Abstract
Levofloxacin is the synthetic L-isomer of the racemic fluoroquinolone, ofloxacin. It interferes with critical processes in the bacterial cell such as DNA replication, transcription, repair, and recombination by inhibiting bacterial topoisomerases. Levofloxacin has broad spectrum activity against several causative bacterial pathogens of community-acquired pneumonia (CAP). Oral levofloxacin is rapidly absorbed and is bioequivalent to the intravenous formulation such that patients can be conveniently transitioned between these formulations when moving from the inpatient to the outpatient setting. Furthermore, levofloxacin demonstrates excellent safety, and has good tissue penetration maintaining adequate concentrations at the site of infection. The efficacy and tolerability of levofloxacin 500 mg once daily for 10 days in patients with CAP are well established. Furthermore, a high-dose (750 mg) and short-course (5 days) of once-daily levofloxacin has been approved for use in the US in the treatment of CAP, acute bacterial sinusitis, acute pyelonephritis, and complicated urinary tract infections. The high-dose, short-course levofloxacin regimen maximizes its concentration-dependent antibacterial activity, decreases the potential for drug resistance, and has better patient compliance.
Keywords: levofloxacin, community-acquired pneumonia, pharmacodynamics, resistance, pharmacokinetics, clinical use
Information resources
The medical literature published in any language since 1980 on levofloxacin was searched using PuBMed, MEDLINE, and EMBASE. Additional citations were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also obtained from Ortho-McNeil Janssen Scientific Affairs, LLC (Titusville, NJ).
Introduction
Community-acquired pneumonia (CAP) is one of the leading causes of morbidity and mortality in adult populations.1–4 The severity and incidence of CAP are significant, especially in the elderly and immunocompromised patients.5–7 CAP affects 6 million people in the US annually.8 Approximately 20% (1.1–1.3 million) of these patients are hospitalized9 with estimated cost of about US$25,000 per hospitalization10 resulting in over US$30 billion annual costs for hospitalizations alone; 12% of patients hospitalized for CAP die.9 In patients with severe CAP requiring admission to the intensive care unit (ICU), mortality increases to up to 30%.11–14 The most common cause of CAP is Streptococcus pneumonia.15–18 Other bacterial causes include Haemophilus influenzae, Moraxella catarrhalis, Klebsiella pneumoniae, and the “atypical” CAP pathogens which include Chlamydia pneumoniae, Mycoplasma pneumoniae, and Legionella pneumophila.2,17,19–22 Severe CAP, generally requiring admission to the ICU for management, is frequently caused by Staphylococcus aureus and Gram-negative bacilli.13,23–25
Epidemiologic studies reveal that pathogenic organisms are not recovered in >50% of patients exhibiting clinical signs and symptoms of CAP. Thus, microbiological information is frequently unavailable to refine initial empiric antibiotic treatment of CAP in either hospitalized and outpatient settings.9,23,25 The guidelines from the Infectious Diseases Society of America/American Thoracic Society recommend initial empiric therapy with a respiratory fluoroquinolone (eg, levofloxacin 750 mg, moxifloxacin, or gemifloxicin) or a β-lactam plus a macrolide. In adults, fluoroquinolones are recommended for the treatment of CAP caused by penicillin-susceptible S. pneumoniae, penicillin-resistant S. pneumoniae, Legionella pneumophilia, H. influenzae, M. pneumoniae, and C. pneumoniae. Levofloxacin combination therapy with an antipseudomonal β-lactam (or aminoglycoside) should be considered if Pseudomonas aeruginosa infection is a likely cause of pneumonia.24 Antibiotic resistance in S. pneumoniae has been a major problem in the US and worldwide for more than a decade.26 Furthermore, increasing rates of antibiotic resistance (most notably, penicillin, cephalosporin, and macrolide resistance) observed in bacteria that commonly cause CAP have resulted in increased treatment failures and inferior clinical outcomes for many patients with CAP.14,15,27–30 Although there are reports of the emergence of resistance to some fluoroquinolones among S. pneumonia,26 the incidence of levofloxacin-resistant organisms has remained steady with resistance rates of <1% worldwide.31–35
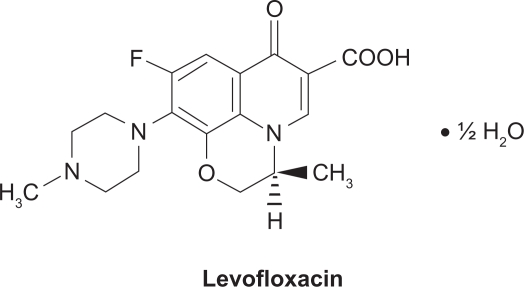
Levofloxacin (Figure 1) is a light yellowish-white crystal or crystalline powder with a molecular weight of 370.38 g/mol. It interferes with critical processes in the bacterial cell, such as DNA replication, transcription, repair, and recombination, by inhibiting bacterial topoisomerases. Human cells lack these topoisomerases, which are essential for bacterial DNA replication, providing specificity against bacterial DNA topoisomerases that are responsible for separating the strands of duplex bacterial DNA, inserting another strand of DNA through the break, and then resealing the originally separated strands.36,37 Levofloxacin is active against a broad range of Gram-positive, Gram-negative, and cell-wall-deficient (atypical) bacteria that may be causative pathogens in community-acquired and nosocomial infections. Levofloxacin is a well-established treatment option for respiratory and urinary tract infections (UTI), particularly since levofloxacin is active against some penicillin – and macrolide-resistant species (eg, S. pneumoniae – the most common causative pathogen for community-acquired bacterial respiratory infections).31–34,38,39 The incidence of penicillin- and macrolide-resistance in many bacterial species is both high and widespread.40 In the US, a high-dose, short-course regimen of levofloxacin (750 mg once daily for 5 days) is approved for the treatment of adults with CAP, acute bacterial sinusitis (ABS), complicated UTI, and acute pyelonephritis (AP). The use of levofloxacin, including some data on the high-dose, short-course treatment regimen, has been reviewed previously.39 This review focuses on the pharmacology of levofloxacin in the treatment of CAP.
Figure 1.
Structure of levofloxacin.
Pharmacodynamic properties
Spectrum of activity
Levofloxacin is the L-isomer of the racemic fluoroquinolone ofloxacin.39,41 Topoisomerase IV is the main target for levofloxacin in Gram-positive bacteria and DNA gyrase (topoisomerase II) is the target in Gram-negative bacteria.42 Levofloxacin has a broad spectrum of antibacterial activity that includes several Gram-positive and Gram-negative aerobes and cell-wall-deficient (atypical) bacteria. The minimum inhibitory concentrations (MIC) of levofloxacin required to inhibit the growth of 90% of clinical isolates (MIC90) are used as assessments of the in vitro activity of levofloxacin. The levofloxacin MIC breakpoints for S. pneumoniae defined by the Clinical and Laboratory Standards Institute are: ≤2 mg/L (susceptible), 4 mg/L, (intermediate), and ≥8 mg/L (resistant).41,43 Also, levofloxacin generally demonstrates good in vitro activity against penicillin-resistant S. pneumoniae strains. S. pneumoniae with reduced susceptibility to penicillin commonly cause CAP. The levofloxacin MIC90 for penicillin-susceptible, -intermediate, and -resistant isolates of S. pneumoniae was 1 mg/L in multiple studies, with >97% of isolates testing susceptible to the drug.31–34,38,44
Levofloxacin has variable activity against S. aureus, depending on methicillin susceptibility. Levofloxacin had MIC90 values of 0.25–4.0 mg/L against methicillin-susceptible S. aureus isolates, whereas methicillin-resistant S. aureus isolates exhibited levofloxacin resistance, MIC90 values ranging from >4 to ≥64 mg/L.38,44–46 The in vitro activity of levofloxacin against Enterococcus faecalis was limited (MIC90 of 8 to ≥32 mg/L in vancomycin-susceptible and -resistant strains). Although levofloxacin has limited activity against coagulase-negative staphylococci (>4 mg/L, 54.1%).45 It has demonstrated good in vitro activity against a range of other Gram-positive bacteria, such as Streptococcus pyogenes (1 mg/L, 99.9%)32,33 and other β-hemolytic streptococci (0.5–1 mg/L, 99.1%–100%).47
Generally, levofloxacin has good in vitro activity against Gram-negative bacteria including the common respiratory tract pathogens H. influenzae.31,35,38,44,48–50 Haemophilus parainfluenzae,50 and M. catarrhalis31,35,44,48–50 as well as urinary tract pathogens (K. pneumoniae,38,44,51 Enterobacter cloacae,38,44,51–53 and Proteus mirabilis38,45,48). The values of MIC90 for levofloxacin against isolates of H. influenzae, H. parainfluenzae, and M. catarrhalis were ≤0.06 mg/L with nearly 100% susceptibility rates. Levofloxacin was also highly active against β-lactamase-positive isolates of H. influenzae31–34,38 and M. catarrhalis,31,44,48–50,54,55 However, the activity of levofloxacin is variable against Escherichia coli and P. aeruginosa. The MIC90 of levofloxacin against E. coli ranged from ≤0.06 mg/L (susceptible) to >8 mg/L (resistant).38,44,45,51,56 Levofloxacin showed lower levels of activity against isolates of P. aeruginosa, MIC90 values ranging from 0.5 mg/L to 64 mg/L and susceptibility rates of 71%–94%.38,44,45,48,51 Levofloxacin also had limited activity against extended-spectrum β-lactamase-producing K. pneumoniae (MIC90 of >8–32 mg/L).45 Levofloxacin has good activity against the cell-wall-deficient (atypical) organisms C. pneumonia.57–60 L. pneumophila,38,44,48,57,61,62, and M. pneumonia,48,57,63–65 MIC90 values being ≤2 mg/L.
Bactericidal activity
The bactericidal activity of levofloxacin is concentration-dependent,66 and the minimum bactericidal concentration (MBC) of levofloxacin was ≤4× the MIC against the majority of isolates for a number of causative pathogens of respiratory tract infections.59,60,64,65,67 The MBC90 of levofloxacin was 1–4× the MIC against the majority of M. pneumoniae isolates (MBC of ≤0.5–1.0 mg/L), as reported by multiple authors.59,60,63–65,67 The MBC of levofloxacin was 1–2× the MIC (≤0.06–4 mg/L) against K. pneumoniae, P. aeruginosa, E. coli, and E. cloacae.51 Levofloxacin has a post-antibiotic effect (PAE) of 2.0–4.5 hours depending on the pathogen.39 The PAE of levofloxacin against S. pneumoniae was up to 4.5 hours at 10× the MIC. Furthermore, levofloxacin has shown PAEs against methicillin-susceptible S. aureus (MSSA), K. pneumoniae, L. pneumophila, and anaerobes39 as well as against erythromycin-resistant and -susceptible strains of L. pneumophila.61
Resistance
Resistance to antibacterial drugs in S. pneumoniae has been a major problem in the US for more than a decade.26 The primary cause of reduced susceptibility of bacteria (particularly S. pneumoniae) to fluoroquinolones is at least one mutation in the parC and parE genes that code for DNA topoisomerase IV or gyrA and gyrB genes that code for DNA gyrase.68,69 Another fluoroquinolone resistance mechanism involves active drug efflux through mutation in the efflux regulatory genes mexR and nfxB.68,70 Although there are reports of the emergence of fluoroquinolone resistance among S. pneumoniae,26 the incidence of levofloxacin-resistant organisms has remained stable to date at ≤1% worldwide.31–35
In the worldwide PROTEKT surveillance program between 1999 and 2000, levofloxacin-resistant isolates of S. pneumoniae were identified; 94% of these isolates had at least one mutation in the genes coding for topoisomerase IV as well as in the genes coding for DNA gyrase.69 The SENTRY surveillance program (1997–2005) identified fluoroquinolone-resistant isolates of β-hemolytic Streptococcus spp. as having significant mutations in the parC or gyrA gene, or both. Only mutations in parC were associated with lower MIC values.47 A report of an in vitro pharmacodynamic model simulating the concentration of levofloxacin in the epithelial lining fluid (ELF) after once daily administration of 500 mg revealed that all five isolates of S. pneumoniae containing the first-step parC mutation had levofloxacin resistance within 48 hours (≥16-fold increase in MIC) and four of the isolates acquired a second-step (gyrA) mutation.71 The acquisition of a second-step mutation appeared to be related with an area under the concentration–time curve (AUC):MIC ratio of ≤256; this indicates that to prevent levofloxacin resistance from being acquired in isolates with a first-step parC mutation, the AUC:MIC ratio target should be >256.71 When the range of free AUCs (fAUCs) of levofloxacin and other fluoroquinolones were simulated, the results demonstrated that fAUC:MIC ratios of ≤82 and ≤86 for levofloxacin were associated with a first-step parC mutation and second-step gyrA mutation in S. pneumoniae. These resistance breakpoints for levofloxacin were significantly higher (P ≤ 0.001) than those for other tested fluoroquinolones (gatifloxacin, gemifloxacin, and moxifloxacin) using post hoc analysis. Furthermore, the higher the fAUC:MIC ratio for each fluoroquinolone, the more delay in the development of first- or second-step mutations was observed.72
In the SENTRY (worldwide, 1997–2004),47 PROTEKT (US and Canada, 1999–2002),32–34 and TRUST (US, 1998–2002)35 surveillance programs, the overall levofloxacin resistance rate in S. pneumoniae isolates was ≤1%; in penicillin-resistant isolates, the overall rate of levofloxacin resistance was 0.9%–2.7%.31,34,35 In the TRUST surveillance program from 2001 to 2005, the rate of S. pneumoniae resistance to levofloxacin changed from 0% to 0.5% and the resistance of these isolates to penicillin resistance increased from 27.4% to 28.9%. Amoxicillin/clavulanic acid resistance increased from 6.5% to 12.9%, and clindamycin resistance increased from 12.1% to 18.6%.73 The levofloxacin 750 mg dose has been directly compared to imipenem–cilastatin in the treatment of nosocomial pneumonia. The average age of the patients was 55 years and 438 patients were randomized. Forty-two percent of patients in the levofloxacin arm were ≥65 years of age. The clinical success rate in the intention-to-treat population was 66.2% in the levofloxacin arm vs 69.4% in the imipenem arm. In the clinically evaluable population, the success rates were 59.3% and 62.5% for levofloxacin and imipenem, respectively.74 Other data from 1998 and 2005 revealed that the levofloxacin-resistant isolates of H. influenzae or M. catarrhalis could not be identified in large worldwide surveillance studies.32–34,49,54,55 However, surveillance studies have demonstrated resistance to levofloxacin in MSSA and methicillin-resistant strains of S. aureus (MRSA) (3.4%–10.1% and 76.6%–79.2%, respectively) and P. aeruginosa (24.7%).45,46,56
Pharmacokinetics and metabolism
Levofloxacin is rapidly absorbed after oral administration and shows linear pharmacokinetics for both single- and multiple-dose (once daily) regimens. The oral solution and tablet formulations are bioequivalent to the intravenous formulation.41 The mean pharmacokinetic parameters obtained in different studies of intravenous and oral levofloxacin in healthy adults75,76 are comparable to those reported in the manufacturer’s US prescribing information.41 The peak plasma concentration (Cmax) after single 750 mg doses of levofloxacin given to healthy volunteers was 11.3 mg/L75 and 12.1 mg/L for intravenous administration, compared with 7.1 mg/L76 and 9.3 mg/L41 for oral administration. When given in multiple doses levofloxacin had Cmax of 12.1 mg/L and 12.4 mg/L for intravenous administration compared with 8.6 mg/L for oral ones.41,76 Levofloxacin steady-state conditions were reached within 48 hours of initiating once-daily intravenous or oral 750 mg.41 After oral administration, the Tmax of levofloxacin is reached within 1–2 hours with an absolute bioavailability of oral levofloxacin 500 mg and 750 mg of approximately 99%.41,75,76 Systemic exposure to levofloxacin was similar for the intravenous and oral formulations upon administering equal doses of levofloxacin.41 The AUC24 was 103 mg h/L75 and 90.7 mg h/L76 at steady state after intravenous or oral administration of levofloxacin 750 mg once daily, respectively.
The in vitro studies revealed that 24%–38% of levofloxacin was bound to plasma proteins (mainly albumin) and the binding was independent of levofloxacin concentration.41 The volumes of distribution obtained in pharmacokinetic studies ranged from 74–112 L after single or multiple doses of levofloxacin 500 mg or 750 mg.75,76 Levofloxacin is distributed extensively in tissues and fluids throughout the body and accumulates in phagocytic cells.39 Furthermore, the mean concentrations of levofloxacin in tissues, ELF, alveolar macrophages, polymorphonuclear leukocytes, paranasal sinus mucosa, and urine, surpass the concentration of levofloxacin in the plasma.39,77–83 It has been reported that the paranasal sinuses mucosa:plasma concentration ratio was 2.56 at Tmax after a single 500 mg oral dose of levofloxacin. The concentration of levofloxacin in the paranasal sinuses mucosa was generally higher than the MIC90 of the common causative pathogens for upper respiratory tract infections (0.008–2.0 mg/L), including penicillin-susceptible, -intermediate, and -resistant isolates of S. pneumoniae.82 In healthy volunteers, oral levofloxacin (500 or 750 mg) had a mean ELF:plasma concentration ratio at steady state of 1.16 using population pharmacokinetic modeling and 3.18 using Monte Carlo simulation.82 At a lower dosage of levofloxacin (500 mg once daily for 3 days), Cmax and AUC24 values for the drug were significantly (P < 0.01) higher in the polymorphonuclear leukocytes than in plasma.84 Reassuringly, the concentrations of levofloxacin in the ELF and alveolar macrophages were 1.5- to 6-fold higher than that in the plasma at steady state after receiving levofloxacin 500 mg once daily for 5 days in older patients undergoing diagnostic bronchoscopy with a mean age of 62 years.80
Levofloxacin is eliminated mainly through the kidneys, 75%–87% of the dose excreted being unchanged in the urine within 48–72 hours of administering oral levofloxacin 500 or 750 mg; <4% is excreted in the feces.41,75,76 After a single dose of levofloxacin 750 mg, the mean drug concentration in the urine was 475 mg/L at 4 hours and 186 mg/L at 24 hours;77 <5% of the dose is excreted in the urine as inactive metabolites of levofloxacin.41 The mean total body clearance (CL) of levofloxacin in healthy volunteers was reported as 8–9.4 L/h75,76 and 8.6–13.6 L/h.41 Levofloxacin appears to undergo glomerular filtration as well as tubular secretion.41 After single or multiple doses of oral or intravenous levofloxacin 750 mg, the mean terminal plasma elimination half-life (t1/2β) is 7.5–8.8 hours in pharmacokinetic studies.75,76 The t1/2β of levofloxacin is increased and the CL reduced in patients with impaired renal function (creatinine clearance CLCR < 50 mL/min); therefore dosage adjustment is required to avoid drug accumulation as shown in Table 1.41 Furthermore, levofloxacin is not cleared effectively by hemodialysis or continuous ambulatory peritoneal dialysis.39,41 The pharmacokinetic properties of levofloxacin are not influenced by age, gender, or race, and they do not show noticeable differences between healthy adults, patients with HIV,39 or patients with severe community-acquired bacterial infections.41 Levofloxacin pharmacokinetics in hepatically-impaired patients have not been investigated; however, because of the limited hepatic metabolism of levofloxacin, hepatic impairment is unlikely to have a prominent effect on the drug pharmacokinetics.41
Table 1.
Dosing in patients with diminished renal function
| Renal status | Initial dose | Subsequent dose |
|---|---|---|
| CLCR ≥ 50 mL/min | 500 mg | 500 mg q24h |
| CLCR 20–49 mL/min | 500 mg | 250 mg q24h |
| CLCR 10–19 mL/min | 500 mg | 250 mg q48h |
| Hemodialysis | 500 mg | 250 mg q48h |
| CAPD | 500 mg | 250 mg q48h |
| CLCR ≥ 50 mL/min | 750 mg | 750 mg q24h |
| CLCR 20–49 mL/min | 750 mg | 750 mg q48h |
| CLCR 10–19 mL/min | 750 mg | 500 mg q48h |
| Hemodialysis | 750 mg | 500 mg q48h |
| CAPD | 750 mg | 500 mg q48h |
Abbreviations: CLCR, creatinine clearance; CAPD, chronic ambulatory peritoneal dialysis; q, every.
Clinical efficacy
The efficacy of levofloxacin 750 mg once daily (intravenous and oral) for 5 days in adults with CAP,66 ABS,85 and complicated UTI86,87 has been assessed in several randomized, double-blind, multicenter, noninferiority trials.66,85–87 The endpoints were the clinical success rate (proportion of patients showing either a clinical cure or improvement with no need for further antimicrobial therapies in both situations) 1–2 weeks after the end of treatment,66 or at 2–3 weeks of the study,85 or the microbiological eradication rate (all pathogens identified in samples at the study entry were eradicated) at 2–3 weeks of the study.86,87 Levofloxacin indications and dosing for patients with normal renal function are summarized in Table 2.
Table 2.
Levofloxacin indications and dosing for patients with upper respiratory tract infections and with normal renal function
| Type of infection | Dose | Frequency | Duration |
|---|---|---|---|
| Community acquired pneumonia | 500 mg | q24h | 7–14 days |
| Community acquired pneumonia | 750 mg | q24h | 5 days |
| Nosocomial pneumonia | 750 mg | q24h | 7–14 days |
| Acute bacterial exacerbation of chronic bronchitis | 500 mg | q24h | 7–14 days |
| Acute bacterial sinusitis | 500 mg | q24h | 10–14 days |
| Acute bacterial sinusitis | 750 mg | q24h | 5 days |
Abbreviation: q, every.
Patients enrolled in the noninferiority trial with CAP were aged ≥18 years and were diagnosed with mild-to-severe CAP. Other inclusion criteria involved one or more signs or symptoms including fever, a white blood cell count of >10,000 cells/mm3, or hypothermia. The exclusion criteria included the following conditions: patients without a confirmed diagnosis of CAP, patients who did not come to the follow-up visit, patients who increased (>120%) or reduced (<80%) the scheduled doses, and patients who had additional antimicrobial therapy during treatment with levofloxacin.66 Patients with mild-to-severe CAP received 750 mg levofloxacin (intravenous or oral) once daily for 5 days or 500 mg once daily for 10 days. Subjects receiving the higher dosage of levofloxacin were given a placebo for the last 5 days of the 10-day treatment regimen.66 Levofloxacin susceptibility testing of the causative pathogens was performed, but initial treatment was empirical. The noninferiority criteria were established as the upper limit of the 2-sided 95% CI for the between-group difference in the clinical success rate <15%, if both treatment groups had a clinical success rate of 80%–90%, or <10%, if both treatment groups had a clinical success rate of ≥90%.66 The results revealed that levofloxacin 750 mg once daily for 5 days was noninferior to 500 mg once daily for 10 days in the treatment of mild-to-severe CAP in the overall patient population,66 as well as for patients with CAP caused by atypical organisms (C. pneumoniae or M. pneumoniae),88 and for elderly patients aged ≥65 years.89
In patients receiving either the levofloxacin 750 mg or 500 mg regimen, baseline characteristics were similar and overall microbiological eradication rates were similar in both groups.66 The eradication rates for both the 750 mg and 500 mg regimens were high for subgroups of micro-biologically evaluable patients infected with aerobic Gram-positive (82.8% vs 85.3%) and Gram-negative (96.2% vs 90.7%) pathogens, as well as other pathogens (93.8% vs 96.2%). Eradication rates for S. pneumoniae, H. influenzae, and H. parainfluenzae in the corresponding post-therapy visit were 86.4% vs 85%, 92.3% vs 85.7% and 100% vs 90%, respectively.66 Retrospective analysis revealed that the clinical success rates in patients with CAP caused by H. influenzae, H. parainfluenzae, or S. pneumoniae were also similar between the levofloxacin 750 mg and 500 mg treatment groups (92.3% vs 92.9%, 100% vs 90%, and 90.9% vs 90%, respectively).66
The efficacy of the high-dose, short-course of levofloxacin in achieving early resolution of symptoms has been studied.90 Resolution of purulent sputum, shortness of breath, chills and cough were 40.6% vs 30.7%, 35.1% vs 27.7%, 54.8% vs 54.2%, and 10% vs 10.1% comparing patients who received the levofloxacin 750 mg or 500 mg regimen, respectively. Furthermore, 99.4% of the 158 pathogens isolated at study entry were susceptible to levofloxacin and there was no significant difference between treatment groups in the time of switching from the intravenous administration of levofloxacin to oral administration of the drug.90 High-dose, short-course of levofloxacin (750 mg once daily for 5 days) also had good efficacy in the subgroup of patients with severe CAP, demonstrating high clinical success rates of >85%. Overall, high microbiological response rates (≥87.5%) were observed in the subgroup of microbiologically evaluable patients receiving levofloxacin regardless of the treatment regimen.91 In the same study, microbiological eradication was observed in 88.2% of typical pathogens identified from respiratory cultures and 90% of atypical pathogens.91
It has been reported that levofloxacin 750 mg once daily for 5 days has good efficacy in patients with CAP caused by atypical organisms.88 The overall clinical success rate of levofloxacin 1–2 weeks after treating CAP caused by a single atypical pathogen, was >95%. Noninferiority of levofloxacin 750 mg once daily for 5 days compared with the 10-day regimen was also established in this study. The overall clinical success rate of the levofloxacin 750 mg regimen was 94.8% for CAP caused by atypical pathogens, compared with 96.5% for the levofloxacin 500 mg regimen.88 Furthermore, the clinical success rates at the 1–2 weeks post-treatment visit for patients with C. pneumoniae, L. pneumophila, and M. pneumoniae were comparable between the groups receiving the levofloxacin 750 mg and 500 mg dosing regimen (90.9% vs 100%, 100% vs 100%, and 95.3% vs 94.4%, respectively).88
Post-marketing surveillance
Post-marketing data demonstrated that levofloxacin simultaneous administered with warfarin may increase the prothrombin time. Therefore, coagulation studies and bleeding should be monitored in patients receiving the two drugs concomitantly.41 Levofloxacin does not currently have a US Food and Drug Administration approved indication in patients aged <18 years. Like other fluoroquinolones, levofloxacin decreases theophylline metabolism and dosage adjustment for theophylline may be required for concurrent administration of both drugs. Concomitant fluoroquinolone administration with cyclosporin resulted in elevated serum concentrations of ciclosporin, but these alterations were not clinically significant.41
Safety and tolerability
Intravenous levofloxacin must be administered slowly as an infusion over a minimum period of 60–90 minutes, depending on the dose. Levofloxacin tablets or oral solution are generally prescribed at dosages of 250, 500, or 750 mg once daily. The tablet formulation of levofloxacin can be taken with or without food; however, the oral solution should be taken 1 hour prior to or 2 hours after meals. In patients receiving levofloxacin, sufficient hydration should be maintained to prevent excessively concentrated urine. Levofloxacin should be administered at least 2 hours apart from some agents such as magnesium- or aluminium-containing antacids, sucralfate, metal cations, zinc-containing multivitamins, or didanosine.
Data from patients aged ≥65 years (phase III clinical trials) demonstrated no difference between elderly and younger patients for safety or effectiveness of levofloxacin. Elderly patients may be more sensitive to levofloxacin, mainly due to the effect of the drug on the QT interval. Thus, caution is required in the simultaneous administration of levofloxacin with drugs that prolong the QT interval such as class IA or class III antiarrhythmics. Although, levofloxacin is a very safe fluoroquinolone, caution and a risk/benefit assessment is required with the use of levofloxacin in the elderly due to the increased risk of severe tendon disorders in this group of patients, particularly if they are receiving corticosteroids.41 However, it should be stated that there is no evidence that tendon rupture is more likely to occur with levofloxacin than with any other fluoroquinolone.92 Blood glucose monitoring is recommended in patients with diabetes mellitus receiving simultaneous hypoglycemic agents and/or insulin, because symptomatic hyperglycemia and hypoglycemia have been reported with levofloxacin administration.41 Concomitant administration of fluoroquinolones (including levofloxacin) with NSAIDs may increase the risk of central nervous system stimulation and convulsive seizures.41
Levofloxacin 750 mg once daily for 5 days is a well-tolerated fluoroquinolone for patients with CAP or UTI.86,87,93 In a pooled analysis of patients with respiratory infections receiving the levofloxacin 750 mg regimen or 500 mg regimen, the results revealed that 4.5% and 4.9% of patients, respectively, had adverse effects during the therapy. The adverse effects in both dosage regimens included nausea, vomiting, diarrhea, dyspepsia, constipation, abdominal pain, headache, insomnia, and dizziness. The incidence of levofloxacin-associated adverse effects was similar between both treatment regimens (8% vs 7.6%).93
The use of fluoroquinolones and exposure to the sun or UV light has been associated with photosensitivity reactions.41 Fluoroquinolones can potentially prolong the QT interval but there are no reported cases of torsade de pointes in any clinical or post-marketing trials.41,93 It has been reported that levofloxacin is associated with Clostridium difficile diarrhea, as are most other antibacterial agents. Severity ranges from mild diarrhea to pseudomembranous colitis.41 The incidence of drug-related adverse effects in patients with CAP or ABS was similar between the levofloxacin 750 mg and 500 mg dosing regimens.93
Regulatory affairs
Levofloxacin is approved for use in the US, Canada, and worldwide in the treatment of CAP, ABS, complicated UTI, and AP.
Conclusion and comments
The respiratory fluoroquinolones are considered to be a substantial component of the anti-infective armamentarium for the treatment of bacterial respiratory infections. Levofloxacin is active against most of the respiratory pathogens and has a good clinical success rate. Its favorable pharmacodynamics, safety, efficacy profile, and tolerability, and also its in vitro activity against the common respiratory pathogens, places levofloxacin among first-line agents for the treatment of community-acquired respiratory tract infections such as CAP.
The Infectious Diseases Society of America/American Thoracic Society guidelines recommend that a respiratory fluoroquinolone (eg, levofloxacin 750 mg) or a β-lactam plus a macrolide be used for the treatment of CAP. The use of fluoroquinolones is a reasonable therapeutic choice for the treatment of respiratory infections caused by penicillin-susceptible S. pneumoniae, penicillin-resistant S. pneumoniae, Legionella pneumophilia, H. influenzae, M. pneumoniae, and C. pneumoniae. Levofloxacin combination therapy with antipseudomonal β-lactam (or aminoglycoside) should be considered if P. aeruginosa is likely to be a causative pathogen of the respiratory infection. S. pneumoniae resistance to antibacterial drugs has been a major problem in the US and worldwide for more than a decade. Although there are reports of the emergence of resistance to some fluoroquinolones among S. pneumoniae, the incidence of levofloxacin-resistant organisms has remained steady at <1% worldwide. In general, levofloxacin shows good in vitro activity against clinically relevant Gram-positive, Gram-negative, and atypical organisms that cause respiratory infections. Levofloxacin is active against penicillin-susceptible and -resistant strains of S. pneumoniae, the Gram-negative species E. cloacae and P. mirabilis, and the atypical organisms C. pneumoniae, L. pneumophila, and M. pneumoniae (MIC90 of ≤2 mg/L). Levofloxacin is highly active against the Gram-negative species H. influenzae, H. parainfluenzae, and M. catarrhalis (MIC90 of ≤0.06 mg/L), including β-lactamase-positive strains of H. influenzae and M. catarrhalis. Because the activity of levofloxacin is concentration-dependent, the most common predictor of microbiological and clinical efficacy is the AUC:MIC ratio. A ratio of >30 was used in some studies to predict in vivo activity, particularly against S. pneumoniae. A higher ratio (>100) is suggested as being predictive of a bactericidal effect, and thus reducing the potential of first-step mutations. Availability of pneumococcal vaccine is decreasing the incidence of pneumococcal infections and decreasing the incidence of infections caused by resistant S. pneumoniae.
In the last 5 years, the rate of resistance of S. pneumoniae to amoxicillin/clavulanic acid, azithromycin, and tetracycline appears to have increased, but the levofloxacin resistance rate of S. pneumoniae remains ≤1% worldwide.94 High-dose, short-term therapy (levofloxacin 750 mg once daily for 5 days) is the standard dosing regimen for levofloxacin in the treatment of CAP worldwide. Increased availability of pneumococcal vaccination programs may decrease the incidence of S. pneumoniae as a cause of CAP in adults over time. Other problematic infections with multidrug-resistant organisms will become the main focus of research in the next 5 years.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Almirall J, Bolibar I, Vidal J, et al. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur Respir J. 2000;15(4):757–763. doi: 10.1034/j.1399-3003.2000.15d21.x. [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez F, Masia M, Rodriguez JC, et al. Epidemiology of community-acquired pneumonia in adult patients at the dawn of the 21st century: a prospective study on the Mediterranean coast of Spain. Clin Microbiol Infect. 2005;11(10):788–800. doi: 10.1111/j.1469-0691.2005.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loh LC, Khoo SK, Quah SY, et al. Adult community-acquired pneumonia in Malaysia: prediction of mortality from severity assessment on admission. Respirology. 2004;9(3):379–386. doi: 10.1111/j.1440-1843.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 4.O’Meara ES, White M, Siscovick DS, Lyles MF, Kuller LH. Hospitalization for pneumonia in the Cardiovascular Health Study: incidence, mortality, and influence on longer-term survival. J Am Geriatr Soc. 2005;53(7):1108–1116. doi: 10.1111/j.1532-5415.2005.53352.x. [DOI] [PubMed] [Google Scholar]
- 5.Gutiérrez F, Masiá M, Mirete C, et al. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect. 2006;53(3):166–174. doi: 10.1016/j.jinf.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan V, Angus DC. Community-acquired pneumonia in the elderly. Crit Care Clin. 2003;19(4):729–748. doi: 10.1016/s0749-0704(03)00057-5. [DOI] [PubMed] [Google Scholar]
- 7.Viegi G, Pistelli R, Cazzola M, et al. Epidemiological survey on incidence and treatment of community acquired pneumonia in Italy. Respir Med. 2006;100(1):46–55. doi: 10.1016/j.rmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Colice GL, Morley MA, Asche C, Birnbaum HG. Treatment costs of community-acquired pneumonia in an employed population. Chest. 2004;125(6):2140–2145. doi: 10.1378/chest.125.6.2140. [DOI] [PubMed] [Google Scholar]
- 9.Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 10.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128(6):3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 11.Bodi M, Rodriguez A, Sole-Violan J, et al. Antibiotic prescription for community-acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis. 2005;41(12):1709–1716. doi: 10.1086/498119. [DOI] [PubMed] [Google Scholar]
- 12.Tejerina E, Frutos-Vivar F, Restrepo MI, et al. Prognosis factors and outcome of community-acquired pneumonia needing mechanical ventilation. J Crit Care. 2005;20(3):230–238. doi: 10.1016/j.jcrc.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Wilson PA, Ferguson J. Severe community-acquired pneumonia: an Australian perspective. Intern Med J. 2005;35(12):699–705. doi: 10.1111/j.1445-5994.2005.00962.x. [DOI] [PubMed] [Google Scholar]
- 14.Woodhead M, Welch CA, Harrison DA, Bellingan G, Ayres JG. Community-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit Care. 2006;10(Suppl 2):S1. doi: 10.1186/cc4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.File TM., Jr Clinical implications and treatment of multiresistant Streptococcus pneumoniae pneumonia. Clin Microbiol Infect. 2006;12(Suppl 3):31–41. doi: 10.1111/j.1469-0691.2006.01395.x. [DOI] [PubMed] [Google Scholar]
- 16.Lauderdale TL, Chang FY, Ben RJ, et al. Etiology of community acquired pneumonia among adult patients requiring hospitalization in Taiwan. Respir. Med. 2005;99(9):1079–1086. doi: 10.1016/j.rmed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Leesik H, Ani U, Juhani A, Altraja A. Microbial pathogens of adult community-acquired pneumonia in Southern Estonia. Medicina (Kaunas) 2006;42(5):384–394. [PubMed] [Google Scholar]
- 18.Luna CM, Famiglietti A, Absi R, et al. Community-acquired pneumonia: etiology, epidemiology, and outcome at a teaching hospital in Argentina. Chest. 2000;118(5):1344–1354. doi: 10.1378/chest.118.5.1344. [DOI] [PubMed] [Google Scholar]
- 19.Huang HH, Zhang YY, Xiu QY, et al. Community-acquired pneumonia in Shanghai, China: microbial etiology and implications for empirical therapy in a prospective study of 389 patients. Eur J Clin Microbiol Infect Dis. 2006;25(6):369–374. doi: 10.1007/s10096-006-0146-7. [DOI] [PubMed] [Google Scholar]
- 20.Saito A, Kohno S, Matsushima T, et al. Prospective multicenter study of the causative organisms of community-acquired pneumonia in adults in Japan. J Infect Chemother. 2006;12(2):63–69. doi: 10.1007/s10156-005-0425-8. [DOI] [PubMed] [Google Scholar]
- 21.Thibodeau KP, Viera AJ. Atypical pathogens and challenges in community-acquired pneumonia. Am Fam Physician. 2004;69(7):1699–1706. [PubMed] [Google Scholar]
- 22.Woodhead M. Community-acquired pneumonia in Europe: causative pathogens and resistance patterns. Eur Respir J Suppl. 2002;36:20s–27s. doi: 10.1183/09031936.02.00702002. [DOI] [PubMed] [Google Scholar]
- 23.File TM, Jr, Garau J, Blasi F, et al. Guidelines for empiric antimicrobial prescribing in community-acquired pneumonia. Chest. 2004;125(5):1888–1901. doi: 10.1378/chest.125.5.1888. [DOI] [PubMed] [Google Scholar]
- 24.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26(6):1138–1180. doi: 10.1183/09031936.05.00055705. [DOI] [PubMed] [Google Scholar]
- 26.Doern GV, Richter SS, Miller A, et al. Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes? Clin Infect Dis. 2005;41(2):139–148. doi: 10.1086/430906. [DOI] [PubMed] [Google Scholar]
- 27.Bonofiglio L, Ojeda MI, de Mier C, et al. Phenotypic and genotypic characterization of macrolide resistant Streptococcus pneumoniae recovered from adult patients with community-acquired pneumonia in an Argentinian teaching hospital. Int J Antimicrob Agents. 2005;25(3):260–263. doi: 10.1016/j.ijantimicag.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Felmingham D. Comparative antimicrobial susceptibility of respiratory tract pathogens. Chemotherapy. 2004;50(Suppl 1):3–10. doi: 10.1159/000079816. [DOI] [PubMed] [Google Scholar]
- 29.Fuller JD, McGeer A, Low DE. Drug-resistant pneumococcal pneumonia: clinical relevance and approach to management. Eur J Clin Microbiol Infect Dis. 2005;24(12):780–788. doi: 10.1007/s10096-005-0059-x. [DOI] [PubMed] [Google Scholar]
- 30.Reinert RR, Reinert S, van der Linden M, Cil MY, Al-Lahham A, Appelbaum P. Antimicrobial susceptibility of Streptococcus pneumoniae in eight European countries from 2001 to 2003. Antimicrob Agents Chemother. 2005;49(7):2903–2913. doi: 10.1128/AAC.49.7.2903-2913.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones RN, Fritsche TR, Sader HS, Stilwell MG. Activity of garenoxacin, an investigational des-F(6)-quinolone, tested against pathogens from community-acquired respiratory tract infections, including those with elevated or resistant-level fluoroquinolone MIC values. Diagn Microbiol Infect Dis. 2007;58(1):9–17. doi: 10.1016/j.diagmicrobio.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Brown SD, Rybak MJ. Antimicrobial susceptibility of Streptococcus pneumoniae, Streptococcus pyogenes and Haemophilus influenzae collected from patients across the USA, in 2001–2002, as part of the PRO-TEKT US study. J Antimicrob Chemother. 2004;54(Suppl 1):i7–i15. doi: 10.1093/jac/dkh313. [DOI] [PubMed] [Google Scholar]
- 33.Doern GV, Brown SD. Antimicrobial susceptibility among community-acquired respiratory tract pathogens in the USA: data from PROTEKT US 2000–2001. J Infect. 2004;48(1):56–65. doi: 10.1016/s0163-4453(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 34.Hoban D, Waites K, Felmingham D. Antimicrobial susceptibility of community-acquired respiratory tract pathogens in North America in 1999–2000: findings of the PROTEKT surveillance study. Diagn Microbiol Infect Dis. 2003;45(4):251–259. doi: 10.1016/s0732-8893(02)00522-9. [DOI] [PubMed] [Google Scholar]
- 35.Karlowsky JA, Thornsberry C, Jones ME, et al. Factors associated with relative rates of antimicrobial resistance among Streptococcus pneumoniae in the United States: results from the TRUST Surveillance Program (1998–2002) Clin Infect Dis. 2003;36(8):963–970. doi: 10.1086/374052. [DOI] [PubMed] [Google Scholar]
- 36.Wang JC. DNA topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 37.Wang JC. A journey in the world of DNA rings and beyond. Annu Rev Biochem. 2009;78:31–54. doi: 10.1146/annurev.biochem.78.030107.090101. [DOI] [PubMed] [Google Scholar]
- 38.Huband MD, Cohen MA, Zurack M, et al. In vitro and in vivo activities of PD 0305970 and PD 0326448, new bacterial gyrase/topoisomerase inhibitors with potent antibacterial activities versus multidrug-resistant gram-positive and fastidious organism groups. Antimicrob Agents Chemother. 2007;51(4):1191–1201. doi: 10.1128/AAC.01321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Croom KF, Goa KL. Levofloxacin: a review of its use in the treatment of bacterial infections in the United States. Drugs. 2003;63(24):2769–2802. doi: 10.2165/00003495-200363240-00008. [DOI] [PubMed] [Google Scholar]
- 40.Keating GM, Scott LJ. Moxifloxacin: a review of its use in the management of bacterial infections. Drugs. 2004;64(20):2347–2377. doi: 10.2165/00003495-200464200-00006. [DOI] [PubMed] [Google Scholar]
- 41.Raritan (NJ): Ortho-McNeil Pharmaceutical, Inc; Aug, 2009. Levaquin® (levofloxacin tablets, oral solution, injection): US prescribing information. [Google Scholar]
- 42.Hurst M, Lamb HM, Scott LJ, Figgitt DP. Levofloxacin: an updated review of its use in the treatment of bacterial infections. Drugs. 2002;62(14):2127–2167. doi: 10.2165/00003495-200262140-00013. [DOI] [PubMed] [Google Scholar]
- 43.Clinical and Laboratory Standards Institute . Clinical and Laboratory Standards Institute Document M7-A7. 2 Vol. 26. Wayne, PA: Clinical and Laboratory Standards Institute; Jan, 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard – seventh edition. [Google Scholar]
- 44.Gordon KA, Sader HS, Jones RN. Contemporary re-evaluation of the activity and spectrum of grepafloxacin tested against isolates in the United States. Diagn Microbiol Infect Dis. 2003;47(1):377–383. doi: 10.1016/s0732-8893(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 45.Fritsche TR, Sader HS, Jones RN. Potency and spectrum of garenoxacin tested against an international collection of skin and soft tissue infection pathogens: report from the SENTRY antimicrobial surveillance program (1999–2004) Diagn Microbiol Infect Dis. 2007;58(1):19–26. doi: 10.1016/j.diagmicrobio.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Goff DA, Dowzicky MJ. Prevalence and regional variation in meticillin-resistant Staphylococcus aureus (MRSA) in the USA and comparative in vitro activity of tigecycline, a glycylcycline antimicrobial. J Med Microbiol. 2007;56(Pt 9):1189–1193. doi: 10.1099/jmm.0.46710-0. [DOI] [PubMed] [Google Scholar]
- 47.Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997–2004) Diagn Microbiol Infect Dis. 2006;55(2):119–127. doi: 10.1016/j.diagmicrobio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Nilius AM, Shen LL, Hensey-Rudloff D, et al. In vitro antibacterial potency and spectrum of ABT-492, a new fluoroquinolone. Antimicrob Agents Chemother. 2003;47(10):3260–3269. doi: 10.1128/AAC.47.10.3260-3269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs MR, Felmingham D, Appelbaum PC, Gruneberg RN, Alexander Project Group The Alexander Project 1998–2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother. 2003;52(2):229–246. doi: 10.1093/jac/dkg321. [DOI] [PubMed] [Google Scholar]
- 50.Soriano F, Granizo JJ, Coronel P, et al. Antimicrobial susceptibility of Haemophilus influenzae, Haemophilus parainfluenzae and Moraxella catarrhalis isolated from adult patients with respiratory tract infections in four southern European countries. The ARISE project. Int J Antimicrob Agents. 2004;23(3):296–299. doi: 10.1016/j.ijantimicag.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 51.Hansen GT, Blondeau JM. Comparison of the minimum inhibitory, mutant prevention and minimum bactericidal concentrations of ciprofloxacin, levofloxacin and garenoxacin against enteric Gram-negative urinary tract infection pathogens. J. Chemother. 2005;17(5):484–492. doi: 10.1179/joc.2005.17.5.484. [DOI] [PubMed] [Google Scholar]
- 52.Deshpande LM, Diekema DJ, Jones RN. Comparative activity of clinafloxacin and nine other compounds tested against 2000 contemporary clinical isolates from patients in United States hospitals. Diagn Microbiol Infect Dis. 1999;35(1):81–88. doi: 10.1016/s0732-8893(99)00020-6. [DOI] [PubMed] [Google Scholar]
- 53.Rolston KV, Frisbee-Hume S, LeBlanc BM, Streeter H, Ho DH. Antimicrobial activity of a novel des-fluoro (6) quinolone, garenoxacin (BMS-284756): compared to other quinolones, against clinical isolates from cancer patients. Diagn Microbiol Infect Dis. 2002;44(2):187–194. doi: 10.1016/s0732-8893(02)00433-9. [DOI] [PubMed] [Google Scholar]
- 54.Thornsberry C, Sahm DF, Kelly LJ, et al. Regional trends in antimicrobial resistance among clinical isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States: results from the TRUST Surveillance Program, 1999–2000. Clin Infect Dis. 2002;34(Suppl 1):S4–S16. doi: 10.1086/324525. [DOI] [PubMed] [Google Scholar]
- 55.Karlowsky JA, Thornsberry C, Critchley IA, et al. Susceptibilities to levofloxacin in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis clinical isolates from children: results from 2000–2001 and 2001–2002 TRUST studies in the United States. Antimicrob Agents Chemother. 2003;47(6):1790–1797. doi: 10.1128/AAC.47.6.1790-1797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhanel GG, Hisanaga TL, Laing NM, et al. Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA) Int J Antimicrob Agents. 2006;27(6):468–475. doi: 10.1016/j.ijantimicag.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Critchley IA, Jones ME, Heinze PD, et al. In vitro activity of levofloxacin against contemporary clinical isolates of Legionella pneumophila, Mycoplasma pneumoniae and Chlamydia pneumoniae from North America and Europe. Clin Microbiol Infect. 2002;8(4):214–221. doi: 10.1046/j.1469-0691.2002.00392.x. [DOI] [PubMed] [Google Scholar]
- 58.Roblin PM, Reznik T, Kutlin A, Hammerschlag MR. In vitro activities of rifamycin derivatives ABI-1648 (Rifalazil, KRM-1648): ABI-1657, and ABI-1131 against Chlamydia trachomatis and recent clinical isolates of Chlamydia pneumoniae. Antimicrob Agents Chemother. 2003;47(3):1135–1136. doi: 10.1128/AAC.47.3.1135-1136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohlhoff SA, Roblin PM, Reznik T, Hawser S, Islam K, Hammerschlag MR. In vitro activity of a novel diaminopyrimidine compound, iclaprim, against Chlamydia trachomatis and C. pneumoniae. Antimicrob Agents Chemother. 2004;48(5):1885–1886. doi: 10.1128/AAC.48.5.1885-1886.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hammerschlag MR, Roblin PM. The in vitro activity of a new fluoroquinolone, ABT-492, against recent clinical isolates of Chlamydia pneumoniae. J Antimicrob Chemother. 2004;54(1):281–282. doi: 10.1093/jac/dkh304. [DOI] [PubMed] [Google Scholar]
- 61.Dubois J, St-Pierre C. Comparative in vitro activity and post-antibiotic effect of gemifloxacin against Legionella spp. J Antimicrob Chemother. 2000;45(Suppl 1):41–46. doi: 10.1093/jac/45.suppl_3.41. [DOI] [PubMed] [Google Scholar]
- 62.Stout JE, Sens K, Mietzner S, Obman A, Yu VL. Comparative activity of quinolones, macrolides and ketolides against Legionella species using in vitro broth dilution and intracellular susceptibility testing. Int J Antimicrob Agents. 2005;25(4):302–307. doi: 10.1016/j.ijantimicag.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 63.Waites KB, Crabb DM, Bing X, Duffy LB. In vitro susceptibilities to and bactericidal activities of garenoxacin (BMS-284756) and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother. 2003;47(1):161–165. doi: 10.1128/AAC.47.1.161-165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waites KB, Crabb DM, Duffy LB. Comparative in vitro activities of the investigational fluoroquinolone DC-159a and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother. 2008;52(10):3776–3778. doi: 10.1128/AAC.00849-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duffy LB, Crabb DM, Bing X, Waites KB. Bactericidal activity of levofloxacin against Mycoplasma pneumoniae. J Antimicrob Chemother. 2003;52(3):527–528. doi: 10.1093/jac/dkg365. [DOI] [PubMed] [Google Scholar]
- 66.Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37(6):752–760. doi: 10.1086/377539. [DOI] [PubMed] [Google Scholar]
- 67.Waites KB, Crabb DM, Duffy LB. Comparative in vitro susceptibilities and bactericidal activities of investigational fluoroquinolone ABT-492 and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother. 2003;47(12):3973–3975. doi: 10.1128/AAC.47.12.3973-3975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Felmingham D, Feldman C, Hryniewicz W, et al. Surveillance of resistance in bacteria causing community-acquired respiratory tract infections. Clin Microbiol Infect. 2002;8(Suppl 2):12–42. doi: 10.1046/j.1469-0691.8.s.2.5.x. [DOI] [PubMed] [Google Scholar]
- 69.Canton R, Morosini M, Enright MC, Morrissey I. Worldwide incidence, molecular epidemiology and mutations implicated in fluoroquinolone-resistant Streptococcus pneumoniae: data from the global PROTEKT surveillance programme. J Antimicrob Chemother. 2003;52(6):944–952. doi: 10.1093/jac/dkg465. [DOI] [PubMed] [Google Scholar]
- 70.Higgins PG, Fluit AC, Milatovic D, Verhoef J, Schmitz FJ. Mutations in GyrA, ParC, MexR and NfxB in clinical isolates of Pseudomonas aeruginosa. Int J Antimicrob. Agents. 2003;21(5):409–413. doi: 10.1016/s0924-8579(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 71.Deryke CA, Du X, Nicolau DP. Evaluation of bacterial kill when modelling the bronchopulmonary pharmacokinetic profile of moxifloxacin and levofloxacin against parC-containing isolates of Streptococcus pneumoniae. J Antimicrob Chemother. 2006;58(3):601–609. doi: 10.1093/jac/dkl292. [DOI] [PubMed] [Google Scholar]
- 72.LaPlante KL, Rybak MJ, Tsuji B, Lodise TP, Kaatz GW. Fluoroquinolone resistance in Streptococcus pneumoniae: area under the concentration-time curve/MIC ratio and resistance development with gatifloxacin, gemifloxacin, levofloxacin, and moxifloxacin. Antimicrob Agents Chemother. 2007;51(4):1315–1320. doi: 10.1128/AAC.00646-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sahm DF, Benninger MS, Evangelista AT, Yee YC, Thornsberry C, Brown NP. Antimicrobial resistance trends among sinus isolates of Streptococcus pneumoniae in the United States (2001–2005) Otolaryngol Head Neck Surg. 2007;136(3):385–389. doi: 10.1016/j.otohns.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 74.West M, Boulanger BR, Fogarty C, et al. Levofloxacin compared with imipenem/cilastatin followed by ciprofloxacin in adult patients with nosocomial pneumonia: a multicenter, prospective, randomized, open-label study. Clin Ther. 2003;25(2):485–506. doi: 10.1016/s0149-2918(03)80091-7. [DOI] [PubMed] [Google Scholar]
- 75.Chow AT, Fowler C, Williams RR, Morgan N, Kaminski S, Natarajan J. Safety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob Agents Chemother. 2001;45(7):2122–2125. doi: 10.1128/AAC.45.7.2122-2125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chien SC, Wong FA, Fowler CL, et al. Double-blind evaluation of the safety and pharmacokinetics of multiple oral once-daily 750-milligram and 1-gram doses of levofloxacin in healthy volunteers. Antimicrob Agents Chemother. 1998;42(4):885–888. doi: 10.1128/aac.42.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stein GE, Schooley SL, Nicolau DP. Urinary bactericidal activity of single doses (250, 500, 750 and 1000 mg) of levofloxacin against fluoroquinolone-resistant strains of Escherichia coli. Int J Antimicrob Agents. 2008;32(4):320–325. doi: 10.1016/j.ijantimicag.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 78.Gotfried MH, Danziger LH, Rodvold KA. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest. 2001;119(4):1114–1122. doi: 10.1378/chest.119.4.1114. [DOI] [PubMed] [Google Scholar]
- 79.Rodvold KA, Danziger LH, Gotfried MH. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob Agents Chemother. 2003;47(8):2450–2457. doi: 10.1128/AAC.47.8.2450-2457.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Capitano B, Mattoes HM, Shore E, et al. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest. 2004;125(3):965–973. doi: 10.1378/chest.125.3.965. [DOI] [PubMed] [Google Scholar]
- 81.Conte JE, Jr, Golden JA, McIver M, Little E, Zurlinden E. Intrapulmonary pharmacodynamics of high-dose levofloxacin in subjects with chronic bronchitis or chronic obstructive pulmonary disease. Int J Antimicrob Agents. 2007;30(5):422–427. doi: 10.1016/j.ijantimicag.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 82.Drusano GL, Preston SL, Gotfried MH, Danziger LH, Rodvold KA. Levofloxacin penetration into epithelial lining fluid as determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother. 2002;46(2):586–589. doi: 10.1128/AAC.46.2.586-589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pea F, Marioni G, Pavan F, et al. Penetration of levofloxacin into paranasal sinuses mucosa of patients with chronic rhinosinusitis after a single 500 mg oral dose. Pharmacol Res. 2007;455(1):38–41. doi: 10.1016/j.phrs.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Garraffo R, Lavrut T, Durant J, et al. In vivo comparative pharmacokinetics and pharmacodynamics of moxifloxacin and levofloxacin in human neutrophils. Clin Drug Investig. 2005;25(10):643–650. doi: 10.2165/00044011-200525100-00003. [DOI] [PubMed] [Google Scholar]
- 85.Poole M, Anon J, Paglia M, Xiang J, Khashab M, Kahn J. A trial of high-dose, short-course levofloxacin for the treatment of acute bacterial sinusitis. Otolaryngol Head Neck Surg. 2006;134(1):10–17. doi: 10.1016/j.otohns.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 86.Peterson J, Kaul S, Khashab M, Fisher AC, Kahn JB. A double-blind, randomized comparison of levofloxacin 750 mg once-daily for five days with ciprofloxacin 400/500 mg twice-daily for 10 days for the treatment of complicated urinary tract infections and acute pyelonephritis. Urology. 2008;71(1):17–22. doi: 10.1016/j.urology.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 87.Klausner HA, Brown P, Peterson J, et al. A trial of levofloxacin 750 mg once daily for 5 days versus ciprofloxacin 400 mg and/or 500 mg twice daily for 10 days in the treatment of acute pyelonephritis. Curr Med Res Opin. 2007;23(11):2637–2645. doi: 10.1185/030079907x233340. [DOI] [PubMed] [Google Scholar]
- 88.Dunbar LM, Khashab MM, Kahn JB, Zadeikis N, Xiang JX, Tennenberg AM. Efficacy of 750-mg, 5-day levofloxacin in the treatment of community-acquired pneumonia caused by atypical pathogens. Curr Med Res Opin. 2004;20(4):555–563. doi: 10.1185/030079904125003304. [DOI] [PubMed] [Google Scholar]
- 89.Shorr AF, Zadeikis N, Xiang JX, Tennenberg AM, Wes Ely E. A multicenter, randomized, double-blind, retrospective comparison of 5- and 10-day regimens of levofloxacin in a subgroup of patients aged ≥65 years with community-acquired pneumonia. Clin Ther. 2005;27(8):1251–1259. doi: 10.1016/s0149-2918(05)80214-0. [DOI] [PubMed] [Google Scholar]
- 90.File TM, Jr, Milkovich G, Tennenberg AM, Xiang JX, Khashab MM, Zadeikis N. Clinical implications of 750 mg, 5-day levofloxacin for the treatment of community-acquired pneumonia. Curr Med Res Opin. 2004;20(9):1473–1481. doi: 10.1185/030079904X2556. [DOI] [PubMed] [Google Scholar]
- 91.Shorr AF, Khashab MM, Xiang JX, Tennenberg AM, Kahn JB. Levofloxacin 750-mg for 5 days for the treatment of hospitalized Fine Risk Class III/IV community-acquired pneumonia patients. Respir Med. 2006;100(12):2129–2136. doi: 10.1016/j.rmed.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 92.Levofloxacin revisited. Med Lett Drugs Ther. 2011;53(1368):55. [PubMed] [Google Scholar]
- 93.Khashab MM, Xiang J, Kahn JB. Comparison of the adverse event profiles of levofloxacin 500 mg and 750 mg in clinical trials for the treatment of respiratory infections. Curr Med Res Opin. 2006;22(10):1997–2006. doi: 10.1185/030079906X132505. [DOI] [PubMed] [Google Scholar]
- 94.Yee YC, Evangelista AT, Obot-Tucker M, et al. Five-year surveillance (2003–2007) of anti-pneumococcal activity of oral agents recommended for the empirical treatment of community-acquired pneumonia (CAP) in adults [Abstract No C2204]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL. Sep 17–20, 2007. [Google Scholar]