996).
Another important ramification of the disturbed K+ homeostasis in old CD-1 mice is the use of this strain for the study of genetic mutations (Cowan et al. 2000). The use of the CD-1 background in the study of the effects of genetic mutations on cochlear function should clearly be performed with young, age-matched controls and interpreted with caution.
In CBA/CaJ mice, the 11 mV positive shift of the −EP in the apical turn is consistent with the previous finding that there was an elevation in the ABR threshold (10 dB) at low frequencies (<10 kHz) in 14-month-old CBA/CaJ mice (Hequembourg and Liberman 2001). The recording location in the present study in the 4.9–7.3 kHz region is within the frequency range of the previous ABR measurements. The shift in −EP is therefore consistent with functional hair cell damage that is reflected in a slight loss of hearing as assessed by the ABR. In striking contrast with the CD-1 strain, unchanged [K+]e with age in the CBA/CaJ strain is consistent with the normal histological appearance of the cochlear lateral wall.
In summary, we have shown for the first time that AHL in CD-1 mice is associated with a significant reduction in [K+]e without a change in normoxic EP. The pathology in the spiral ligament may be related to this decreased [K+]e and appears similar to metabolic human presbycusis. The ahl gene was speculated to be involved in the AHL of CD-1, considering the similar pathology between CD-1 and C57BL/6J. By contrast, the CBA/CaJ strain has minimal AHL and does not display an age-related change in [K+]e.
Acknowledgements
This work was supported by research grant number 5R01-DC00212 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health. We thank Shelly Christenson and Shon Koenig of the KSU Histology Diagnostic Laboratory for their excellent assistance in preparation of histologic samples.
References
- 1.Adams JC, Schulte BA. Histopathologic observations of the aging gerbil cochlea. Hear. Res. 1997;104:101–111. doi: 10.1016/S0378-5955(96)00184-0. [DOI] [PubMed] [Google Scholar]
- 2.Brown SD, Steel KP. Genetic deafness—progress with mouse models. Hum. Mol. Genet. 1994;3 Spec No:1453–1456. doi: 10.1093/hmg/3.suppl_1.1453. [DOI] [PubMed] [Google Scholar]
- 3.Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 4.Dallos P. Overview: cochlear neurobiology. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer-Verlag; 1996. pp. 1–43. [Google Scholar]
- 5.Ehret G. Peripheral Anatomy and Physiology II. In: Willott JF, editor. The Auditory Psychobiology of the Mouse. Springfield, IL: Charles C. Thomas; 1983. pp. 169–200. [Google Scholar]
- 6.Gratton MA, Smyth BJ, Schulte BA, Vincent Jr DA. Na,K–ATPase activity decreases in the cochlear lateral wall of quiet-aged gerbils. Hear. Res. 1995;83:43–50. doi: 10.1016/0378-5955(94)00188-V. [DOI] [PubMed] [Google Scholar]
- 7.Gratton MA, Schmiedt RA, Schulte BA. Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis [corrected and republished article originally printed in Hear. Res. 1996 May;94(1–2):116–24]. Hear. Res. 1996;102:181–190. doi: 10.1016/S0378-5955(96)00130-X. [DOI] [PubMed] [Google Scholar]
- 8.Gratton MA, Smyth BJ, Lam CF, Boettcher FA, Schmiedt RA. Decline in the endocochlear potential corresponds to decreased Na,K–ATPase activity in the lateral wall of quiet-aged gerbils. Hear. Res. 1997;108:9–16. doi: 10.1016/S0378-5955(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 9.Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J. Assoc. Res. Otolaryngol. 2001;2:118–129. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichimiya I, Suzuki M, Mogi G. Age-related changes in the murine cochlear lateral wall. Hear. Res. 2000;139:116–122. doi: 10.1016/S0378-5955(99)00170-7. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez AM, Stagner BB, Martin GK, Lonsbury–Martin BL. Age-related loss of distortion product otoacoustic emissions in four mouse strains. Hear. Res. 1999;138:91–105. doi: 10.1016/S0378-5955(99)00154-9. [DOI] [PubMed] [Google Scholar]
- 12.Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- 13.Kiang NY, Liberman MC, Levine RA. Auditory-nerve activity in cats exposed to ototoxic drugs and high-intensity sounds. Ann. Otol. Rhinol. Laryngol. 1976;85:752–768. doi: 10.1177/000348947608500605. [DOI] [PubMed] [Google Scholar]
- 14.Konishi T, Kelsey E, Singleton GT. Negative potential in scala media during early stage of anoxia. Acta Otolaryngol. 1967;64:107–118. doi: 10.3109/00016486709139097. [DOI] [PubMed] [Google Scholar]
- 15.Konishi T, Hamrick PE, Walsh PJ. Ion transport in guinea pig cochlea. I. Potassium and sodium transport. Acta Otolaryngol. (Stockh.) 1978;86:22–34. doi: 10.3109/00016487809124717. [DOI] [PubMed] [Google Scholar]
- 16.Konishi T, Salt AN, Hamrick PE. Effects of exposure to noise on ion movements in guinea pig cochlea. Hear. Res. 1979;1:325–342. doi: 10.1016/0378-5955(79)90004-2. [DOI] [PubMed] [Google Scholar]
- 17.Konishi T, Salt AN. Electrochemical profile for potassium ions across the cochlear hair cell membranes of normal and noise-exposed guinea pigs. Hear. Res. 1983;11:219–233. doi: 10.1016/0378-5955(83)90080-1. [DOI] [PubMed] [Google Scholar]
- 18.Le Calvez S, Avan P, Gilain L, Romand R. CD1 hearing-impaired mice. I: Distortion product otoacoustic emission levels, cochlear function and morphology. Hear. Res. 1998a;120:37–50. doi: 10.1016/s0378-5955(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 19.Le Calvez S, Guilhaume A, Romand R, Aran JM, Avan P. CD1 hearing-impaired mice. II. Group latencies and optimal f2/f1 ratios of distortion product otoacoustic emissions, and scanning electron microscopy. Hear. Res. 1998b;120:51–61. doi: 10.1016/s0378-5955(98)00051-3. [DOI] [PubMed] [Google Scholar]
- 20.Lim DJ, Rueda J. Structural development of the cochlea. In: Romand R, editor. Development of Auditory and Vestibular Systems, Vol 2. New York: Elsevier; 1992. pp. 33–58. [Google Scholar]
- 21.Marcus DC. Nonsensory electrophysiology of the cochlea: stria vascularis. In: Altschuler RA, Hoffman DW, Bobbin RP, editors. Neurobiology of Hearing: The Cochlea. New York: Raven; 1986. pp. 123–137. [Google Scholar]
- 22.Marcus DC, Wu T, Wangemann P, Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am. J. Physiol. Cell Physiol. 2002;282:C403–C407. doi: 10.1152/ajpcell.00312.2001. [DOI] [PubMed] [Google Scholar]
- 23.Ohlemiller KK, Lear PM, Verges DK. The mouse Ahl gene may not affect the cochlear lateral wall, and does not lead to reduction of the endocochlear potential (EP). Assoc. Res. Otolaryngol. Abstr. 2002;264:. [Google Scholar]
- 24.Pujol R, Hilding D. Anatomy and physiology of the onset of auditory function. Acta Otolaryngol. 1973;76:1–10. doi: 10.3109/00016487309121476. [DOI] [PubMed] [Google Scholar]
- 25.Salt AN, Konishi T. Effects of noise on cochlear potentials and endolymph potassium concentration recorded with potassium-selective electrodes. Hear. Res. 1979;1:343–363. doi: 10.1016/0378-5955(79)90005-4. [DOI] [PubMed] [Google Scholar]
- 26.Schmiedt RA. Effects of aging on potassium homeostasis and the endocochlear potential in the gerbil cochlea. Hear. Res. 1996;102:125–132. doi: 10.1016/S0378-5955(96)00154-2. [DOI] [PubMed] [Google Scholar]
- 27.Schuknecht HF. Pathology of the Ear. Cambridge, MA: Harvard University Press; 1974. pp. 388–403. [Google Scholar]
- 28.Schuknecht HF, Watanuki K, Takahashi T, Belal Jr AA, Kimura RS, Jones DD, Ota CY. Atrophy of the stria vascularis, a common cause for hearing loss. Laryngoscope. 1974;84:1777–1821. doi: 10.1288/00005537-197410000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Schulte BA, Schmiedt RA. Lateral wall Na,K–ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear. Res. 1992;61:35–46. doi: 10.1016/0378-5955(92)90034-K. [DOI] [PubMed] [Google Scholar]
- 30.Sellick PM, Johnstone BM. Changes in cochlear endolymph Na+ concentration measured with Na+ specific microelectrodes. Pflugers Arch. 1972;336:11–20. doi: 10.1007/BF00589137. [DOI] [PubMed] [Google Scholar]
- 31.Shone G, Raphael Y, Miller JM. Hereditary deafness occurring in cd/1 mice. Hear. Res. 1991;57:153–156. doi: 10.1016/0378-5955(91)90084-M. [DOI] [PubMed] [Google Scholar]
- 32.Spicer SS, Schulte BA. Differentiation of inner ear fibrocytes according to their ion transport related activity. Hear. Res. 1991;56:53–64. doi: 10.1016/0378-5955(91)90153-Z. [DOI] [PubMed] [Google Scholar]
- 33.Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Ann. Otol. Rhinol. Laryngol. Suppl. 1984;112:76–82. doi: 10.1177/00034894840930s415. [DOI] [PubMed] [Google Scholar]
- 34.Steel KP, Brown SD. Genetics of deafness. Curr. Opin. Neurobiol. 1996;6:520–525. doi: 10.1016/S0959-4388(96)80059-6. [DOI] [PubMed] [Google Scholar]
- 35.Syka J, Melichar I, Ulehlova L. Longitudinal distribution of cochlear potentials and the K+ concentration in the endolymph after acoustic trauma. Hear. Res. 1981;4:287–298. doi: 10.1016/0378-5955(81)90013-7. [DOI] [PubMed] [Google Scholar]
- 36.Van Laer L, Van Camp G. Genes in the ear: what have we learned over the last years? Scand. Audiol. 2001;Suppl.:44–53. doi: 10.1080/010503901750166646. [DOI] [PubMed] [Google Scholar]
- 37.Walton JP, Frisina RD, Meierhans LR. Sensorineural hearing loss alters recovery from short-term adaptation in the C57BL/6 mouse. Hear. Res. 1995;88:19–26. doi: 10.1016/0378-5955(95)00093-J. [DOI] [PubMed] [Google Scholar]
- 38.Wangemann P. K+ cycling and the endocochlear potential. Hear. Res. 2002;165:1–9. doi: 10.1016/S0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 39.Wangemann P, Schacht J. Homeostatic mechanisms in the cochlea. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer-Verlag; 1996. pp. 130–185. [Google Scholar]
- 40.Weber PC, Cunningham III CD, Schulte BA. Potassium recycling pathways in the human cochlea. Laryngoscope. 2001;111:1156–1165. doi: 10.1097/00005537-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Willott JF. Effects of aging, hearing loss, and anatomical location on thresholds of inferior colliculus neurons in C57BL/6 and CBA mice. J. Neurophysiol. 1986;56:391–408. doi: 10.1152/jn.1986.56.2.391. [DOI] [PubMed] [Google Scholar]
- 42.Yamasaki M, Komune S, Shimozono M, Matsuda K, Haruta A. Development of monovalent ions in the endolymph in mouse cochlea. ORL J. Otorhinolaryngol. Relat. Spec. 2000;62:241–246. doi: 10.1159/000027753. [DOI] [PubMed] [Google Scholar]
- 43.Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999;130:94–107. doi: 10.1016/S0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


