Abstract
Human locomotor adaptive learning is thought to involve the cerebellum, but the neurophysiological mechanisms underlying this process are not known. While animal research has pointed to depressive modulation of cerebellar outputs, a direct correlation between adaptive learning and cerebellar depression has never been demonstrated. Here, we used transcranial magnetic stimulation to assess excitability changes occurring in the cerebellum and primary motor cortex (M1) after individuals learned a new locomotor pattern on a split-belt treadmill. To control for potential changes associated to task performance complexity, the same group of subjects was also assessed after performing 2 other locomotor tasks that did not elicit learning. We found that only adaptive learning resulted in reduction of cerebellar inhibition. This effect was strongly correlated with the magnitude of learning (r = 0.78). In contrast, M1 excitability changes were not specific to learning but rather occurred in association with task complexity performance. Our results demonstrate that locomotor adaptive learning in humans is proportional to cerebellar excitability depression. This finding supports the theory that adaptive learning is mediated, at least in part, by long-term depression in Purkinje cells. This knowledge opens the opportunity to target cerebellar processes with noninvasive brain stimulation to enhance motor learning.
Keywords: adaptation, cerebellum, locomotion, rehabilitation, TMS
Introduction
The human nervous system has the remarkable ability to control complex movements in the face of changing environmental demands, muscle fatigue, and even injury. Consider the ease with which we can transition from walking on a hard surface road to a soft sandy beach. We initially react to these types of demands and correct for unplanned disturbances. If the demands persist, it is more efficient to learn to predict the correct motor commands required under the new circumstances. The behavioral and neural mechanisms involved in this form of learning, commonly referred as adaptation or adaptive learning, are not fully understood. However, the adaptation process is thought to be dependent on the cerebellum and is especially important for this type of behavioral flexibility.
Adaptation has been defined as a trial and error short timescale motor learning process that is used to adjust motor commands for new predictable demands on a timescale of minutes to hours (Martin et al. 1996; Bastian 2008). Behavioral studies of adaptation show that it is a ubiquitous process that affects virtually all kinds of movements, such as walking (Reisman et al. 2005), standing (Kluzik et al. 2007), reaching (Shadmehr and Mussa-Ivaldi 1994), and a variety of eye movements (Ito 1998). It allows us to more effectively control movement by learning to anticipate perturbations that would normally interfere with a given movement.
Several lines of research have suggested that the cerebellum plays a crucial role in motor adaptation (Ito 1982; Martin et al. 1996; Diedrichsen et al. 2005; Chen et al. 2006). People with cerebellar damage have difficulty adapting to novel environmental demands (Martin et al. 1996; Smith and Shadmehr 2005; Morton and Bastian 2006), whereas individuals with damage to other motor structures typically adapt normally (Weiner et al. 1983; Reisman et al. 2007). Neurophysiological studies in animals indicated that motor adaptation may be mediated, in part, via long-term depression (LTD) in cerebellar Purkinje cells (Gilbert and Thach 1977; Medina and Lisberger 2008). However, less is known about the underlying neural mechanisms by which humans adapt and no study has related the extent of cerebellar excitability changes to that of adaptive motor learning.
Here, we used transcranial magnetic stimulation (TMS) to investigate the neurophysiological correlates specific to locomotor adaptation associated with the cerebellum, while controlling for changes related to complex motor performance. We used a well-studied split-belt walking adaptation task that is known to be cerebellum dependent (Morton and Bastian 2006) and 2 control walking tasks. We hypothesized that adaptation in a split-belt walking paradigm would change the pattern of cerebellar brain inhibition (CBI) normally seen using paired pulse TMS (Ugawa, Useka et al. 1995; Pinto and Chen 2001). More specifically, we predicted a reduction in CBI, which is what would be expected if LTD in Purkinje cells is a physiological mechanism involved in adaptive learning. If so, the magnitude of adaptation should also correlate with a decrement in the amount of CBI measured with TMS. In contrast, we predicted that M1 excitability would increase nonspecifically as a result of performing a complex motor task (i.e., walking in a challenging task that does not require adaptation to a predictable perturbation) but not exclusively due to adaptation.
Materials and Methods
Nine healthy subjects (3 female, 6 male) with no known neurological disorder participated in the main experiment (mean age 23, range: 19–25). A second group of 6 naive healthy participants took part in an additional experiment (all male). The investigation was approved by the Johns Hopkins University Institutional Review Board. All methods conformed to the Declaration of Helsinki, and all participants provided written informed consent.
Experimental Design
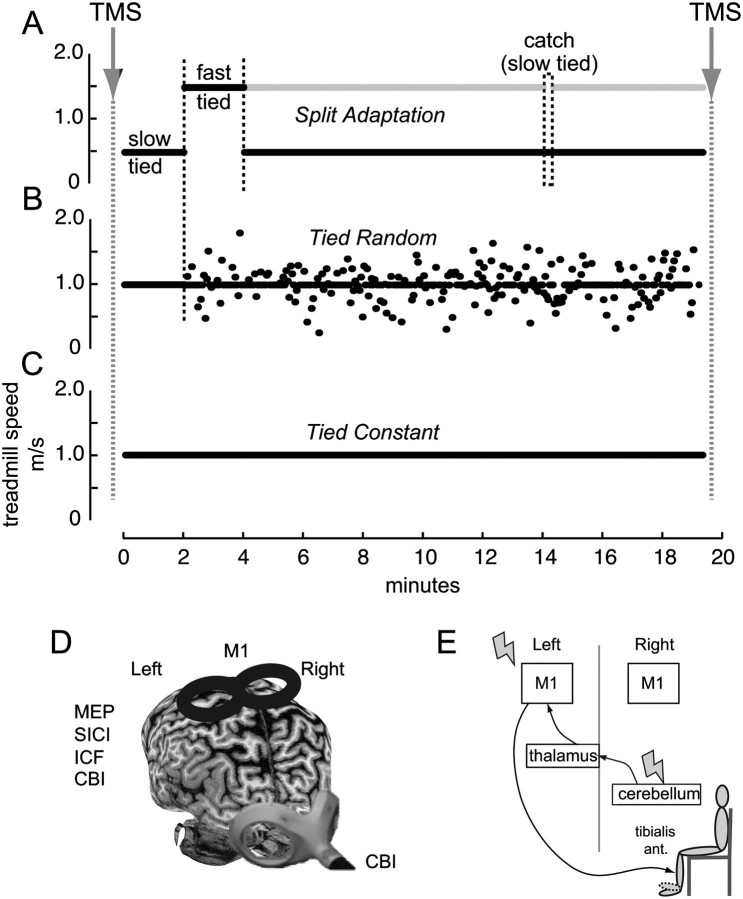
Subjects in the main experiment participated in 3 separate randomized, crossover counterbalanced sessions. In all sessions, we tested excitability of M1 and cerebral–cerebellar connectivity before and after 20 min walking on a custom split-belt treadmill (Woodway). This treadmill comprised 2 separate belts driven by independent motors that allow independent speed control of each belt (leg) through a custom-written computer interface in MATLAB (MathWorks). Sessions were separated by at least 1 week. During each session, participants were exposed to one of the different locomotor conditions (Fig. 1):
Figure 1.
The figure shows the schematic representations of the experimental setup. Subjects participated in 3 different sessions. TMS measures were obtained before and after subjects walked in a split-belt treadmill with (A) belts moving at different speeds where the fast leg (gray line) moves 3 times faster than the slow leg (black line), split adaptation, (B) both belts moving at the same speed but with unpredictable speed changes, tied random, and (C) both belts moving at the same constant speed, tied constant. In the split adaptation session, symmetry was assessed at 2 different baseline speeds. Ten minutes into adaptation, a catch trial consisting of tying the belts at the same slow speed was introduced for 10 s. After this, subjects returned to the split adaptation condition for another 5 min. (D) Representation of the TMS coils and positions used for the different excitability measures over the left leg representation of the primary motor cortex (M1, MEPs threshold and amplitude, SICI, ICF) and over the right cerebellar cortex (CBI). (E) Schematic representation of the CBI pathway and assessment. A conditioning TMS pulse is delivered over the right cerebellar hemisphere 5 ms prior to a test pulse applied over the left M1. MEP amplitudes are recorded from the right TA muscle during minimal muscle contraction.
Split adaptation consisted of a 4-min baseline period of tied-belt walking at both slow (0.5 m/s) and fast (1.5 m/s) speeds. After this, participants were exposed to a 10-min adaptation period, where one belt moved at 1.5 m/s and the other at 0.5 m/s. Split-belt walking initially disrupts coordination between the legs such that the fast and slow leg steps are asymmetric and the fast leg's motion is phase advanced relative to that of the slow leg. In other words, subjects walk with a “limp.” We refer to the limb on the slow belt in the split-belt period as the slow limb and the limb on the fast belt as the fast limb. The split-belt perturbation is predictable, so adaptive mechanisms act to eliminate the limp in about 10 min (Reisman et al. 2005). Ten minutes into the adaptation period a brief catch trial (10 s) with the belts tied at the same slow speed used at baseline was introduced to assess how much the subjects had learned. When subjects are reexposed to tied-belt walking, they limp in the opposite way. This occurs because the newly adapted split-belt pattern is now being used for tied-belt walking and demonstrates storage of the new locomotor pattern. Subsequent to this “catch trial” subjects returned to the adaptation period for another 5 min (Fig. 1a). Finally, and after the physiological assessments were completed (see below), participants were exposed to a postadaptation period (10 min), where they walked with the belts tied at the slow speed.
The tied random condition required walking for 20 min with both belts tied but moving at variable unpredictable speeds changing every 3 s and centered around 1 m/s with a standard deviation of 0.8 m/s. This task is more complex than walking at a constant speed due to the sudden changes in walking speed. Importantly, however, no adaptive learning can occur (i.e., learning to predict the split-belt perturbation) because both legs always move in a symmetric pattern and the change in walking speed is randomly introduced (Fig. 1b). The tied constant condition consisted of 20 min of walking with both belts moving at the same speed of 1 m/s (Fig. 1c). Again, there was no adaptation in this task since no perturbations were introduced.
In all sessions, subjects wore a safety harness and were positioned in the middle of the treadmill with their arms folded across their chest. They were instructed not look down at the belts when walking on the treadmill and were allowed to watch television. TMS measurements were performed before baseline walking and immediately after the entire adaptation period (including the 5 min of adaptation after presentation of a “catch trial period”) in the split-belt condition and before and after the walking in the tied random or tied constant condition.
Electromyography
We recorded subjects’ electromyography (EMG) using a bipolar electrode configuration and 3M Red Dot surface Ag/AgCl EMG electrodes (3M) placed over the dominant tibialis anterior (TA) muscle belly. The ground electrode was placed over the right external malleolus. EMG data were sampled at 2000 Hz, amplified (1000), and band-pass filtered (10–500 Hz) using an amplifier (Motion Lab Systems). EMG and stimulator trigger pulse data were recorded using Spike2 software (Cambridge Electronic Design). Prior to the initiation of the study, we measured the amplitude of TA EMG activity during 3 maximum voluntary contractions (MVCs) performed against resistance allowing for a brief rest period in between. During all excitability measures, subjects were instructed to maintain a contraction of the TA at 20% of their MVC using visual feedback (Madhavan and Stinear 2010). Using a custom-written Spike2 script, TMS pulses were triggered only when the EMG activity was in the target range (20 ± 1% of MVC). All data were stored on a computer for off-line analysis using a custom Matlab program (MathWorks).
Measures of Cerebellar Excitability
In each session, we determined cerebellar excitability by assessing CBI before and after the performance of the locomotor tasks. To this end, we delivered TMS using a Magstim double-cone coil (110 mm mean diameter, Magstim) centered over the cerebellar cortex ipsilateral to the target muscle and 3 cm lateral to the inion on the line joining the inion and the external auditory meatus (Fig. 1d). The coil was oriented to induce an inferior–superior current flow in cortex. Similar to previous studies, we assessed CBI by independently triggering a TMS conditioning stimulus (CS) over the cerebellar cortex ipsilateral to the fast leg 5 ms prior to a test stimulus (TS) over the contralateral M1 (Fig. 1e) (Ugawa, Terao, et al. 1995; Ugawa 1999; Pinto and Chen 2001; Daskalakis et al. 2004; Galea et al. 2009). We gave 10 CS + TS stimuli to measure CBI along with 10 unconditioned TS stimuli in a random order. CBI was calculated for each subject by measuring the percent change of the mean motor evoked potential (MEP) amplitude in the CS + TS relative to TS. To avoid direct activation of the corticospinal tract, the intensity for cerebellar stimulation was set at 5% below the brainstem active motor threshold (aMT) (Fisher et al. 2009; Ugawa 2009). For this, the double-cone coil was placed over the inion, and subjects preactivated their TA, a muscle involved in the locomotor tasks, at 20% of their MVC. Threshold was defined as the nearest 5% stimulator output that elicited an MEP of 100 μV in the preactivated TA muscle in 5 of 10 trials. When MEPs from brainstem stimulation could not be elicited in the TA (4 subjects), the CS intensity was based on the brainstem threshold of the first dorsal interosseus muscle. However, in 3 subjects, the brainstem threshold was not observed at 80% of the maximum stimulator output and therefore 70% intensity was used for the CS. The MEP amplitudes of 10 single-pulse TMS responses over M1, as tested during M1 excitability measures (see below), and 10 paired-test plus conditioned responses were averaged before and after walking in each session. The intensity of stimulation for the TS were adjusted to elicit similar MEP amplitudes (mean stimulus intensity adjustment before and after walking were less than 1%).
Measures of Primary Motor Cortex (M1) Excitability
To assess M1 excitability, we measured in each session the aMT, MEP amplitude, short intracortical inhibition (SICI), and intracortical facilitation (ICF) of the TA. Thus, using a 70-mm-diameter figure-of-eight coil, we applied TMS over the motor cortex in each session. First, we determined the optimal location of the leg representation of the primary motor cortex (M1) to elicit MEP in the contralateral TA muscle (hot spot). Then the aMT was defined as the lowest intensity of magnetic stimulation required to evoke 100 μV MEPs in 5 of 10 trials. After this, we established the stimulator intensity to obtain MEP of 1 mV amplitude. Then, SICI and ICF were assessed using paired-pulse TMS with subthreshold CS at 80% of aMT intensity and suprathrehsold TS set to elicit ∼1 mV MEPs (Kujirai et al. 1993). SICI was tested with a 2ms interstimulus interval and ICF with 12 ms. After the locomotor tasks were completed, we assessed MEP amplitudes changes by stimulating at the same intensity as used to elicit 1 mV MEP at baseline. We also repeated the measures of SICI and ICF, but for these, the TS intensity was adjusted to ensure that the MEP amplitudes remained at the same size as before walking. For each measurement before and after the locomotor tasks, we recorded and then averaged 10 MEPs.
Additional Experimental Session
To further determine consistency of the cerebellar excitability changes observed in the main experiment, a second group of individuals participated in a single session assessing excitability before and after locomotor adaptation. Here, a naive group of 6 healthy subjects were exposed to the split-adaptation walking task (see experiment 1 methods for details). Before and after split adaptation, we assessed CBI in the preactivated TA muscle as previously described. Of note, in this addition session, we were able to obtain brainstem aMT in all subjects.
Kinematic Data
We collected kinematic data during walking at 100 Hz using Optotrak (Northwen Digital). We placed bilateral infrared-emitting markers over the following joints: foot (fifth metatarsal head), ankle (lateral malleolus), knee (lateral femoral epicondyle), hip (greater trochanter), pelvis (iliac crest), and shoulder (acrominion process). The coordinate system was aligned such that the x-axis was parallel to the treadmill belts, the y-axis was parallel to the vertical line, and the z-axis was parallel to the horizontal line perpendicular to the x–y plane.
Data Processing and Analysis
Motor Evoked Potentials
MEP amplitudes were measured peak to peak for each trial and averaged before and after walking for each session. SICI, ICF, and CBI were calculated as the ratio of the conditioned to the test MEP amplitudes. We also analyzed the pretrigger root mean square EMG amplitude (40 ms prior to stimulus onset) to compare the level of background activation between the baseline and postwalking TMS data.
Optotrack Motion Analysis Data
Three-dimensional marker position data were low-pass filtered at 6 Hz. Custom software in MATLAB (Mathworks) was used for all analyses. Based on our previous work, we calculated spatial walking parameters that were expected to change using adaptive mechanisms (Reisman et al. 2005). Specifically, we assessed step length symmetry as an indicator of adaptation. Each step length is calculated as the anterior–posterior distance between the ankle marker of each leg at heel strike of the leading leg; fast step length refers to the step length measured at fast leg heel strike and slow step length refers to the step length at slow leg heel strike. Step symmetry (SS) was calculated as the difference in fast (SLf) and slow (SLs) step lengths, normalized to their sum to allow for comparisons across subjects of different sizes (Equation 1). We then calculated the magnitude of step symmetry for each pair of steps occurring during adaptation, the catch trial, and of the after-effect during deadaptation (Morton and Bastian 2006).
| (1) |
Statistical Analysis
We use separate repeated measures analyses of variance (ANOVARM) in MEP amplitude, SICI, ICF, and CBI with factors SESSION (tied constant, tied random, and split adaptation) and TIME (prewalking and postwalking). When significant differences were found, post hoc analyses were performed using paired t-tests. Data are expressed as mean ± standard error of the mean, and effects were considered significant if P < 0.05.
To determine association between physiological changes and behavior, we performed correlation analysis between CBI and 1) step length symmetry for the catch trial and 2) adaptation magnitude. Step length symmetry was calculated as the difference between the fast and slow step lengths divided by the sum of them. Magnitude of adaptation is the difference in the step symmetry for the first 5 steps of the adaptation period and the last 30 s of the adaptation period.
Results
Locomotor Tasks
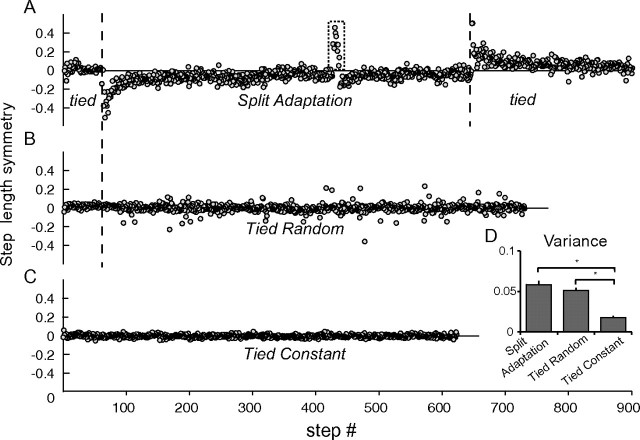
All subjects completed the 3 sessions and none experienced complications. During the split adaptation condition, all subjects showed adaptation as demonstrated by large asymmetric step lengths early during the adaptation period rapidly returning to baseline symmetry and opposite asymmetry during the catch trial and postadaptation period (Fig. 2a, Supplementary Fig. 1). In contrast, performance was more variable in the tied random, but no signs of adaptation were present (i.e., sudden change in step symmetry that returns to baseline over time and or presence of after-effects indicated by step symmetry changes in the opposite direction after the perturbation is removed; Fig. 2b). Finally, step lengths were symmetric in the tied constant condition with little variation from step to step (Fig. 2c). To quantify the degree of performance complexity, we calculated the step symmetry variance in each session. ANOVA showed a main effect of session (F2,20 = 23.4, P < 0.001), with no post hoc difference between split adaptation and tied random (P = 0.18, Fig. 2d). However, both split adaptation and tied random were different from tied constant (P < 0.001). Thus, the complexity of the task was similar in the split adaptation and tied random conditions but larger than the tied constant condition.
Figure 2.
Each graph shows single subject behavioral data describing the step length symmetry of sequential strides in each condition. (A) The rectangular dotted area demonstrates the step symmetry during the catch trial after 10 min of split adaptation; this reflects retention of the new locomotor pattern. (B) Note that during the tied random condition asymmetric steps were present. These occurred when there were transitions between speeds. (C) During the tied constant condition, there is little variability in step symmetry. (D) Group data showing the step symmetry variance during each condition. Please note that variance during split adaptation and tied random conditions were similar and higher than tied constant condition. *P < 0.05.
Adaptation, but Not Performance, Modulates Cerebellar Excitability
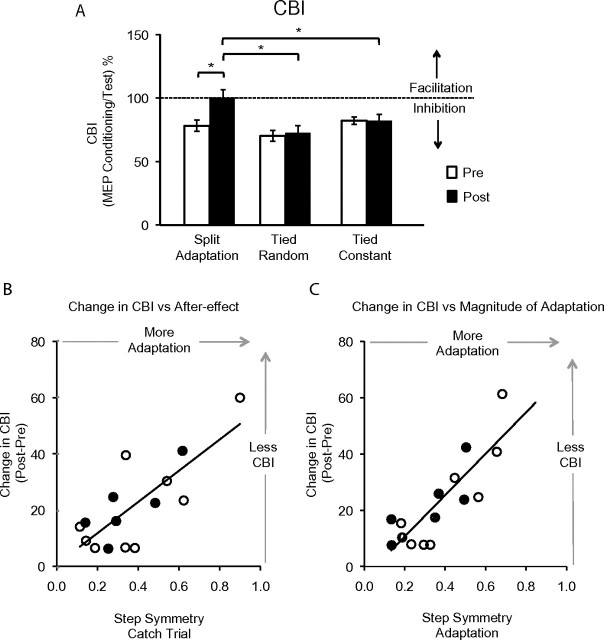
Adaptation to the split-belt condition reduced the magnitude of CBI, in the absence of similar changes in the random perturbation and tied-belt walking sessions (Figs 3 and 4a). Since the cerebellum normally exerts an inhibitory tone over M1, the hypothesized reduction in CBI as a consequence of learning would be reflected by larger evoked potential amplitudes after the learning has occurred. Repeated measures ANOVA (ANOVARM) revealed a significant effect of SESSION, TIME, and a TIME by SESSION interaction on CBI. Importantly, the TIME by SESSION interaction indicated that CBI changed from pre split adaptation to postadaptation but not during the tied random or tied constant conditions (Table 1). Paired t-tests revealed a significant decrease of CBI, which is observed as an increase in MEP response to the CS + TS, after split adaptation (Fig. 4a). This change in CBI was larger than that observed after the tied random session or tied constant session. Finally, the findings on CBI (CS + TS) were not due to simple modifications of TS MEP amplitudes (Table 1).
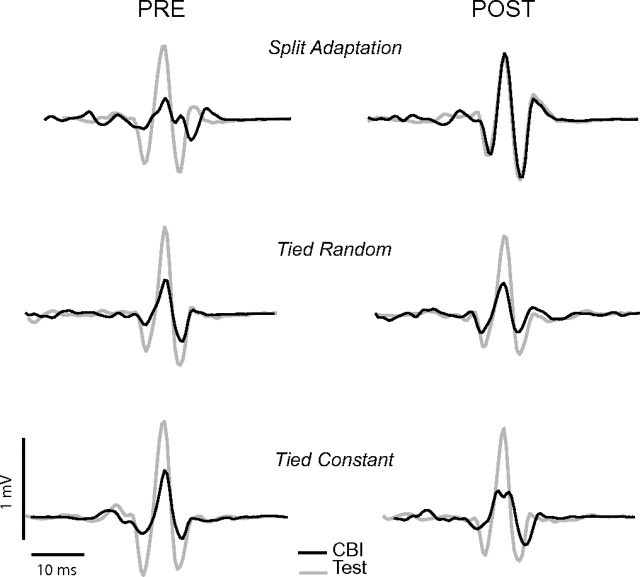
Figure 3.
The figure shows representative EMG traces depicting MEPs before and after performance of the behavioral tasks during test stimulation only (Test, TMS over M1, gray line) and CBI assessment (CBI, conditioning pulse over right cerebellum and test pulse over left M1, black line). Please note the presence of cerebellar inhibition in all conditions at baseline (CBI MEP amplitudes are smaller than the Test M1 amplitudes) that is only reduced after split adaptation (CBI and Test amplitudes are similar) but not in tied constant and tied random conditions.
Figure 4.
(A) The histogram shows the mean CBI amplitudes during pre (white) and post (black) performance under the different behavioral conditions relative to test (100%). Please note the significant reduction of CBI only after split adaptation but not in the other conditions, *P < 0.01. (B) The mean step symmetry during the catch trial, a measure indicative of retention of the new locomotor pattern, was strongly correlated with the reduction in CBI, where subjects that learned more had greater reductions in CBI (r = 0.78). (C) The magnitude of adaptation, a variable that reflects storing of the new pattern and calculated as the step symmetry delta from the beginning of adaptation to the end, was also strongly correlated with the reduction in CBI (r = 0.84). Please note that open circles represent the 9 subjects that participated in the main crossover designed study. Black circles represent the 6 subjects who took part in the additional split adaptation session only.
Most importantly, the reduction in CBI following split-belt walking was strongly correlated with step symmetry in the catch trial. The catch trial, brief return to tied-belt condition, was used to assess how much of the new locomotor pattern has been stored. Larger asymmetry indicates that more storage has occurred. Here, subjects with more asymmetry during the catch trial also had larger reductions in CBI (r = 0.78; Fig. 4b). Recall that a reduction in CBI is what would be expected from depression of Purkinje cells excitability in cerebellar cortex. We also found that the subjects who adapted the most during the split-belt period (i.e., changed the most throughout adaptation) showed the largest reduction of CBI (r = 0.84; Fig. 4c). On the other hand, performing similar correlation analysis between magnitude of adaptation and step symmetry in the tied random and constant conditions did not show any significant differences. These findings are thus consistent with the interpretation that the magnitude of CBI is related to the amount of adaptation.
Finally, we evaluated cerebellar excitability changes before and after adaptation in an additional group of individuals to determine reliability of the findings. Here, a separate group of subjects showed locomotor adaptation as in experiment 1. Again, performance of this task resulted in a clear reduction of CBI in the TA muscle, as found in experiment 1. Paired t-test with factor TIME (pre-, postadaptation) revealed a significant effect of TIME on CBI (P < 0.002). Interestingly, the reduction in CBI following split-belt walking was also strongly correlated with step symmetry in the catch trial and adaptation amount (r = 0.93, r = 0.75; Fig. 4b,c dark circles).
Task complexity, but Not Adaptation, Affects Primary Motor Cortex Excitability
Both split adaptation and tied random conditions caused significant changes in M1 excitability, whereas simple tied-belt walking did not. We determined M1 excitability in each session by assessing aMT, MEP amplitude, SICI, and ICF of the fast leg TA cortical representation using standard TMS procedures (see Materials and Methods). There was no change in motor threshold after split adaptation, tied random, or tied constant walking conditions. We found that MEP amplitudes increased significantly over TIME (pre- to postwalking) but not across all SESSIONS. There was a TIME by SESSION interaction (Table 1) and post hoc paired t-tests revealed that the MEP increase over TIME was significant only during the split adaptation and the tied random conditions (Fig. 5a).
Table 1.
Excitability values before (pre) and after (post) performance of the different locomotor tasks
| SESSION | Split adaptation |
Tied random |
Tied constant |
ANOVARM |
Post hoc (pre to post) |
|||||||
| TIME | Pre | Post | Pre | Post | Pre | Post | TIME (pre, post) | SESSION | TIME × SESSION | Split adapt | Tied random | Tied belt |
| Cerebellar excitability | ||||||||||||
| CBI (%) | 78.1 ± 4.4 | 100.1 ± 6.2 | 70.1 ± 4.3 | 72.4 ± 5.8 | 82.1 ± 3.0 | 85.7 ± 3.8 | F1,16 = 10.8, P = 0.011 | F2,16 = 4.0, P = 0.039 | F2,16 = 7.7, P = 0.005 | t(8) = −3.6, P = 0.007 | t(8) = −0.9, P = 0.40 | t(8) = 0.04, P = 0.97 |
| TS MEP (mV) | 1.43 ± 0.18 | 1.43 ± 0.17 | 1.53 ± 0.23 | 1.66 ± 0.22 | 1.25 ± 0.31 | 1.21 ± 0.23 | F1,16 = 0.4, P = 0.56 | F2,16 = 0.8, P = 0.42 | F2,16 = 1.1, P = 0.36 | — | — | — |
| M1 excitability | ||||||||||||
| aMT (%) | 55 | 53 | 56 | 55 | 54 | 54 | F1,16 = 0.13, P = 0.878 | F2,16 = 0.26, P = 0.77 | F2,16 = 0.23, P = 0.80 | — | — | — |
| MEP (mV) | 0.93 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.4 ± 0.2 | 1.0 ± 0.13 | 1.0 ± 0.11 | F1,16 = 25.2, P = 0.001 | F2,16 = 1.4, P = 0.29 | F2,16 = 6.1, P = 0.01 | t(8) = −4.9, P = 0.001 | t(8) = −5.38, P = 0.001 | t(8) = 0.23, P = 0.82 |
| SICI (%) | 77.4 ± 6.5 | 100.2 ± 6.0 | 78.3 ± 4.9 | 101.5 ± 11.5 | 82.5 ± 2.9 | 83.9 ± 2.3 | F1,14 = 16.9, P = 0.004 | F2,12 = 0.26, P = 0.78 | F2,12 = 3.3, P = 0.06 | t(7) = −2.8, P = 0.016 | t(7) = −3.04, P = 0.025 | t(7) = −0.005, P = 0.996 |
| ICF (%) | 148.9 ± 25.8 | 116 ± 8.3 | 117.8 ± 9.2 | 120.6 ± 10.3 | 141.7 ± 14.1 | 125.8 ± 9.3 | F1,8 = 4.8, P = 0.09 | F2,8 = 5.4, P = 0.03 | F2,8 = 2.9, P = 0.099 | — | — | — |
CBI, SICI, and ICF are presented as percent of the conditioned plus test stimuli (CS + TS) of the test stimulation over M1 alone. The TS intensity during CBI measures was adjusted to maintain similar MEP amplitudes (TS MEP). MEP amplitudes from M1 were obtained separately from the SICI and ICF measures. aMT units represent the percent of the stimulator output. Post hoc values were calculated using paired t-test.
Figure 5.
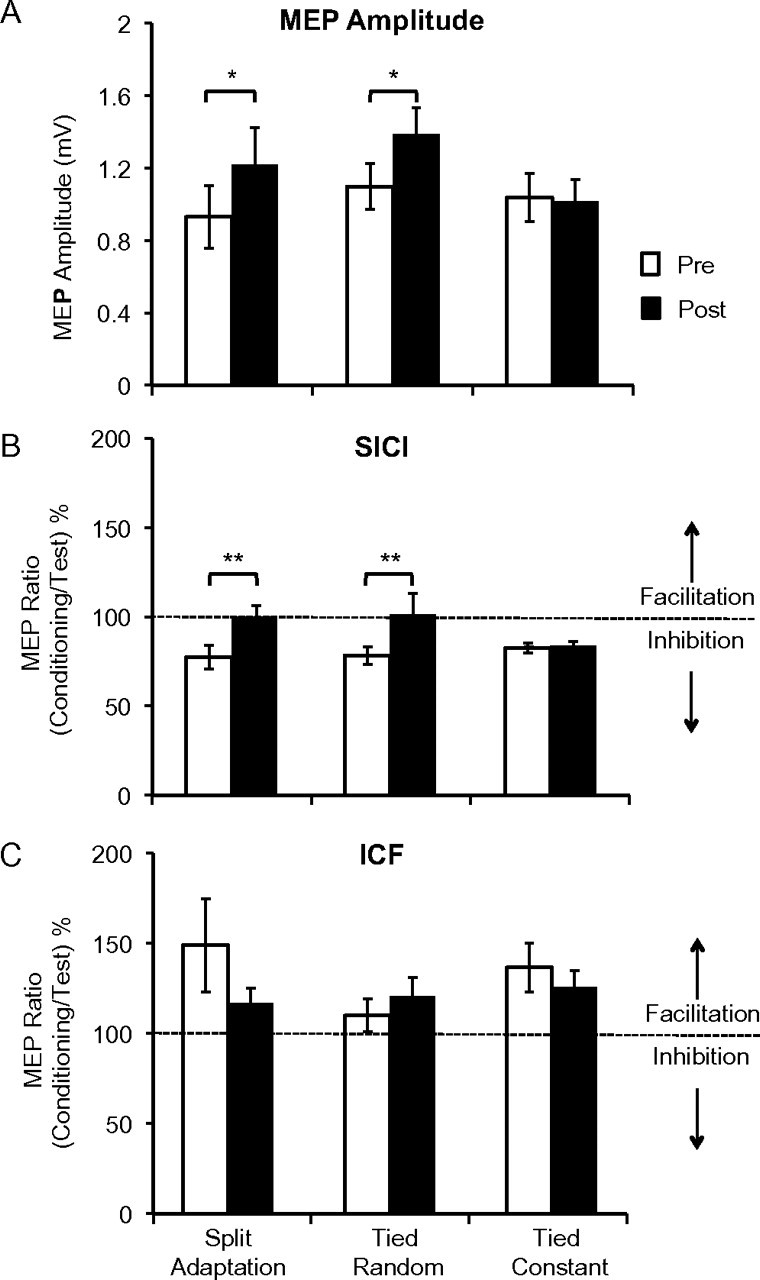
The histograms show measures of M1 excitability: MEP amplitude in millivolts (mV, A), SICI (B), and ICF (C). The latter 2 are calculated as the ratio of the MEP amplitudes of conditioned over test MEP times 100 and expressed relative to the test MEP (100%). MEP amplitudes increased significantly (*P < 0.05). Intracortical inhibition had a strong trend toward significant reduction (ANOVARM P = 0.06) following split adaptation and tied random only, as demonstrated by exploratory post hoc t-tests (**P < 0.05). There were no significant changes in ICF.
We were able to obtain SICI in all sessions in only 8 of the 9 subjects. ANOVARM showed significant changes in SICI across TIME but not SESSION; there was also a strong trend toward a TIME by SESSION interaction (P = 0.06; Table 1; Fig. 5b). Due to this trend, we performed exploratory post hoc paired t-tests, which revealed a significant reduction in SICI for the split adaptation and tied random conditions but not for the tied constant session. Thus, the SICI results largely paralleled the MEP amplitude findings. ICF could only be assessed in all sessions on 5 subjects. ANOVARM revealed a significant change in ICF across sessions but no effect of TIME or TIME by SESSION interaction (Table 1; Fig. 5c). Finally, although we found changes in motor cortex excitability measures, correlation analysis did not reveal significant relationships between these and the magnitude of locomotor adaptation.
Discussion
Our results show specific neurophysiological involvement of the cerebellum during locomotor adaptation in humans. In particular, we found a reduction of the normal inhibitory tone the cerebellum exerts over the primary motor cortex only as a consequence of learning a new locomotor pattern, but not during performance of a complex locomotor task. This reduction of inhibition strongly correlated with the magnitude of adaptation; the subjects who experienced the most adaptation (either assessed by improvement in symmetric walking during the perturbation or magnitude of after-effect when the perturbation was removed) had the largest reduction of CBI. On the other hand, the changes in primary motor cortex excitability appeared to be associated with performance of complex motor behavior rather than adaptive learning. In other words, we dissociated neurophysiological changes due to locomotor adaptation from those related to complex motor performance in humans.
Previous lesion studies have shown that the cerebellum is important for adaptation. For example, healthy individuals can adapt movements to predictable new demands, whereas patients with cerebellar disorders show impairments in adapting and storing a new pattern (Weiner et al. 1983; Martin et al. 1996; Morton and Bastian 2003; Tseng et al. 2007). Of particular relevance, our prior work has shown that the cerebellum is required for adaptive learning of split-belt locomotion, but is not critical for reacting to changes in treadmill speeds using feedback control (Morton and Bastian 2006). Based on that result and the current findings, we suggest that the cerebellum is most important for learning motor commands that anticipate a predictable change in the environment and less critical for reacting to unpredictable events.
There is little information regarding the neurophysiological mechanisms by which humans adapt and no direct relationship between neural mechanisms and adaptive learning behaviors has been shown. Animal studies, however, have indicated that the development of LTD in Purkinje cells is associated with adaptive learning (Gilbert and Thach 1977; Medina and Lisberger 2008). Similarly, blocking cerebellar LTD abolishes locomotor adaptation (Yanagihara and Kondo 1996). Thus, in this study, we reasoned that if LTD is the mechanism by which humans adapted their locomotor pattern, then the excitability of Purkinje cells as reflected by CBI after adaptation should decrease in proportion to the extent of adaptation. Our results demonstrate this relationship and therefore support the hypothesis of LTD-mediated cerebellar adaptive learning.
Paired-pulse TMS studies have described the existence at rest of a normal inhibitory tone that the cerebellum exerts on the primary motor cortex (Ugawa, Terao, et al. 1995; Pinto and Chen 2001; Daskalakis et al. 2004). In these investigations, a conditioning pulse delivered over one cerebellar cortex 5–7 ms prior to a test pulse over the contralateral M1 results in smaller MEP amplitude in a hand muscle relative to single TMS pulse over the same M1. The decreased MEP amplitudes reflect inhibition of M1. This effect has been attributed to TMS activation of Purkinje cells resulting in inhibition of the dentate nucleus, which in turn has a disynaptic excitatory connection through the ventral thalamus to the contralateral M1 (Ugawa, Uesaka, et al. 1995; Middleton and Strick 1999; Pinto and Chen 2001; Daskalakis et al. 2004; Reis et al. 2008). Given this pathway, we predicted that LTD changes in Purkinje cells after locomotor adaptation should result in decreased activation of these cells when the conditioning TMS pulse is delivered over the cerebellum, thus reducing inhibition of the dentate nucleus and ultimately not affecting the primary motor cortex (i.e., the test MEP amplitudes are similar to the conditioned plus test MEP amplitudes). Alternatively, it is possible that LTD could have occurred in the synapses between Purkinje cells and deep cerebellar nuclei. Indeed, our findings showing decreased CBI only after learning the new locomotor pattern suggest that LTD changes have occurred affecting activation and or downstream transmission of Purkinje cell activity and provides evidence of this process in humans. The strong correlation between behavioral changes and the amount of CBI further support this idea; those individuals adapting (delta between early and late adaptation) or storing (catch trial adaptation) the new locomotor pattern the most had the largest decrease of CBI.
It is important to note that changes in the amount of inhibition of M1 via CBI are likely to be reflective of the state of Purkinje cell excitability (Galea et al. 2009) but could also reflect changes anywhere along the cerebellar-thalamo-cortical pathway. It is also important to note that the cerebellar-thalamo-cortical connections may not be the only pathway utilized during locomotor adaptation. For instance, cerebellar influences on brainstem motor pathways (i.e., vestibulospinal, reticulospinal) (Morton and Bastian 2006) may also be important for adapting this behavior. However, the possible participation of other circuits in mediating the adaptation studied here in no way diminish the importance of the correlation found between the magnitude of CBI and the degree of locomotor adaptation. Indeed, we replicated in a second group of healthy individuals our main experimental findings demonstrating the robustness of the physiological and behavioral correlation.
M1 excitability, on the other hand, increased with split adaptation and tied random walking but not with tied belt constant speed walking. These findings suggest that M1 changes are the result of complex motor performance similar to what has been observed in functional imaging studies, where more complex task performance is associated with increased activation (Rao et al. 1993; Shibasaki et al. 1993). Similar observations have also been made in a TMS study assessing performance of different complex hand tasks (Tinazzi and Zanette 1998). Interestingly, previous investigations have also shown an increase in M1 excitability associated with learning different upper or lower extremity tasks (i.e., piano sequence, wrist motions, tracing with the foot) but no changes following passive training or repetition of nonskilled tasks (Pascual-Leone et al. 1995; Lotze et al. 2003; Perez et al. 2004; Rosenkranz et al. 2007). However, since these studies did not control for task performance complexity as a possible source of change in excitability, it remains an open question whether the observed M1 changes were specific to task learning or increased task complexity.
The observed trend toward reduction of intracortical excitability in the split adaptation and tied random sessions are likely related to strengthening the networks mediating complex task execution, rather than mediating the acquisition of a new internal model, as only cerebellar excitability changes were specifically found and correlated with adaptation to a new locomotor pattern. Indeed, modulation of SICI reflecting γ-aminobutyric acid (GABA) A neurotransmission (Ziemann et al. 1996; Ilic et al. 2002; Di Lazzaro et al. 2005) is thought to be pivotal in M1 plasticity (Jacobs and Donoghue 1991; Pascual-Leone et al. 1995; Bütefisch et al. 2000). Similar reduction in SICI has also been observed in leg muscles following skilled training but not unskilled repetitive movements (Perez et al. 2004), a finding consistent with our results showing no clear SICI changes following regular tied-belt walking. These results suggest that during the performance of complex motor tasks reduction in GABAergic inhibition may facilitate the strengthening of corticocortical connections.
In contrast to SICI, ICF did not change significantly following any of the 3 walking tasks. ICF is known to be significantly weaker following tonic contraction than at rest (Ridding et al. 1995). Thus, it is possible that subtle changes in facilitatory circuitry were masked by the muscle contraction required during testing. Alternatively, it is possible that ICF does not reflect crucial changes related to motor performance or adaptation, as suggested by others (Perez et al. 2004). In addition, it is possible that unlike Oliveri et al. (2005) who found changes in ICF after cerebellar inhibition with repetitive TMS, we did not find these changes due to our choice of measuring ICF at a 12ms interstimulus interval rather than 15 ms. Finally, it is also possible that the lack of significant difference was due to small sample size.
To our knowledge, this is the first time that CBI and its changes relative to motor behavior are reported for leg muscles. We used a similar approach to study the leg cerebellar representation as has been previously done for the hand. It should be noted that the leg representation is located immediately anterior to the hand (Diedrichsen et al. 2005). Therefore, some methodological issues of this study need to be considered. First, we used a double-cone coil to ensure that we reached the deeper leg representation. Second, in 3 subjects, we could not elicit brainstem MEPs in any of the 3 sessions. While not ideal, the consistency within each subject should not produce the confound of changed excitability across sessions. Third, one of the main reasons to search for brainstem MEPs is to avoid concerns of stimulating directly the corticospinal tract or other possible brainstem pathways during cerebellar conditioning stimulation (Fisher et al. 2009; Ugawa 2009). Thus, it is unlikely that other brainstem structures were stimulated when assessing CBI because we used intensities below brainstem threshold and in few subjects we could not even elicit brainstem MEPs. Fourth, we found that our CBI assessments were stable in 2 conditions (tied walk and random tied) and only changed after adaptation of split-belt walking, a behaviorally specific effect. Thus, it is unlikely that the nature of inhibition (or lack of it) originated from nonspecific mechanisms, such as skin afferents from the neck (Gerschlager et al. 2002) or direct activation of the corticospinal tract (Fisher et al. 2009). Fifth, in this study, we could not use a neuronavigational system. Due to this limitation, we went to great lengths to mark the coil position on the scalp of each subject using techniques that were used preceding the widespread use of neuronavigation technology. The data suggest that we did not introduce a consistent bias in coil position since we had stable MEPs and CBI assessments as mentioned above. Finally, any potential variability due to coil localization should have affected the 3 behavioral conditions equally.
Our results have important ramifications for understanding neural mechanisms that may be involved in or facilitate rehabilitation. Strategies that promote and enhance adaptive learning and retention are of considerable interest. The strong relationship between inhibition of cerebellar outputs and adaptation of a complex behavior suggest that this mechanism may be useful for individuals with damage outside the cerebellum. Indeed, our prior work has shown that while cerebellar damage impairs adaptive changes in split-belt locomotion (Morton and Bastian 2006), cerebral damage may not (Reisman et al. 2007). Individuals with cerebral damage can show after-effects that improve the symmetry of stepping (Reisman et al. 2007), which is compelling evidence that their compromised nervous systems are still able to learn a “normal” pattern of movement. Those results combined with those of the current study suggest that the cerebellum may be an ideal site to stimulate noninvasively during adaptive learning. As such, we have recently showed that transcranial direct current stimulation is also capable of modulating cerebellar excitability (Galea et al. 2009), suggesting that this strategy is plausible. In sum, understanding the neurophysiological underpinnings of motor adaptation will allow the rational application of brain stimulation interventions to improve behavioral gains in patients with neurological conditions.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
Supported by R21 HD060169, The Johns Hopkins Brain Science Institute, F31NS062503, R01HD040289, and R01HD053793.
Supplementary Material
Acknowledgments
Conflict of Interest: None declared.
References
- Bastian AJ. Understanding sensorimotor adaptation. Current Opin Neurol. 2008;21:628–633. doi: 10.1097/WCO.0b013e328315a293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hua SE, Smith MA, Lenz FA, Shadmehr R. Effects of human cerebellar thalamus disruption on adaptive control of reaching. Cereb Cortex. 2006;16:1462–1473. doi: 10.1093/cercor/bhj087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Paradiso GO, Christensen BK. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci. 2005;25:9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone A, Ranieri F, Musumeci G, Fiorilla T, Tonali P. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol. 2005;564:661–668. doi: 10.1113/jphysiol.2004.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KM, Lai HM, Baker MR, Baker SN. Corticospinal activation confounds cerebellar effects of posterior fossa stimuli. Clin Neurophysiol. 2009;120:2109–2113. doi: 10.1016/j.clinph.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea J, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific non-invasive direct current stimulation. J Neurosci. 2009;29:9115. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschlager W, Christensen LO, Bestmann S, Rothwell JC. rTMS over the cerebellum can increase corticospinal excitability through a spinal mechanism involving activation of peripheral nerve fibres. Clin Neurophysiol. 2002;113:1435–1440. doi: 10.1016/s1388-2457(02)00156-6. [DOI] [PubMed] [Google Scholar]
- Gilbert PF, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Cerebellar control of the vestibulo-ocular reflex—around the flocculus hypothesis. Ann Rev Neurosci. 1982;5:275–297. doi: 10.1146/annurev.ne.05.030182.001423. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar learning in the vestibulo-ocular reflex. Trends Cogn Sci. 1998;2:313–321. doi: 10.1016/s1364-6613(98)01222-4. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Kluzik J, Peterka RJ, Horak FB. Adaptation of postural orientation to changes in surface inclination. Exp Brain Res. 2007;178:1–17. doi: 10.1007/s00221-006-0715-0. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain. 2003;126:866–872. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain. 1996;119(Pt 4):1199–1211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- Madhavan S, Stinear JW. Focal and bi-directional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimul. 2010;3:42. doi: 10.1016/j.brs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–1192. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton F, Strick P. The cerebellum: an overview. Trends Cogn Sci. 1999;2:305–306. doi: 10.1016/s1364-6613(98)01224-8. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Relative contributions of balance and voluntary leg-coordination deficits to cerebellar gait ataxia. J Neurophysiol. 2003;89:1844–1856. doi: 10.1152/jn.00787.2002. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri M, Koch G, Torriero S, Caltagirone C. Increased facilitation of the primary motor cortex following 1Hz reptitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci Lett. 2005;376:188–193. doi: 10.1016/j.neulet.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Dang N, Cohen LG, Brasil-Neto J, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- Pinto AD, Chen R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res. 2001;140:505–510. doi: 10.1007/s002210100862. [DOI] [PubMed] [Google Scholar]
- Rao S, Binder J, Bandettini P, Hammeke T, Yetkin F, Jesmanowicz A, Lisk L, Morris G, Mueller W, Estkowski L. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–2318. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Inter-limb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94:2403–2415. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Kacar A, Rothwell J. Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci. 2007;27:12058–12066. doi: 10.1523/JNEUROSCI.2663-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H, Sadato N, Lyshkow Y, Yonekura Y, Honda M, Nagamine T, Suwazono S, Magata Y, Ikedo A, Miyazaki M. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain. 1993;116:138. doi: 10.1093/brain/116.6.1387. [DOI] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Zanette G. Modulation of ipsilateral motor cortex in man during unimanual finger movements of different complexities. Neurosci Lett. 1998;244:121–124. doi: 10.1016/s0304-3940(98)00150-5. [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- Ugawa Y. Magnetic cerebellar stimulation. Electroencephalogr Clin Neurophysiol Suppl. 1999;49:222–225. [PubMed] [Google Scholar]
- Ugawa Y, Terao Y, Nagai C, Nakamura K, Kanazawa I. Electrical stimulation of the cerebellum normally suppresses motor cortical excitability in a patient with ataxia due to a lesion of the middle cerebellar peduncle. Eur Neurol. 1995;35:243–244. doi: 10.1159/000117140. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- Ugawa Y. Can we see the cerebellar activation effect by TMS over the back of the head? Clin Neurophysiol. 2009;120:1999–2120. doi: 10.1016/j.clinph.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology. 1983;33:766–772. doi: 10.1212/wnl.33.6.766. [DOI] [PubMed] [Google Scholar]
- Yanagihara D, Kondo I. Nitric oxide plays a key role in adaptive control of locomotion in cat. Proc Natl Acad Sci. 1996;93:13292–13297. doi: 10.1073/pnas.93.23.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.