Abstract
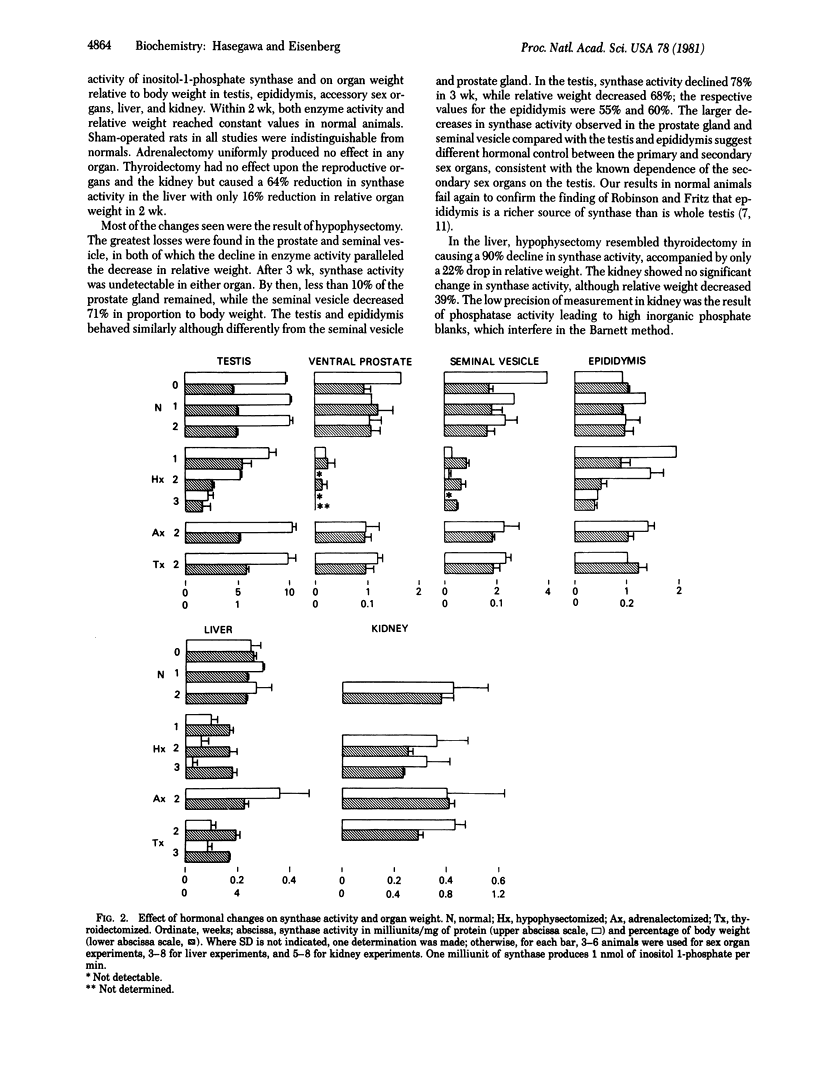
myo-Inositol biosynthesis has been examined in hypophysectomized and thyroidectomized male rats. After hypophysectomy, inositol-1-phosphate synthase [1L-myo-inositol-1-phosphate lyase (isomerizing), EC 5.5.1.4] in the reproductive organs and liver decreased markedly. At the same time, testicular acid phosphatase [orthophosphoric-monoester, phosphohydrolase (acid optimum), EC 3.1.3.2] and beta-glucuronidase (beta-D-glucuronide glucuronosohydrolase, EC 3.2.1.31) increased. Thyroidectomy caused a similar decrease in inositol-1-phosphate synthase in the liver but not in the reproductive organs. Follicle-stimulating in the liver but not in the reproductive organs. Follicle-stimulating hormone (follitropin) and luteinizing hormone (lutropin) restored the activity to at least normal levels in the testis, prostate, and seminal vesicle but not in the liver of hypophysectomized animals. Triiodothyronine and thyroxine stimulated liver synthase 30-fold in hypophysectomized animals. We conclude that inositol-1-phosphate synthase in the reproductive organs is under more or less direct control of the pituitary; in the liver, the control is mediated through the thyroid.
Full text
PDF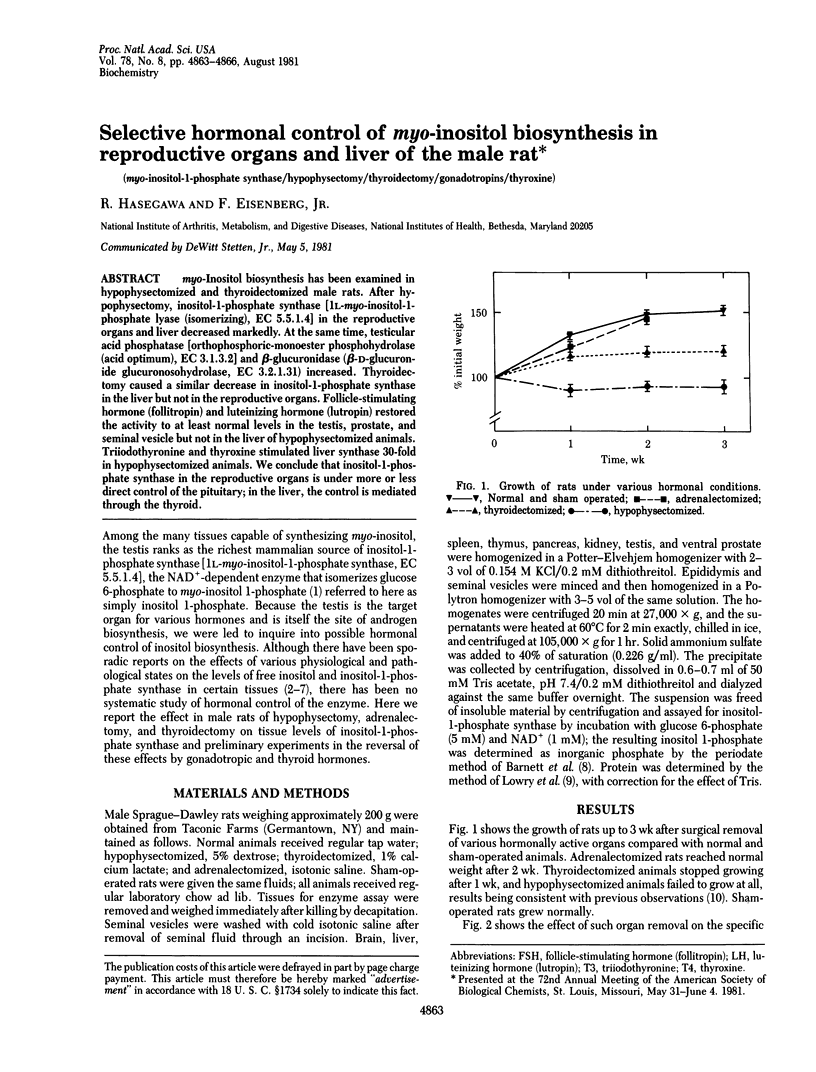


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong E. G., Feigelson M. Effects of hypophysectomy and triiodothyronine on de novo biosynthesis, catalytic activity, and estrogen induction of rat liver histidase. J Biol Chem. 1980 Aug 10;255(15):7199–7203. [PubMed] [Google Scholar]
- Barnett J. E., Brice R. E., Corina D. L. A colorimetric determination of inositol monophosphates as an assay for D-glucose 6-phosphate-1L-myoinositol 1-phosphate cyclase. Biochem J. 1970 Sep;119(2):183–186. doi: 10.1042/bj1190183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton L. E., Wells W. W. Studies on the developmental pattern of the enzymes converting glucose 6-phosphate to myo-inositol in the rat. Dev Biol. 1974 Mar;37(1):35–42. doi: 10.1016/0012-1606(74)90167-5. [DOI] [PubMed] [Google Scholar]
- EISENBERG F., Jr, BOLDEN A. H. REPRODUCTIVE TRACT AS SITE OF SYNTHESIS AND SECRETION OF INOSITOL IN THE MALE RAT. Nature. 1964 May 9;202:599–600. doi: 10.1038/202599a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg F., Jr D-myoinositol 1-phosphate as product of cyclization of glucose 6-phosphate and substrate for a specific phosphatase in rat testis. J Biol Chem. 1967 Apr 10;242(7):1375–1382. [PubMed] [Google Scholar]
- Kumar S., Das D. K., Dorfman A. E., Asato N. Stimulation of the synthesis of hepatic fatty acid synthesizing enzymes of hypophysectomized rats by 3,5,3'-l-triiodothyronine. Arch Biochem Biophys. 1977 Jan 30;178(2):507–516. doi: 10.1016/0003-9861(77)90221-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MELAMPY R. M., MASON R. B. Androgen and myo-inositol content of male accessory organs of the rat. Proc Soc Exp Biol Med. 1957 Nov;96(2):405–408. doi: 10.3181/00379727-96-23492. [DOI] [PubMed] [Google Scholar]
- Maeda T., Eisenberg F., Jr Purification, structure, and catalytic properties of L-myo-inositol-1-phosphate synthase from rat testis. J Biol Chem. 1980 Sep 25;255(18):8458–8464. [PubMed] [Google Scholar]
- Majumder A. L., Eisenberg F., Jr The formation of cyclic inositol 1,2-monophosphate, inositol 1-phosphate, and glucose 6-phosphate by brain preparations stimulated with deoxycholate and calcium: a gas chromatographic study. Biochem Biophys Res Commun. 1974 Sep 9;60(1):133–139. doi: 10.1016/0006-291x(74)90182-x. [DOI] [PubMed] [Google Scholar]
- Middleton A., Setchell B. P. The origin of inositol in the rete testis fluid of the ram. J Reprod Fertil. 1972 Sep;30(3):473–475. doi: 10.1530/jrf.0.0300473. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Hruska K. A., Fishman N., Daughaday W. H. Effects of glucose and parathyroid hormone on the renal handling of myoinositol by isolated perfused dog kidneys. J Clin Invest. 1979 Jun;63(6):1110–1118. doi: 10.1172/JCI109403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. N., Collins A. C. Biosynthesis of myo-inositol by rat testis following surgically induced cryptorchidism or treatment with triethylenemelamine. J Reprod Fertil. 1971 Nov;27(2):201–210. doi: 10.1530/jrf.0.0270201. [DOI] [PubMed] [Google Scholar]
- Nixon D. A. The effect of sex hormones upon the inositol concentration of the rat seminal vesicle. J Reprod Fertil. 1970 Feb;21(1):187–189. doi: 10.1530/jrf.0.0210187. [DOI] [PubMed] [Google Scholar]
- PAULUS H., KENNEDY E. P. The enzymatic synthesis of inositol monophosphatide. J Biol Chem. 1960 May;235:1303–1311. [PubMed] [Google Scholar]
- Rancour T. P., Wells W. W. myo-Inositol metabolism in rat testis in response to streptozotocin-induced diabetes. Arch Biochem Biophys. 1980 Jun;202(1):150–159. doi: 10.1016/0003-9861(80)90417-8. [DOI] [PubMed] [Google Scholar]
- Robinson R., Fritz I. B. Myoinositol biosynthesis by Sertoli cells, and levels of myoinositol biosynthetic enzymes in testis and epididymis. Can J Biochem. 1979 Jun;57(6):962–967. doi: 10.1139/o79-117. [DOI] [PubMed] [Google Scholar]
- Setchell B. P., Dawson R. M., White R. W. The high concentration of free myo-inositol in rete testis fluid from rams. J Reprod Fertil. 1968 Oct;17(1):219–220. doi: 10.1530/jrf.0.0170219. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Widnell C. C. Ribonucleic acid synthesis during the early action of thyroid hormones. Biochem J. 1966 Feb;98(2):604–620. doi: 10.1042/bj0980604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P. H., Palmano K. P., Hawthorne J. N. Enzymes of myo-inositol and inositol lipid metabolism in rats with streptozotocin-induced diabetes. Biochem J. 1979 Jun 1;179(3):549–553. doi: 10.1042/bj1790549. [DOI] [PMC free article] [PubMed] [Google Scholar]



