Abstract
During rodent corticogenesis, a sizeable subpopulation of γ-aminobutyric acid (GABA)ergic interneurons arises extracortically from the medial ganglionic eminence (MGE). These neurons progressively acquire responsiveness to GABA in the course of corticopetal tangential migration, a process regulated by ambient GABA and mediated by GABAA receptors. Here, we combined patch clamp electrophysiology and single-cell reverse transcription–polymerase chain reaction to examine GABAA receptor expression in green fluorescent MGE-derived (eGFP+) cells in telencephalic slices from gestational day 14.5 BAC-Lhx6 embryos. GABA concentration–response curves revealed lower apparent affinity and efficacy in eGFP+ cells in and around the MGE than their counterparts in the cortex. Pharmacological tests revealed subunit-selective response profiles in the MGE and cortex consistent with differential expression of GABAA receptor isoforms. Profiling of GABAA receptor subunit transcripts (α1–5, β1–3, and γ1–3, δ) uncovered increased expression of the α1-, α2-, α5-, γ2-, and γ3-subunit messenger RNAs in the cortex. We propose that the dynamic expression of certain GABAA receptor subunits contributes to assembling receptor isoforms that confer functional attributes important in regulating the migration and maturation of primordial GABAergic cortical interneurons.
Keywords: cortex, GABAA receptor subunits, medial ganglionic eminence, single-cell expression profiling, tangential migration
Introduction
Progenitor cells located in the embryonic ganglionic eminences and preoptic area migrate tangentially into the neocortical anlage and give rise to distinct subpopulations of γ-aminobutyric acid (GABA)ergic interneurons in the adult cerebral cortex (Tamamaki et al. 1997; Lavdas et al. 1999; Anderson et al. 2001; Gelman et al. 2009; Miyoshi et al. 2010). Notably, the medial ganglionic eminence (MGE) is the principal source of GABAergic cortical interneurons that contain somatostatin, parvalbumin, calbindin, and, to a lesser extent, neuropeptide-Y (NPY) (Xu et al. 2004; Fogarty et al. 2007). A variety of extrinsic navigational cues orchestrate neuronal migration from the MGE to the dorsal telencephalon (reviewed by Marin and Rubenstein 2003; Wang and Kriegstein 2009). Among them, ambient GABA, present along the tangential migratory path, tonically activates GABAA receptors in cortex-bound MGE-derived cells, and this has been shown to modulate their migration into the cortical anlage during corticogenesis (Cuzon et al. 2006).
Indeed, GABA has been implicated in cortical development to regulate the proliferation, migration, and differentiation of developing neurons well before morphological signs of synaptogenesis (LoTurco et al. 1995; Behar et al. 1996, 1998, 2000; Ikeda et al. 1997; Manent et al. 2005; Cuzon et al. 2006). MGE-derived cells express functional GABAA receptors and acquire responsiveness to GABA as they migrate from the MGE into the cortex (Cuzon et al. 2006). During corticogenesis, immature cells express GABAA receptors that bind GABA with higher affinity than more mature cells, and their activation elicits depolarizing and slowly desensitizing responses (Owens et al. 1999). Numerous studies have demonstrated brain region–specific yet overlapping and developmentally regulated expression of the GABAA receptor subunits (α1–6, β1–3, γ1–3, δ, ϵ, θ, π, and ρ1–3; e.g., Gambarana et al. 1991; Araki et al. 1992; Laurie et al. 1992; Cheng et al. 2006; Peden et al. 2008; Yu et al. 2009). In addition, the functional and kinetic properties of GABAA receptor isoforms are subunit dependent (Pritchett et al. 1989; Angelotti and Macdonald 1993; Tia et al. 1996; Hutcheon et al. 2000; Boileau et al. 2003; Lagrange et al. 2007; reviewed by Macdonald and Olsen 1994; Sieghart and Sperk 2002). These considerations, taken together, point to immature neurons expressing functionally different GABAA receptor isoforms as they mature.
In this study, we tested the hypothesis that primordial cortical interneurons express multiple GABAA receptor subunits that can form functionally distinct receptor isoforms in the course of tangential migration. To this end, we combined single-cell reverse transcription–polymerase chain reaction (RT-PCR) with whole-cell patch clamp recording to compare the expression profile of GABAA receptor subunit transcripts and the pharmacological properties of GABA-activated responses between cortex-bound MGE-derived cells migrating in the subpallium and their cohorts that have arrived in the cortical anlage. Our results revealed dynamic changes consistent with MGE-derived cells favoring the expression of more mature patterns of GABAA receptor subunits and functional receptor isoforms as they migrate from their site of origin to their destinations in the cortex.
Materials and Methods
All procedures involving animals were in full compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Dartmouth Medical School Institutional Animal Care and Use Committee. Transgenic mice were generated by National Institute of Neurological Disorders and Stroke Gene Expression Nervous System Atlas Bacterial Artificial Chromosome (BAC) Transgenics Project using a BAC vector in which the entire transcription unit and associated regulatory domain for the Lhx6 gene controlled the expression of eGFP (Gong et al. 2003). Since the transcription factor Lhx6 identifies postmitotic neurons generated from the MGE and is required for cortical migration and specification of these cells (Lavdas et al. 1999; Alifragis et al. 2004; Liodis et al. 2007), they fluoresce green, facilitating their identification in acute slices for electrophysiological analysis and harvesting for single-cell expression profiling. The eGFP+-expressing MGE-derived cells are heretofore referred to as eGFP+ cells.
Acute Embryonic Slice Preparation
On embryonic day 14.5 (E14.5), dams were asphyxiated with CO2, and fetuses were removed by caesarian section. BAC-Lhx6 embryos were genotyped by the presence of eGFP fluorescence in the mouth region, visualized using ultraviolet (UV) goggles, since Lhx6 has been implicated in tooth development and palate formation (Grigoriou et al. 1998; Zhang et al. 2002; Denaxa et al. 2009). The brains of BAC-Lhx6 GFP embryos were isolated and immersed in ice-cold oxygenated artificial cerebral spinal fluid (aCSF) containing (in millimolars) NaCl 124, KCl 5.0, MgCl2 2.0, CaCl2 2.0, glycine 0.01, NaH2PO4 1.25, NaHCO3 26, and glucose 10. The brains were then embedded in 3.5% low-melting point agarose (Invitrogen), and coronal slices (250 μm) from the anterior half of the cerebral hemisphere were obtained using a vibroslicer (WPI). For consistency, only slices in which the MGE and lateral ganglionic eminence are demarcated by the ganglionic sulcus and clearly distinguishable were used.
Electrophysiology
The slices were stored at room temperature in a reservoir of oxygenated aCSF prior to electrophysiological recording. Embryonic slices were transferred to a recording chamber, stabilized by an overlaying platinum ring strung with plastic string mesh, and maintained at 32–34 °C on a heated stage fit onto an upright microscope (BX51WI; Olympus). Slices were perfused at a rate of 0.5 mL/min with oxygenated aCSF. eGFP+ cells were identified under fluorescence illumination and Hoffman Modulation Optics (Modulation Optics) using a 40× water immersion objective (3-mm working distance; Olympus). Real-time images were captured using an analog video camera attached to a video frame grabber (Integral Technologies) and displayed on a computer monitor, which also aided the navigation and placement of the drug and recording pipettes. Patch clamp recording pipettes were pulled from borosilicate glass capillaries (1.5-mm outer diameter and 0.86-mm inner diameter; Sutter Instrument Co.) and filled with recording solution composed of (in millimolars) KCl 140, CaCl2 1.8, MgCl2 1.0, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid 5.0, and Mg2+ ATP 3.0. When filled with recording solution, the patch pipettes had resistances of 8–10 MΩ. Recordings were made using an AxoPatch 200A amplifier (Molecular Devices). Whole-cell membrane currents were filtered at 5 kHz, digitized using Clampex v9.0 and analyzed with Clampfit v9.0 (Molecular Devices). Statistical analysis was performed using Sigma Stat 3.0 (SPSS Inc.). Mean peak current amplitude of drug-evoked currents was analyzed using Student's t-Test. Data were reported as mean ± standard error of the mean (mean ± SEM).
Drug Application
GABA (0.1–500 μM; Sigma), diazepam (3 μM; Sigma), L655,708 (10 μM; Tocris), loreclezole (10 μM; Sigma), methyl-6,7-dimethoxy-4-ethyl-beta-carboline-3-carboxylate (DMCM, 100 nM; Tocris), Ro15-4513 (1 μM; Tocris), 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP, 10 μM; Tocris), zinc chloride (100 μM; Sigma), and zolpidem (100 nM; Tocris) were dissolved in aCSF, stored as frozen stock, and diluted to working concentrations with aCSF immediately prior to each recording session. The drug solutions were loaded into separate barrels of an 8-barrel drug pipette assembly and applied using regulated pulses of pressure (≤3 psi, Picospritzer; General Valve Corporation) within 10 μm of the soma of the cell under study. The timing and the duration of the pressure pulses were controlled by a digital timing unit (Pulse train 1831; WPI). One of the barrels of the multibarrel assembly was routinely filled with aCSF, which was applied between drug applications to clear drugs from the vicinity of the cell. This also served to control for any mechanical artifacts that occur occasionally due to bulk flow.
Profiling Expression of GABAA Receptor Subunit Messenger RNAs
Expression profiles of candidate GABAA receptor subunit messenger RNAs were obtained from embryonic MGE and neocortical tissue as well as individual eGFP+ cells employing a RT-PCR–based protocol modified from that published previously (Yeh et al. 2002; Cuzon et al. 2006). For profiling embryonic tissue, E13.5–E14.5 embryonic brains were isolated, and a parasagittal incision was made bilaterally along the dorsal rostral-to-caudal extent of the cortex, thereby splaying the cortical mantle and revealing the V-shaped ridges comprising the lateral, medial, and caudal ganglionic eminences on the floor of the lateral ventricles. The MGE and cortical tissue were microdissected and processed separately. Tissue was homogenized in TRI reagent (Molecular Research Center Inc.); RNA was extracted using bromochloropropane; precipitated by adding isopropanol, ammonium acetate (3 N), and glycogen (5 mg/mL); washed in 75% ethanol; and then solubilized in ribonuclease-free water. First-strand cDNA was synthesized by the addition of RT reaction mix consisting of reverse transcriptase (RT-SSIII; Invitrogen), 5× first-strand buffer, 2.5 mM dNTPs, 300 ng Oligo dT, RNasin inhibitor, and 100 mM dithiothreitol in a final volume of 30 μL and incubated for 1 h at 42 °C.
For profiling single cells, individual cells were aspirated into the glass-recording pipette. The tip of the pipette was then broken inside an RT reaction tube, and the contents were expelled by positive pressure into a PCR reaction tube. First-strand cDNA was synthesized by the addition of RT reaction mix in a final volume of 20 μL and incubated overnight at 37 °C. The RT-SSIII was inactivated at 90 °C for 15 min. The first-round multiplex PCR reaction was performed for 20 cycles (94 °C for 20 s, 60 °C for 60 s, and 72 °C for 60 s) in a cocktail of primer sets for GFP, β-actin, and GABAA receptor α1–5, β1–3, γ1–3, and δ-subunits (10 pM each). Three microliters of the first-round PCR product was then used as template for the second-round of PCR amplification in the presence of individual primer sets.
PCR amplification of reverse transcribed cDNA template obtained from tissue samples or first-round PCR product from single cells was performed using a programmable mastercycler (Mastercycler ES realplex; Eppendorf) in a solution containing 2× Sybr Greener mix (Eppendorf), 50 pmol of either GABAA receptor subunit–specific primers (α1–5, β1–3, γ1–3, and δ) or β-actin, and 1 μL of cDNA. A standard curve was generated for each PCR run using adult mouse whole brain cDNA at concentrations ranging between 100 μg/mL and 1 pg/mL and probed for β-actin. Samples were run in duplicates. A set of “no-RT” controls with water added in lieu of the sample were routinely included and run in parallel. A 15-μL aliquot of the reaction product was then electrophoresed in 1% agarose parallel to a molecular weight ladder and visualized under UV illumination after staining with ethidium bromide. The GABAA receptor subunit–specific primer sequences and expected molecular weight of the PCR amplicons were the same as those previously reported (Liu and Burt 1998; Cuzon et al. 2006). Semiquantitative expression profiling data were expressed as mean ± SEM, and significant differences were determined by Student's t-test.
Results
Apparent Potency and Affinity of GABAA Receptors in eGFP+ Cells Change With Advancing Migration
In acute E14.5 BAC-Lhx6 slices, an incremental series of GABA concentrations (0.1–500 μM) were focally applied to eGFP+ cells in the region of the MGE or the intermediate zone of the cortex (Fig. 1A). The current traces in the inset of Figure 1B illustrate representative whole-cell current responses to 100 μM GABA obtained from a cell in the region of the MGE (upper trace) and from one recorded in the cortex (lower trace). The mean peak amplitudes of GABA-activated whole-cell current responses were normalized to those monitored in eGFP+ cells in the MGE region and plotted semilogarithmically as a function of the GABA concentrations tested (0.1–500 μM; Fig. 1B). In the same slices, GABA at concentrations ≥10 μM consistently evoked responses of larger amplitude in eGFP+ cells recorded in the cortex compared with those elicited in their counterparts in the MGE region. Consequently, the concentration–response curve constructed from eGFP+ cells in the cortex shifted leftward (EC50 of 188.0 μM in and around the MGE vs. 30.7 μM in cortex), reflecting an increase in both apparent potency and affinity to GABA. This implies increased expression of GABAA receptors, either of the same or of the different isoforms, in the course of tangential migration.
Figure 1.
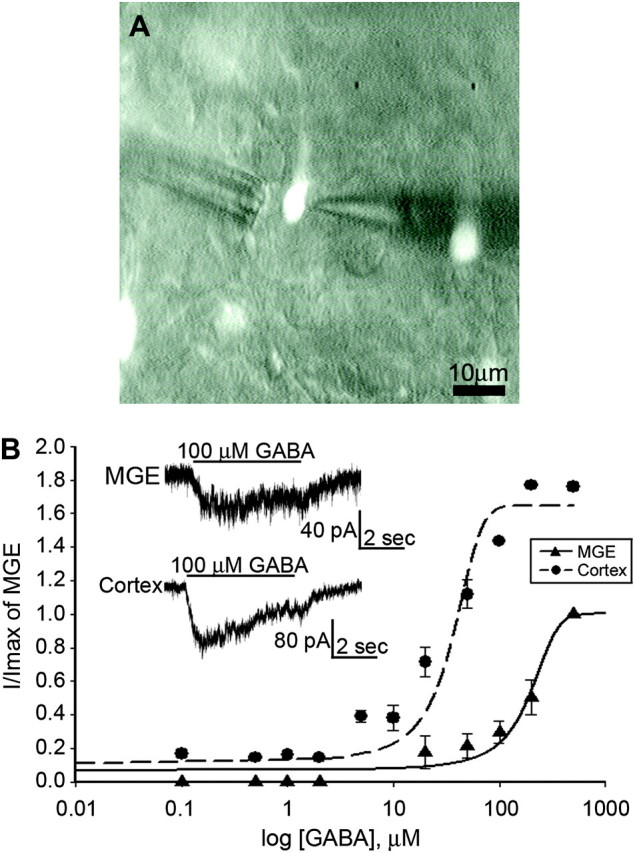
MGE-derived primordial GABAergic cortical interneurons acquire sensitivity to GABA as they migrate into the cortex. (A) An eGFP+ cell in the intermediate zone of an E14.5 BAC-Lhx6 slice visualized under epifluoresence and Hoffman Modulation. The recording pipette located on the right is used to monitor whole-cell current responses to drugs applied by an 8-barrel drug pipette assembly located on the left. Scale bar = 10 μm. (B) GABA concentration–response curves for eGFP+ cells recorded in the MGE area and intermediate zone of the cortex of acute slices obtained from E14.5 BAC-Lhx6 embryos. The amplitude of responses to each concentration of GABA was normalized to the maximal response amplitude recorded in the MGE. Inset: current responses to 100 μM GABA applied to eGFP+ cells located in the MGE (top trace) and the cortex (bottom trace).
GABAA Receptor Subunit Transcripts in the Developing MGE and Cortex
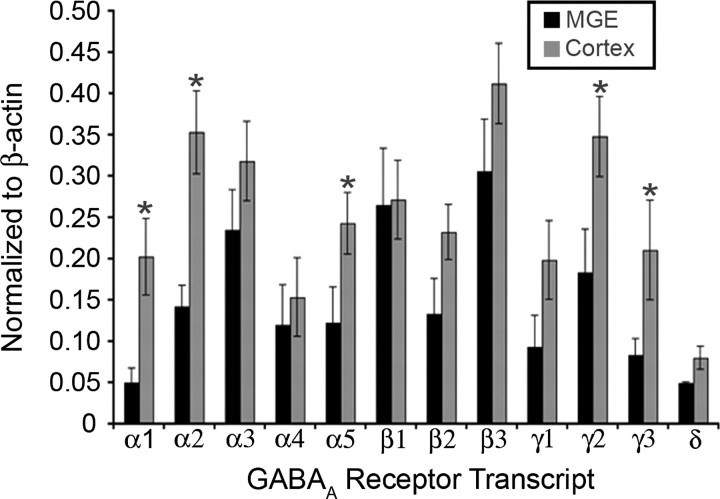
Since subunit composition can account for functional and kinetic differences in GABAA receptor properties (Verdoorn et al. 1990; Hutcheon et al. 2000; Devor et al. 2001), we asked whether the difference in concentration–response profiles to GABA reflected a regionally dependent variation in the expression of GABAA receptor subunits. We first analyzed the expression pattern of 12 GABAA receptor subunit transcripts (α1–5, β1–3, γ1–3, and δ) in the MGE and neocortical tissue microdissected from E14.5 brains. The α6- and ρ(1–3) subunits were not profiled since their expression is largely limited to the cerebellum and visual system, respectively (Varecka et al. 1994; Yeh et al. 1996; Albrecht et al. 1997; Alakuijala et al. 2005). Semiquantitative comparison, with the abundance of each GABAA receptor subunit transcript normalized to that of β-actin in the same sample, revealed a conspicuous increase in the expression of α1-, α2-, α5-, γ2-, and γ3-subunit transcripts in tissue derived from the cortex compared with the MGE tissue (Fig. 2).
Figure 2.
Upregulated expression of GABAA receptor subunit transcripts in the developing cortex. Semiquantitative RT-PCR expression of GABAA receptor subunit transcripts (α1–5, β1–3, γ1–3, and δ) of tissue microdissected from E14.5 BAC-Lhx6 MGE (n = 14) and cortex (n = 14). Data are normalized to β-actin and expressed as mean ± SEM. Asterisks denote a significant difference in relative abundance between the MGE and cortex (Student's t-test; p < 0.05).
GABAA Subunit Transcripts in Individual Migrating MGE-Derived Cells Before and After Entry Into the Cortex
The above results demonstrating differential expression of certain GABAA receptor subunit transcripts were obtained from microdissected MGE and cortical tissue, both of which contained heterogeneous populations of neuronal and nonneuronal cell types. To optimize selection of migrating MGE-derived cells in the acute telencephalic slices that are destined to become GABAergic cortical interneurons, we targeted eGFP+ cells situated in the area proximal to the corticostriate juncture (CSJ) with leading edge directed dorsally toward the cortex and excluded those located away from the CSJ and appearing to migrate ventrally in the direction of the striatum. We characterized the pharmacological properties of GABA-mediated responses using agents that have been reported to modulate GABAA receptor function in a subunit-dependent manner (Table 1) and profiled the expression of GABAA receptor subunits in these cells. The results of these pharmacological and expression profiling parameters were compared between individual eGFP+ corticopetal GABAergic interneurons and those that have already migrated into the developing cortex.
Table 1.
Pharmacological agents employed to assess expression of GABAA receptor isoforms
| Compound | Action | Reference |
| Zolpidem | Positive allosteric modulator α1- and γ-subunit–containing GABAA receptors. | Sieghart (1995); Mohler et al. (1996) |
| DMCM | Inverse agonist at α1-subunit–containing GABAA receptors. | Sieghart (1995); Mohler et al. (1996) |
| Ro15-4513 | Weak partial inverse agonist at α4-subunit–containing GABAA receptors. | Wisden et al. (1991); Wafford et al. (1996); Knoflach et al. (1996) |
| L655-708 | Inverse agonist at α5-subunit–containing GABAA receptors. | Pritchett and Seeburg (1990) |
| Loreclezole | Positive allosteric modulator of β2- or β3-subunit–containing GABAA receptors. | Stevenson et al. (1995) |
| Diazepam | Positive allosteric modulator of γ-subunit–containing GABAA receptors. | Pritchett et al. (1989); Yang et al. (1995); Saxena and Macdonald (1996) |
| Zinc | Inhibits GABAA receptor function at receptors without the γ-subunit. | Draguhn et al. (1990) |
| THIP | GABAA receptor agonist selective for δ-subunit–containing receptor isoforms. | Adkins et al. (2001); Brown et al. (2002) |
α-Subunits
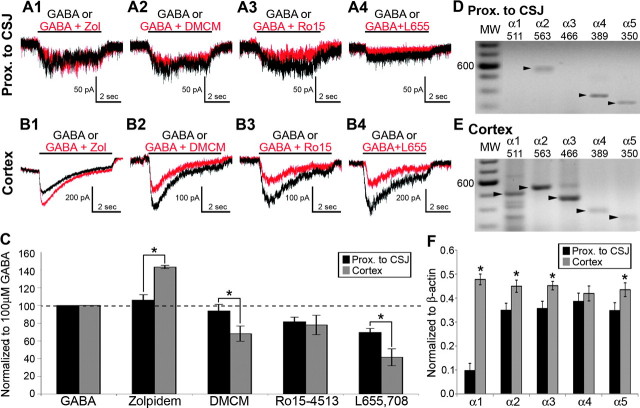
Zolpidem potentiates GABA-activated current responses in GABAA receptor isoforms containing the α1- and γx-subunits (Sieghart 1995; Mohler et al. 1996). DMCM, on the other hand, is an inverse agonist with greatest affinity for α1-subunit–containing GABAA receptor isoforms (Sieghart 1995; Mohler et al. 1996). The current traces in Figure 3A illustrate that focal application of GABA (100 μM; 5 s) elicited modest slowly desensitizing responses in eGFP+ cells proximal to the CSJ as reported previously (Cuzon et al. 2006). The majority of these eGFP+ cells displayed GABA responses that were insensitive to potentiation by zolpidem (100 nM; 16 of 18 cases; Fig. 3A1) or attenuation by DMCM (100 nM; 16 of 18 cases; Fig. 3A2). The mean peak amplitude of the GABA response during exposure to zolpidem was statistically similar to control (Fig. 3C; 106 ± 6.2%; P = 0.26) as was the DMCM-induced attenuation (Fig. 3C; 93.8 ± 7.4%; P = 0.34). By contrast, zolpidem potentiated (18 of 20 cases; Fig. 3B1) and DMCM attenuated (16 of 18 cases; Fig. 3B2) GABA responses monitored in the majority of eGFP+ cells recorded in the cortex. Zolpidem potentiated the mean amplitude of the GABA-activated current responses by 143.4 ± 1.9% (Fig. 3C; P = 0.03), while DMCM suppressed the same GABA responses by 68.2 ± 8.6% (Fig. 3C; P < 0.001).
Figure 3.
Expression of GABAA receptor α-subunits in individual BAC-Lhx6 eGFP+ cells in the area before the CSJ and cortex. (A and B) Whole-cell responses of eGFP+ cells in the area proximal to the CSJ (A) or the cortex (B) to focal coapplication of GABA either alone (100 μM; black traces) or in conjunction with zolpidem (100 nM; A1 and B1; red traces), DMCM (100 nM; A2 and B2; red traces), Ro15-4513 (1 μM; A3 and B3; red traces), and L655,708 (10 μM; A4 and B4; red traces). (C) Percentage change in the amplitude of GABA-induced current responses in the presence of GABAA receptor modulators in eGFP+ cells examined in the area proximal to the CSJ (black bars) and cortex (gray bars). Data are expressed as mean ± SEM. The dashed line denotes no change from the current elicited by the application of GABA alone. (D and E) Example of individual eGFP+ cells harvested from the area proximal to the CSJ (D) and the cortex (E) and profiled for their expression of GABAA receptor α1–5 subunits. The darker bands in the molecular weight (MW) ladder lanes denote the 600-bp position. Each arrowhead points to the predicted size of the amplicon detected for the corresponding α-subunit transcript, which are also given at the top of each lane. (F) Semiquantitative determination of the abundance of GABAA receptor α-subunit transcripts (α1–5) relative to β-actin in the same eGFP+ cells harvested from the area proximal to the CSJ and the cortex. Data are expressed as mean ± SEM. Asterisk denotes a significant difference in the normalized GABA + modulator response amplitude between eGFP+ cells from the area proximal to the CSJ and the cortex (C) or transcript abundance (F) relative to that found in the CSJ (p < 0.05, Student's t-test).
Ro15-4513 (1 μM), the partial inverse agonist for α4-subunit–containing recombinant GABAA receptors (Wisden et al. 1991; Knoflach et al. 1996; Wafford et al. 1996) attenuated GABA-activated current responses in the majority of eGFP+ cells all along the tangential migratory route (CSJ: Fig. 3A4; 17 of 17 cells and cortex: Fig. 3B4; 17 of 20). The mean magnitude of the Ro15-4513-induced attenuation in cells recorded in the area proximal to the CSJ was not significantly different from that recorded in cells located in the cortex (Fig. 3C; proximal to CSJ: 81.6 ± 5.2% and cortex: 78.0 ± 11.0%; P = 0.27). The α5-subunit–selective inverse agonist L655-708 (10 μM; Pritchett and Seeburg 1990) also attenuated GABA responses in eGFP+ cells regardless of location (proximal to CSJ: 69.5 ± 4.5%; 14 of 14 cells and cortex 41.5 ± 9.6%; 15 of 15 cells). However, eGFP+ cells in the cortex were attenuated to a significantly greater extent than their counterparts proximal to the CSJ (Student's t-test; P = 0.01). These results, taken together, suggest that migrating eGFP+ cells already express functional α4- and α5-subunit–containing GABAA receptor isoforms prior to entry into the cortex and begin to incorporate the α1-subunit in assembling functional GABAA receptors once they have reached the cortical anlage.
Expression profiling of individual eGFP+ cells proximal to the CSJ revealed the presence of the α2- (31 of 36 cells), α4- (30 of 36 cells), and α5- (30 of 36 cells) subunits in a majority of the eGFP+ cells (Fig. 3D). The α1-subunit transcript was notably absent in 27 of 36 cells (Fig. 3D). However, the α1-subunit transcript was consistently present in eGFP+ cells located in the cortex of the same acute slice preparation (Fig. 3E; 44 of 44 cells). Overall, although the α1-subunit was detectable in a few cells harvested proximal to the CSJ, its expression was dramatically upregulated in individual eGFP+ cells situated in the cortex as were the α2-, α3-, and α5-subunits (Fig. 3F). It should be noted that the increase in the α3 transcript was not evident in MGE- and cortex-derived tissues (Fig. 2). It may be that α3 transcript expression is limited to the MGE-derived eGFP+ cells (proximal to CSJ: 31 of 36 cells and cortex: 43 of 44 cells), masking a relatively modest (albeit significant) increase in relative abundance when assayed at the tissue level.
β-Subunits
Loreclezole is a broad-spectrum anticonvulsant with positive allosteric action on β2- or β3-subunit–containing GABAA receptor isoforms (Wafford et al. 1994; Wingrove et al. 1994). Loreclezole (10 μM) potentiated GABA (100 μM) responses of eGFP+ cells in the area proximal to the CSJ (Fig. 4A; 126.0 ± 5.6%; P = 0.04, n = 18) and cortex (Fig. 4B; 130.2 ± 8.3%; P = 0.03; n = 17). Expression profiling in individual eGFP+ cells indicated varied patterns of β1-, β2-, and β3-subunit expression. Figure 4D illustrates an eGFP+ cell harvested from the area proximal to the CSJ that expressed the β2- and β3-subunit transcripts (β1-subunit in 21 of 26 cells, β2-subunit in 32 of 36 cells, and β3-subunit in 28 of 36 cells; Fig. 4D). The example of another cell harvested from the cortex displayed transcripts for all three β-subunits (β1: 23 of 26 cells, β2: 40 of 44 cells, and β3: 42 of 44 cells; Fig. 4E). Of the β-subunit transcripts, the expression of the β3 transcript increased significantly in cortical eGFP+ cells compared with those from the area proximal to the CSJ (Fig. 4F; area proximal to the CSJ: n = 36 and cortex: n = 44).
Figure 4.
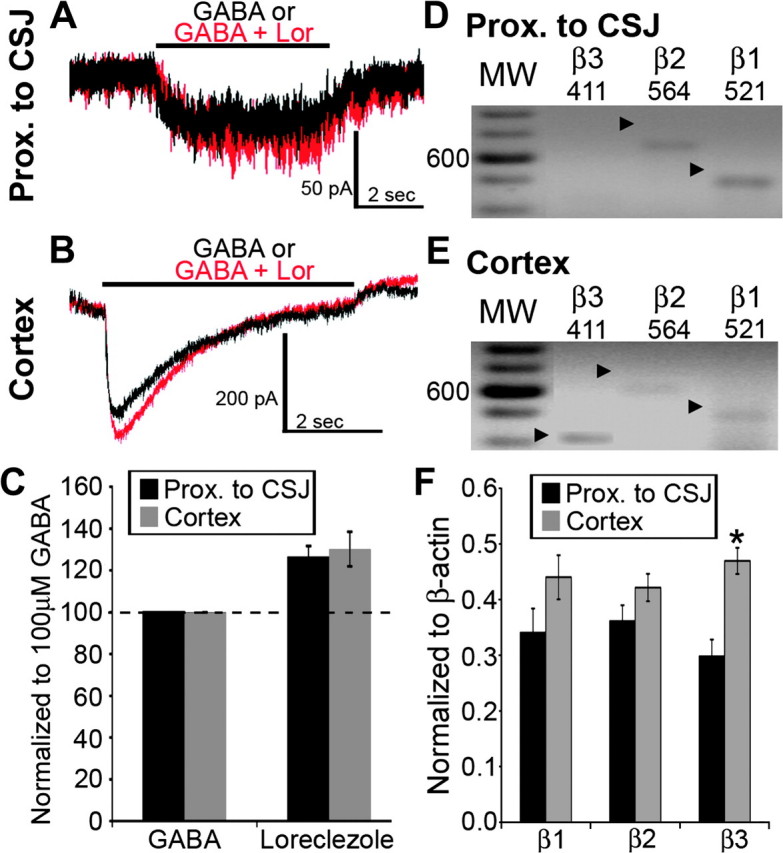
Expression of GABAA receptor β-subunits in individual BAC-Lhx6 eGFP+ cells in the area proximal to the CSJ and cortex. (A and B) Whole-cell current responses of eGFP+ cells in the area proximal to the CSJ (A) or the cortex (B) to focal application of GABA either alone (100 μM; black traces) or in conjunction with loreclezole (10 μM; red traces). (C) Percentage change in the amplitude of GABA-induced current responses in the presence of loreclezole in eGFP+ cells examined in the area proximal to the CSJ (black bars) and cortex (gray bars). Data are expressed as mean ± SEM. The dashed line denotes no change from the current response amplitude elicited by application of GABA alone. (D and E) Expression profiling of the GABAA receptor β1–3 subunits in individual eGFP+ cells located in the area proximal to the CSJ (D) and the cortex (E). The darker bands in the molecular weight (MW) ladder lanes correspond to the 600-bp position. The arrowheads point to PCR-amplified products of the corresponding β-subunit transcripts, which are also given at the top of each lane. (F) Semiquantitative determination of the abundance of GABAA receptor β-subunit transcripts (β1–3) relative to β-actin in the same eGFP+ cells harvested from the area proximal to the CSJ (black bars) and the cortex (gray bars). Data are expressed as mean ± SEM. Asterisk in (F) denotes a significant increase in cortical cells of transcript abundance relative to that detected in the CSJ (P < 0.05, Student's t-test).
γ- and δ-Subunits
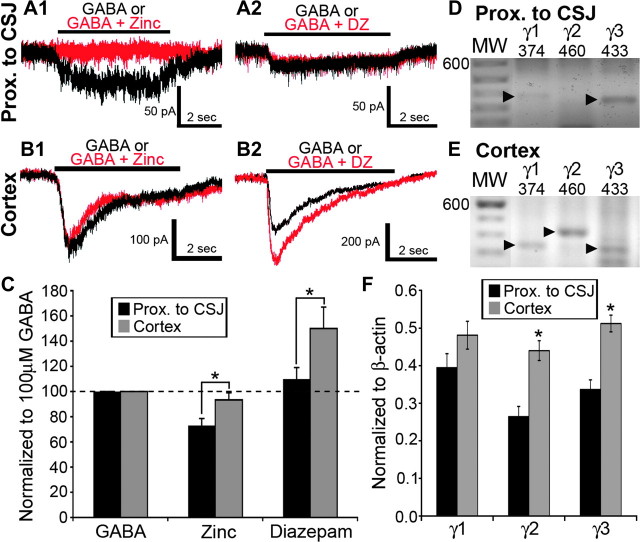
Since eGFP+ cells proximal to the CSJ expressed GABAA receptors with lower affinity to GABA (Fig. 1B) and since the γ2- and γ3-subunit transcripts are upregulated in the cortex (Fig. 2), we hypothesized that MGE-derived cells differentially incorporated γ-subunits with advancing migration and maturation. Zinc inhibits GABAA receptor function in recombinant receptor isoforms lacking γ-subunits, while diazepam potentiates GABA action at receptors containing γ-subunits (Pritchett et al. 1989; Kirshek et al. 1998; Hosie et al. 2003). In the present study, zinc chloride (100 μM) attenuated GABA responses monitored in 12 of 14 eGFP+ cells recorded in the area proximal to the CSJ (Fig. 5A1 and C; 73.1 ± 5.5%; P < 0.01). The same 12 cells were insensitive to potentiation by diazepam (3 μM; Fig. 5A2 and C; 109.8 ± 9.3%; P = 0.39; 12 of 14). The remaining 2 cells tested in the area proximal to the CSJ exhibited zinc and diazepam response profiles that resembled those of cortical eGFP+ cells. Specifically, in the cortex, the great majority of eGFP+ cells (14 of 15 cells) displayed GABA-induced currents that were insensitive to modulation by zinc (Fig. 5B1; 93.3 ± 5.7%; P = 0.30) but were potentiated by diazepam (Fig. 5B2; 150.0 ± 17.0%; P = 0.004). These results indicated that MGE-derived cells switch from expressing GABAA receptor isoforms with γ-less to γ-containing pharmacology in the course of corticopetal migration. However, expression profiling of the family of γ-subunits (γ1–γ3) yielded a different outcome. Specifically, all 36 eGFP+ cells harvested and profiled in the area proximal to the CSJ and all 44 of their counterparts in the cortex expressed transcripts for one or more of the γ-subunit transcripts. The relative abundance of the γ2- and γ3-subunit transcripts increased markedly in MGE-derived cells that have entered the cortex (Fig. 5F).
Figure 5.
Individual BAC-Lhx6 eGFP+ cells in the embryonic cortex, but not those in the area proximal to the CSJ, display GABAA receptor γ-subunit pharmacology and expression profiles. (A and B) Whole-cell current responses of eGFP+ cells in the area proximal to the CSJ (A) or the cortex (B) to focal application of GABA either alone (100 μM; black traces) or in conjunction with zinc chloride (Zinc; 100 μM; A1 and B1; red traces) or diazepam (DZ; 3 μM; A2 and B2; red traces). The GABA response of the cell in the area proximal to the CSJ was abolished in the presence of zinc (A1) but was insensitive to modulation by diazepam (A2). The GABA response of the cell recorded in the cortex, however, was insensitive to zinc (B1) but potentiated in the presence of diazepam (B2). (C) Percentage change in the amplitude of GABA-induced current responses in the presence of zinc or diazepam in eGFP+ cells examined in the area proximal to the CSJ (black bars) and cortex (gray bars). Data are expressed as mean ± SEM. The dashed line denotes no change from the current response amplitude elicited by application of GABA alone. (D and E) The amplicons corresponding to the GABAA receptor γ1–3 subunits in individual eGFP+ cells located in the CSJ (D) and the cortex (E) are electrophoresed parallel to a molecular weight (MW) ladder. The 600-bp position is indicated in the lanes containing the molecular weight ladder. The arrowheads point to the predicted sizes of the amplicons corresponding to the γ-subunit transcripts, which are also given at the top of each lane. (F) Semiquantitative results of GABAA receptor γ-subunit transcripts (γ1–3) of single eGFP+ cells harvested from the area proximal to the CSJ (black bars) and the cortex (gray bars). Amount of transcript was normalized to the amount of β-actin in the individual cell. Data are expressed as mean ± SEM. Asterisk denotes a significant difference in the normalized GABA + modulator response amplitude between eGFP+ cells from the area proximal to the CSJ and the cortex (C) or transcript abundance (F) relative to that found in the area proximal to the CSJ (P < 0.05, Student's t-test).
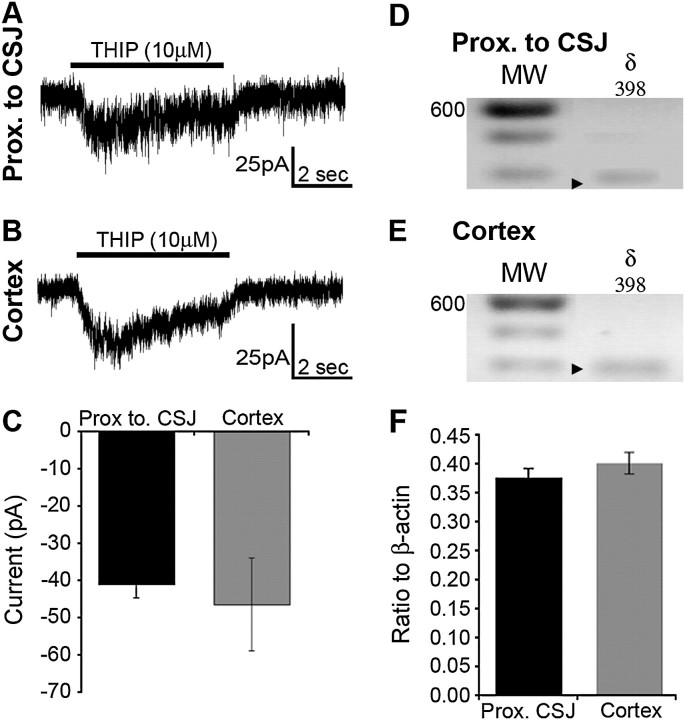
In certain extrasynaptic GABAA receptor isoforms, the γ-subunit is replaced with the δ-subunit (Nusser et al. 1998). We asked whether this might be the case in MGE-derived cells that displayed γ-less GABAA receptors. Figure 6 illustrates that THIP (10 μM), a GABAA receptor agonist with preference for δ-containing receptors, induced an inward current response in an eGFP+ cells recorded in the area proximal to the CSJ (Fig. 6A; 13 of 13 cases) and the cortex (Fig. 6B; 14 of 14 cases). THIP induced currents of comparable amplitude in cells regardless of location (Fig. 6C; CSJ: −41.06 ± 3.64 pA; cortex: −46.46 ± 12.43 pA; P = 0.23). Consistent with this, the δ-subunit transcript was present at a similar relative expression level in every cell profiled (Fig. 6F, P = 0.32) in the area proximal to the CSJ (Fig. 6D; 36 of 36 cells) and cortex (Fig. 6E; 44 of 44 cells). This suggested that, unlike the family of γ-subunits, the δ-subunit is not developmentally regulated in MGE-derived cells.
Figure 6.
The relative expression of the δ-subunit of the GABAA receptor in BAC-Lhx6 eGFP+ cells is unchanged regardless of location along the tangential migratory. (A and B) Whole-cell current responses of BAC-Lhx6 eGFP+ cells recorded in the area proximal to the CSJ (A) or the cortex (B) to focal application of THIP (10 μM). (C) The mean amplitude of the current response to THIP is not significantly different between cells recorded in the area proximal to the CSJ and in the cortex (Student's t-test; p = 0.23). Data expressed as mean ± SEM. (D and E) Single-cell expression profiling of individual eGFP+ cells in the area proximal to the CSJ (D) and the cortex (E) reveals the presence of the GABAA receptor δ-subunit. The darker bands in the molecular weight (MW) ladder lanes denote the 600-bp position. The arrowheads point to the predicted size of amplicon corresponding to the δ-subunit transcript, which is also indicated at the top of each lane. (F) Abundance of the GABAA receptor δ-subunit transcript in single BAC-Lhx6 eGFP+ cells harvested from the area proximal to the CSJ and the cortex relative to that of β-actin in the same cell. Data are expressed as mean ± SEM.
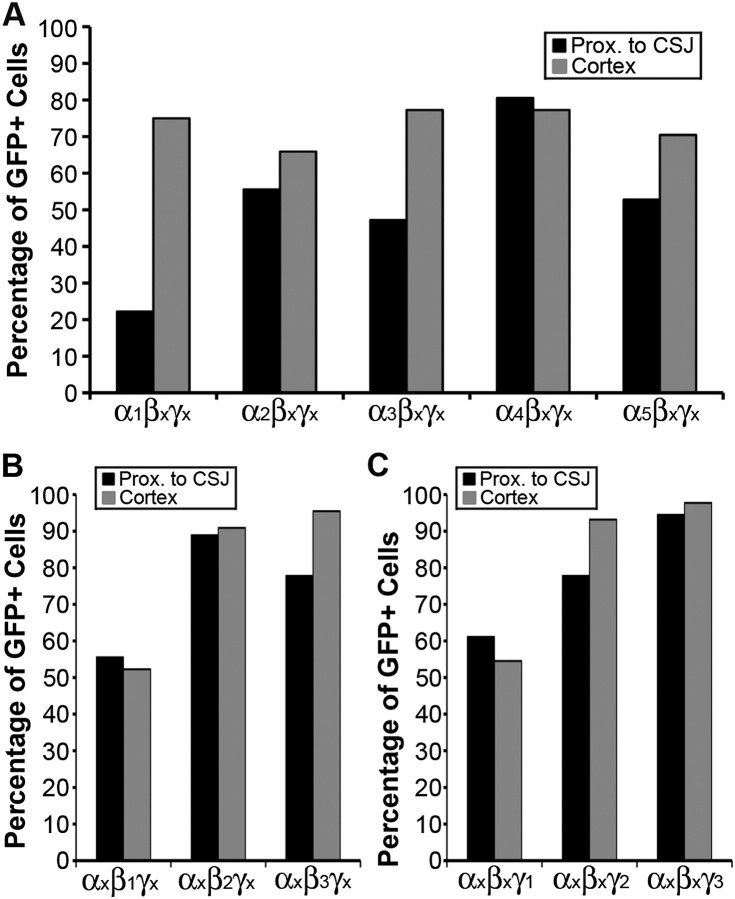
Our profiling of GABAA receptor subunit expression in the MGE-derived cells revealed heterogeneity not only among but also within the family of α-, β-, and γ-subunits. A striking trend was for individual migrating MGE-derived cells to incorporate more GABAA subunit isomers that can potentially assemble into pharmacologically distinct receptor isoforms. This was particularly evident in the expression profile of the α1-subunit. Specifically, assuming a hypothetical α/β/γ subunit stoichiometric motif, only 26% of the MGE-derived cells proximal to the CSJ could potentially express the combination of α1/βx/γx subunits, while it could be expressed in 75% of those located in the cortex (Fig. 7A). An increased potential was also observed for expressing the α3/βx/γx and α5/βx/γx subunit combinations but not for expressing the α2/βx/γx or α4/βx/γx subunit combinations (Fig. 7A) nor those containing the β1–3 or γ1–2 subunits (Fig. 7B and C, respectively). Overall, although the putative GABAA receptor subunit combinations expressed in any given MGE-derived cortical interneuronal subpopulation await elucidation, our results underscore the potential for dynamic expression of select members of the α-subunit family in tangentially migrating MGE-derived cells.
Figure 7.
Percentage of eGFP+ MGE-derived cells expressing hypothetical α1–5/βxγx, αx/β1–3γx, and αx/βxγ1–3 GABAA receptor subunit combinations. Summary of the potential for individual eGFP+ cells expressing any one of the α1-5 GABAA receptor subunit transcripts to form α/β/γ receptor isoforms in combination with βxγx subunits profiled in the same cells (A) for β1–3 subunit transcripts in combination with αxγx (B) and for γ1–3 in combination with αxβx (C). The percentages reflect the potential for a given cell to express multiple GABAA receptor isoforms since each cell profiled expressed multiple α, β, and γ isomers.
Discussion
In this study, we demonstrate that primordial GABAergic cortical interneurons migrating from the region of the MGE to the embryonic cortical anlage display regionally selective differences in pharmacological GABA response properties as well as profiles of GABAA receptor subunit transcripts. The use of acute telencephalic slices from E14.5 BAC-Lhx6 embryos permitted us to target corticopetal eGFP+ cells of MGE origin when tangential migration is at its height. We reasoned that their location and distribution in any given E14.5 telencephalic slice should reflect the progression of the tangential migratory process. Specifically, those situated in the cortex presumably would be more mature than those found in the region of the MGE since they were ahead of the migratory exodus and, thus, were born earlier. In this light, we propose that, as part of their maturational process, primordial GABAergic cortical interneurons acquire increasing responsiveness to GABA and switch to expressing more mature GABAA receptor isoforms with advancing migration into the cortex. Indeed, while the literature is replete with observations of either changes in neuronal responsiveness to GABA (Shen et al. 1988; LoTurco and Kriegstein, 1991; Owens et al. 1999; Hutcheon et al. 2000) or expression of GABAA receptor subunits in the immature brain (Gambarana et al. 1991; Araki et al. 1992; Laurie et al. 1992; Cheng et al. 2006; Peden et al. 2008; Yu et al. 2009), our study integrated these developmental processes into perspective with the embryonic development of an identified neuronal population, notably, that of the GABAergic cortical interneurons.
MGE-Derived Cells Increase Receptivity to GABA With Tangential Migration
In the present study, GABA concentration–response profiles, coupled with assessing the effectiveness of GABAA receptor subunit–selective modulators, facilitated a pharmacological analysis of the GABA response monitored in tangentially migrating MGE-derived cells. We found that the concentration–response curve derived from eGFP+ neurons in the region of the MGE shifted leftward relative to that obtained from their cohorts in the embryonic cortex. This suggested that the MGE-derived neurons en route to the embryonic cortex acquire GABAA receptors with increasing apparent efficacy and affinity to GABA. While apparent efficacy provides an indication of receptor number, apparent affinity reflects in part the subunit makeup of functional GABAA receptor isoforms. Thus, we propose that MGE-derived neurons upregulate and incorporate functional GABAA receptors of the same isoforms, isoforms with higher affinity to GABA, or both as they migrate from the MGE to the embryonic cortex. MGE-derived cells located in the MGE region also displayed GABA-activated currents characterized by slow kinetics and decay time with little to no desensitization compared with those recorded in the cortex. In addition, profiling of GABAA receptor subunit transcripts in individual migrating cells revealed differential expression of a number of α- and γ-subunit transcripts. These observations reinforce the notion that different subunits contribute to the makeup of GABAA receptors in MGE-derived cells at different locations along the tangential migratory route.
MGE-Derived Cells in the Cortex Express More Mature GABAA Receptor Isoforms
The expression of many GABAA receptor subunits is developmentally regulated (Gambarana et al. 1991; Araki et al. 1992; Laurie et al. 1992; Cheng et al. 2006; Peden et al. 2008). We focused on analyzing GABAA receptor subunits reported to be expressed in the developing cortex, notably the α1–α5, β1–β3, γ1–γ3, and δ-subunits (Gambarana et al. 1991; Araki et al. 1992; Laurie et al. 1992; Cheng et al. 2006; Peden et al. 2008). Although not an exhaustive analysis, the present data illustrate the dynamic expression and potential for functional diversity of GABAA receptors in migrating MGE-derived neurons.
In individual primordial GABAergic cortical interneurons located proximal to the CSJ, there was robust expression of the α2- and α3-subunit transcripts vis-a-vis the α1-subunit, reminiscent of type II benzodiazepine GABAA receptor isoforms. As these neurons enter the developing cortex, the α2- and α3-subunit transcripts continue to be expressed, but a dramatic upregulation in the α1- and γ1–3 subunit transcripts occurs, suggesting a switch to a predominantly type I benzodiazepine pharmacology. Corroborating these findings was electrophysiological results, indicating that zolpidem and DMCM were ineffective in modulating GABA-activated current responses in eGFP+ neurons in the area proximal to the CSJ but potentiated and suppressed, respectively, the responses elicited in the same population of neurons situated in the developing cortex. These results are consistent with previous studies reporting the predominant expression of the α2- and α3-subunits early during development, which subsequently is replaced by the α1-subunit with advancing neuronal migration (Laurie et al. 1992; Fritschy et al. 1994; Hornung and Fritschy 1996; Bosman et al. 2002; Liu and Wong-Riley 2004; Takayama and Inoue 2004).
Along a similar developmental trend, our electrophysiological results employing diazepam and zinc, combined with transcript profiling, indicate that MGE-derived neurons first express γ-subunit–less GABA receptor isoforms as they migrate in the subpallium and then express γ-subunit–containing isoforms once they enter and traverse the developing cortex. This scheme of a γ-less to γ-containing switch in GABAA receptor isoform is reminiscent of our previous study that demonstrated expression of γ-less GABAA receptors in embryonic but not in postnatal Cajal–Retzius cells during corticogenesis (Cheng et al. 2006) and is consistent with the notion that MGE-derived neurons switch from expressing immature to more mature GABAA receptor isoforms as they migrate. However, our expression profiling revealed that virtually all MGE-derived cells, regardless of location, expressed at least one of the γ-subunit transcripts. It may be that MGE-derived cells express γ-subunit transcripts early in the course of migration, but the encoded proteins are not translated and assembled into functionally demonstrable GABAA receptor isoforms until they have reached the cortical anlage. Such a developmental relationship between transcript and protein expression may also apply to other GABAA receptor subunits. The present study contributes to the groundwork for a comprehensive immunohistochemical analysis to resolve these and other issues related to the assembly of functionally distinct GABAA receptor isoforms in migrating and mature MGE-derived cortical interneurons.
Tangentially Migrating MGE-Derived Cells Express functional “Synaptic” and “Extrasynaptic” GABAA Receptor Isoforms
Subdomains of the MGE give rise to Nkx2.1/Lhx6–expressing precursor GABAergic cortical interneurons, including basket and Martinotti interneurons that either express or coexpress parvalbumin, somatostatin, calbindin, and NPY (Xu et al. 2004; Fogarty et al. 2007). Our experimental approach precluded ascribing the different GABAA receptor pharmacological and subunit transcript profiles of eGFP+ cells to their origin within subdomains of the MGE (e.g., dorsal vs. lateral MGE) and to their specification into specific GABAergic interneuronal subtypes later in development. Future studies would need to incorporate genetic fate mapping strategies to refine the identification of tangentially migrating MGE-derived cells and to establish GABAA receptor subunit expression profiles of GABAergic interneuronal subtypes in the adult cortex.
The above caveats notwithstanding, our results of expression profiling implicate migrating MGE-derived cells expressing more subunit transcripts than necessary to constitute one functional isoform of GABAA receptor. In this light, our pharmacological analyses of whole-cell GABA-activated current responses would be expected to reflect a net outcome of simultaneous activation of multiple GABAA receptor isoforms. Although the subunit combinations of GABAA receptor isoforms expressed in adult GABAergic cortical interneurons await elucidation, it is reasonable to postulate that, in addition to α4-, α5- and δ-containing GABAA receptor isoforms (e.g., α4βxδ and α5βxγ2/3), which are relatively evenly expressed in MGE-derived cells regardless of location, there is upregulation in the cortex of isoforms that include the α1- and γ-subunits, such as those of the α1βxγ2/3 combination. These GABAA receptor isoforms have been implicated to mediate either “synaptic” or “extrasynaptic” actions of GABA (reviewed by Sieghart et al. 1999; Barnes 2001; Kittler et al. 2002). At E14.5, synapses have not yet formed on tangentially migrating MGE-derived cells, but the results of our study indicate that synaptic and extrasynaptic types of GABAA receptors are already present and functional. Extrasynaptic GABAA receptor isoforms, those of the α5βxγ2/3 subunit combination in particular, have been shown to be highly sensitive to GABA (Burgard et al. 1996; Nusser and Mody 2002; Caraiscos et al. 2004; Bonin et al. 2007). In this light, they are in a favorable position to mediate GABA tone and, thus, play an important role in regulating the migration of MGE-derived neurons.
Functional Implications
GABA is present in the embryonic brain, and immature neurons express GABAA receptors well before the first signs of synaptogenesis (van Eden et al. 1989; Imamoto et al. 1994; Zecevic and Milosevic 1997; Cuzon et al. 2006). As a “developmental neurotransmitter” (Redburn and Rowe-Rendleman 1996; Redburn-Johnson 1998; Owens and Kriegstein 2002; Represa and Ben-Ari 2005; Heng et al., 2007; Manent and Represa 2007), GABA maintained at an ambient level is trophic for migrating neurons. This trophism has been reported to be mediated by GABAA receptors (Behar et al. 1996, 1998, 2000; Bolteus and Bordey 2004; Manent et al. 2005; Cuzon et al. 2006), but whether subunit composition comes into play has not been addressed. Here, we provide evidence that MGE-derived neurons express GABAA receptors of different subunit compositions and functional properties in the course of their migration. We propose that these differences may impart attributes to embryonic MGE-derived neurons that are important in conferring the role of GABA as a trophic factor. Specifically, the upregulation of receptor isoforms with higher affinity to GABA may be important in signaling cortical entry of MGE-derived cells and beyond. At the time point examined in this study (E14.5), GABAA receptor activation induces membrane depolarization that activates voltage-gated calcium channels (Cherubini et al. 1991; Walton et al. 1993; Lin et al. 1994; LoTurco et al. 1995; Owens et al. 1996). Since rises in intracellular calcium have been shown to be involved in numerous gene regulatory processes, including those leading to cellular differentiation (Holliday et al. 1991; Gu and Spitzer 1995), it may be that activation of GABAA receptors in MGE-derived cells triggers calcium influx to trigger the transcription of genes encoding GABAA receptor subunits, leading to cellular differentiation, maturation, and synaptogenesis.
Funding
Public Heath Service Grants RO1 MH069826 (H.H.Y.) and F31 AA014698 (V.C.C.C.).
Acknowledgments
Conflict of Interest: None declared.
References
- Adkins CE, Pillai GU, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. alpha4beta3delta GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276(42):38934–38939. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- Alakuijala A, Palgi M, Wegelius K, Schmidt M, Enz R, Paulin L, Saarma M, Pasternak M. GABA receptor rho subunit expression in the developing rat brain. Dev Brain Res. 2005;154(1):15–23. doi: 10.1016/j.devbrainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Albrecht BE, Breitenbach U, Stuhmer T, Harvey RJ, Darlison MG. In situ hybridization and reverse transcription-polymerase chain reaction studies on the expression of the GABAC receptor rho1- and rho2-subunit genes in avian and rat brain. Euro J Neurosci. 1997;9(11):2414–2422. doi: 10.1111/j.1460-9568.1997.tb01658.x. [DOI] [PubMed] [Google Scholar]
- Alifragis P, Liapi A, Parnavelas JG. Lhx6 regulates the migration of cortical interneurons from the ventral telencephalon but does not specify their GABA phenotype. J Neurosci. 2004;24(24):5643–5648. doi: 10.1523/JNEUROSCI.1245-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Angelotti TP, Macdonald RL. Assembly of GABAA receptor subunits: α1β1 and α1β1γ2s subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci. 1993;13(4):1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Kiyama H, Tohyama M. GABAA receptor subunit messenger RNAs show differential expression during cortical development in the rat brain. Neuroscience. 1992;51(3):583–591. doi: 10.1016/0306-4522(92)90298-g. [DOI] [PubMed] [Google Scholar]
- Barnes EM. Assembly and intracellular trafficking of GABAA receptors. Int Rev Neurobiol. 2001;48:1–29. doi: 10.1016/s0074-7742(01)48012-3. [DOI] [PubMed] [Google Scholar]
- Behar T, Li Y, Tran HT, Ma W, Dunlap V, Scott C, Barker JL. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16:1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10(9):899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, O'Connell C, Barker JL. Differential response of cortical plate and ventricular zone cells to GABA as a migration stimulus. J Neurosci. 1998;18(16):6378–6387. doi: 10.1523/JNEUROSCI.18-16-06378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau AJ, Li T, Benkwitz C, Czajkowski C, Pearce RA. Effects of γ2s subunit incorporation on GABAA receptor macroscopic kinetics. Neuropharmacol. 2003;44(8):1003–1012. doi: 10.1016/s0028-3908(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24(35):7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Martin LJ, MacDonald JF, Orser BA. α5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons. J Neurophysiol. 2007;98:2244–2254. doi: 10.1152/jn.00482.2007. [DOI] [PubMed] [Google Scholar]
- Bosman LW, Rosahl TW, Brussaard AB. Neonatal development of the visual cortex: synaptic function of GABAA receptors alpha subunits. J Physiol. 2002;545(1):169–181. doi: 10.1113/jphysiol.2002.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABAA receptors. Br J Pharmacol. 2002;136(7):965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard EC, Tietz EI, Neelands TR, Macdonald RL. Properties of recombinant gamma-aminobutyric acid A receptor isoforms containing the alpha 5 subunit subtype. Mol Pharmacol. 1996;50(1):119–127. [PubMed] [Google Scholar]
- Caraiscos VC, Elliott EM, You T, Cheng VY, Belelli D, Newell JF, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. PNAS. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yeh PWL, Yeh HH. Cajal-Retzius cells switch from expressing γ-less to γ-containing GABAA receptors during corticogenesis. Eur J Neurosci. 2006;24:2145–2151. doi: 10.1111/j.1460-9568.2006.05122.x. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14(12):515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PWL, Cheng Q, Yeh HH. Ambient GABA promotes cortical entry of tangentially migrating cells derived from the medial ganglionic eminence. Cereb Cortex. 2006;16(10):1577–1588. doi: 10.1093/cercor/bhj084. [DOI] [PubMed] [Google Scholar]
- Denaxa M, Sharpe PT, Pachnis V. The LIM homeodomain transcription factor Lhx6 and Lhx7 are key regulators of mammalian dentition. Dev Biol. 2009;333(2):324–336. doi: 10.1016/j.ydbio.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Fritschy JM, Yarom Y. Spatial distribution and subunit composition of GABA(A) receptors in the inferior olivary nucleus. J Neurophysiol. 2001;85(4):1686–1696. doi: 10.1152/jn.2001.85.4.1686. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5(6):781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic epithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27(41):10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14(9):5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambarana C, Beattie CE, Rodríguez ZR, Siegel RE. Region-specific expression of messenger RNAs encoding GABAA receptor subunits in the developing rat brain. Neuroscience. 1991;45(2):423–432. doi: 10.1016/0306-4522(91)90238-j. [DOI] [PubMed] [Google Scholar]
- Gelman DM, Martini FJ, Nóbrega-Pereira A, Pierani A, Kessais N, Marín A. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Grigoriou M, Tucker AS, Sharpe PT, Pachnis V. Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development. 1998;125:2063–2074. doi: 10.1242/dev.125.11.2063. [DOI] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Heng J, Moonen G, Nguyen L. Neurotransmitters regulate cell migration in the telencephalon. Eur J Neurosci. 2007;26:537–546. doi: 10.1111/j.1460-9568.2007.05694.x. [DOI] [PubMed] [Google Scholar]
- Holliday J, Adams RJ, Sejnowski TJ, Spitzer NC. Calcium-induced release of calcium regulates differentiation of cultured spinal neurons. Neuron. 1991;7(5):787–796. doi: 10.1016/0896-6273(91)90281-4. [DOI] [PubMed] [Google Scholar]
- Hornung JP, Fritschy JM. Developmental profile of GABAA receptors in the marmoset monkey: expression of distinct subtypes in pre- and postnatal brain. J Comp Neurol. 1996;367(3):413–430. doi: 10.1002/(SICI)1096-9861(19960408)367:3<413::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABA(A) receptors: discrete binding sites underlie subtype specificity. Nat Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- Hutcheon B, Morley P, Poulter MO. Developmental change in GABAA receptor desensitization kinetics and its role in synapse function in rat cortical neurons. J Physiol. 2000;522(1):3–17. doi: 10.1111/j.1469-7793.2000.t01-5-00003.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Nishiyama N, Saito H, Katsuki H. GABAA receptor stimulation promotes survival of embryonic rat striatal neurons in culture. Dev Brain Res. 1997;98(2):253–258. doi: 10.1016/s0165-3806(96)00183-6. [DOI] [PubMed] [Google Scholar]
- Imamoto K, Karasawa N, Isomura G, Nagatsu I. Cajal-Retzius neurons identified by GABA immunohistochemistry in layer I of the rat cerebral cortex. Neurosci Res. 1994;20(1):101–105. doi: 10.1016/0168-0102(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Kirshek BJ, Moss SJ, Smart TG. Interaction of H+and Zn2+on recombinant and native rat neuronal GABAA receptors. J Physiol (Lond) 1998;507:639–652. doi: 10.1111/j.1469-7793.1998.639bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking: implications fort he modulation of inhibitory neurotransmission. Mol Neurobiol. 2002;26:251–268. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scherer L, Luddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acid A receptors alpha4beta2gamma2 and alpha6beta2gamma2. Mol Pharmacol. 1996;50(5):1253–1261. [PubMed] [Google Scholar]
- Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response for α4 subtype-containing GABAA receptors to synaptic and extrasynaptic GABA. J Physiol. 2007;578(3):655–676. doi: 10.1113/jphysiol.2006.122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Panchnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;12:4151–4172. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MH, Takahashi MP, Takahashi Y, Tsumoto T. Intracellular calcium increase induced by GABA in visual cortex of fetal and neonatal rats and its disappearance with development. Neurosci Res. 1994;20(1):85–94. doi: 10.1016/0168-0102(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27(12):3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits α1, α2, and α3 in the rat pre-Botzinger complex. J Appl Physiol. 2004;96(5):1825–1831. doi: 10.1152/japplphysiol.01264.2003. [DOI] [PubMed] [Google Scholar]
- Liu ZF, Burt DR. A synthetic standard for competitive RT/PCR quantification of 13 GABA receptor type A subunit RNAs in rats and mice. J Neurosci Methods. 1998;85:89–98. doi: 10.1016/s0165-0270(98)00125-3. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Kriegstein AR. Clusters of coupled neuroblasts in embryonic neocortex. Science. 1991;252(5005):563–566. doi: 10.1126/science.1850552. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath JS, Davis MBE, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L, Repressa A. A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci. 2005;25(35):8027–8036. doi: 10.1523/JNEUROSCI.0553-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Represa A. Neurotransmitters and brain maturation: early paracrine actions of GABA and glutamate modulate neuronal migration. Neuroscientist. 2007;13(3):268–279. doi: 10.1177/1073858406298918. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa V, Butt S, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces and large and diverse population o f superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Ludenns B, Rudolph U, Benson J, Benke D. The GABAA receptors: from subunits to diverse functions. Ion Channels. 1996;4:89–113. [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18(5):1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MBE, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16(20):6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Owens DF, Liu X, Kriegstein AR. Changing properties of GABAA receptor-mediated signaling during early neocortical development. J Neurophysiol. 1999;82:570–583. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- Peden DR, Petitjean CM, Herd MB, Durakoglugil MS, Rosahl TW, Wafford K, Homanics GE, Belelli D, Fritschy JM, Lambert JJ. Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurons. J Physiol. 2008;586(4):965–987. doi: 10.1113/jphysiol.2007.145375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Seeburg PH. Gamma-aminobutyric acid A receptor alpha 5-subunit creates novel type II benzodiazepine receptor. J Neurochem. 1990;54(5):1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Redburn DA, Rowe-Rendleman C. Developmental neurotransmitters. Signals for shaping neuronal circuitry. Invest Ophthalmol Vis Sci. 1996;37(8):1479–1482. [PubMed] [Google Scholar]
- Redburn-Johnson D. GABA as a developmental neurotransmitter in the outer plexiform layer of the vertebrate retina. Perspect Dev Neurobiol. 1998;5:261–267. [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28(6):278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol Pharmacol. 1996;49(3):567–579. [PubMed] [Google Scholar]
- Shen JM, Hugenard JR, Kriegstein AR. Development of GABA responsiveness in embryonic turtle cortical neurons. Neurosci Lett. 1988;89(3):335–341. doi: 10.1016/0304-3940(88)90549-6. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acid A receptor subtypes. Pharmacol Rev. 1995;47(2):181–234. [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Trettler V, Ebert V, Jechilinger M, Hoger H, Adamiker D. Structure and subunit composition of GABAA receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution, and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2(8):795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Stevenson A, Wingrove PB, Whiting PJ, Wafford KA. beta-carboline gamma aminobutyric acid A receptor inverse agonists modulate gamma-aminobutyric acid via the loreclezole binding site as well as the benzodiazepine site. Mol Pharmacol. 1995;48(6):965–969. [PubMed] [Google Scholar]
- Takayama C, Inoue Y. Transient expression of GABAA receptors α2 and α3 subunits in differentiating cerebellar neurons. Dev Brain Res. 2004;148(2):169–177. doi: 10.1016/j.devbrainres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori KE, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J Neurosci. 1997;17(21):8313–8323. doi: 10.1523/JNEUROSCI.17-21-08313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology. 1996;35:1375–1382. doi: 10.1016/s0028-3908(96)00018-4. [DOI] [PubMed] [Google Scholar]
- van Eden CG, Mrzljak L, Voorn P, Uylings HBM. Prenatal development of GABA-ergic neurons in the neocrotex of the rat. J Comp Neurol. 1989;289:213–227. doi: 10.1002/cne.902890204. [DOI] [PubMed] [Google Scholar]
- Varecka L, W Ch Rotter A, Frostholm A. GABAA/benzodiazepine receptor alpha 6 subunit mRNA in granule cells of the cerebellar cortex and cochlear nuclei: expression in developing mutant mice. J Comp Neurol. 1994;339(3):341–352. doi: 10.1002/cne.903390304. [DOI] [PubMed] [Google Scholar]
- Verdoorn TA, Draguhn A, Ymer S, Seeburg PH, Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990;4:919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Quirk K, Mckernan RM, Wingrove PB, Whiting PJ, Kemp JA. A novel allosteric modulatory site on the GABAA receptor beta subunit. Neuron. 1994;12(4):775–782. doi: 10.1016/0896-6273(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human gamma-aminobutyric acid A receptors containing the alpha 4 subunit. Mol Pharmacol. 1996;50(3):670–678. [PubMed] [Google Scholar]
- Walton MK, Schaffner AE, Barker JL. Sodium channels, GABAA receptors, and glutamate receptors develop sequentially on embryonic rat spinal cord cells. J Neurosci. 1993;13(5):2068–2084. doi: 10.1523/JNEUROSCI.13-05-02068.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Kriegstein AR. Defining the role of GABA in cortical development. J Physiol. 2009;587(9):1873–1879. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove PB, Wafford KA, Bain C, Whiting PJ. The modulatory action of loreclezole at the γ-aminobutyric acid type A receptor is determined by a single amino acid in the β2 and β3 subunit. PNAS. 1994;91:4569–4573. doi: 10.1073/pnas.91.10.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Herb A, Wieland H, Keinanen K, Luddens H, Seeburg PH. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor alpha 4 subunit. FEBS Lett. 1991;289(2):227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, DeLaCruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24(11):2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Drewe JA, Lan NC. Cloning and characterization of the human GABAA receptor alpha 4 subunit: identification of a unique diazepam-insensitive binding site. Eur J Pharmacol. 1995;291(3):319–325. doi: 10.1016/0922-4106(95)90072-1. [DOI] [PubMed] [Google Scholar]
- Yeh HH, Grigorenko EV, Veruki ML. Correlation between a bicuculline-resistant response to GABA and GABAA receptor rho 1 subunit expression in single rat retinal bipolar cells. Vis Neurosci. 1996;13(2):283–292. doi: 10.1017/s0952523800007525. [DOI] [PubMed] [Google Scholar]
- Yeh HH, Lu S-M, Therianos S. Combining patch-clamp recording and gene profiling in single neurons. In: Liu Y, Lovinger D, editors. Methods in alcohol-related research. Boca Raton (FL): CRC press; 2002. pp. 83–98. [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 2009;1099(1):73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Milosevic A. Initial development of gamma-aminobutyric acid immunoreactivity in the human cerebral cortex. J Comp Neurol. 1997;380(4):495–506. doi: 10.1002/(sici)1096-9861(19970421)380:4<495::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mori T, Takaki H, Takeuch M, Iseki K, Hagino S, Murakawa M, Yokoya S, Wanaka A. Comparison of the expression patters of two LIM-homeodomain genes, Lhx6 and L3/Lhx8, in the developing palate. Orthod Craniofac Res. 2002;5(2):65–70. doi: 10.1034/j.1600-0544.2002.02198.x. [DOI] [PubMed] [Google Scholar]