Abstract
The quantitative significance of reserves and current assimilates in regrowing tillers of severely defoliated plants of perennial ryegrass (Lolium perenne L.) was assessed by a new approach, comprising 13C/12C and 15N/14N steady-state labeling and separation of sink and source zones. The functionally distinct zones showed large differences in the kinetics of currently assimilated C and N. These are interpreted in terms of ”substrate” and ”tissue” flux among zones and C and N turnover within zones. Tillers refoliated rapidly, although C and N supply was initially decreased. Rapid refoliation was associated with (a) transient depletion of water-soluble carbohydrates and dilution of structural biomass in the immature zone of expanding leaves, (b) rapid transition to current assimilation-derived growth, and (c) rapid reestablishment of a balanced C:N ratio in growth substrate. This balance (C:N, approximately 8.9 [w/w] in new biomass) indicated coregulation of growth by C and N supply and resulted from complementary fluxes of reserve- and current assimilation-derived C and N. Reserves were the dominant N source until approximately 3 d after defoliation. Amino-C constituted approximately 60% of the net influx of reserve C during the first 2 d. Carbohydrate reserves were an insignificant source of C for tiller growth after d 1. We discuss the physiological mechanisms contributing to defoliation tolerance.
Photosynthesis decreases drastically when a plant is defoliated as a consequence of grazing or mowing (Davidson and Milthorpe, 1966b; Richards, 1993). Also, root growth (Davidson and Milthorpe, 1966b) and, with some delay, N uptake (Jarvis and Macduff, 1989) and assimilation (Macduff et al., 1989) are transiently depressed, most likely as a result of decreased assimilate availability to roots. Clearly, refoliation and, thus, the restoration of active photosynthesis are the crucial elements of a plant's response to severe defoliation. Refoliation may be controlled by the availability of substrate to the (re)growing shoot (source control) or by meristematic constraints (sink control). Sink control may be important where defoliation involves the removal of all or most of the currently expanding leaf tissue and active shoot meristems, thus requiring the activation of dormant meristems for refoliation (such as in dicots with orthotropic stems, e.g. alfalfa or caespitose grasses after apex elevation, compare with Richards, 1993). Conversely, where defoliation is severe but all active meristems and expanding leaf tissue are left behind intact (such as in grasses during vegetative growth), source control of regrowth is likely.
A critical role of carbohydrate and N reserves as the substrates for refoliation has been advocated previously (Sullivan and Sprague, 1943). Extensive mobilization of nonstructural carbohydrates and nitrogenous compounds (Alberda, 1957; Prud'homme et al., 1992; Ourry et al., 1994; Volenec et al., 1996) regularly occurs in the residual parts of defoliated plants. However, the supply of mobilized carbohydrates and N to the regrowing shoots has seldom been studied with quantitative methods. Quantification of reserve-derived C or N accumulation in regrowing shoots requires steady-state labeling of all pre- or postdefoliation fixed C or N (de Visser et al., 1997). Since N redistribution occurs mainly in the form of amino acids (Ourry et al., 1989; Bigot et al., 1991), a significant fraction of the reserve-derived C influx in regrowing shoots may be associated with the import of amino acids (Avice et al., 1996). Thus, estimation of the reserve-derived carbohydrate influx from C-labeling data alone would lead to wrong conclusions, if transport of redistributed amino-C is not accounted for. As yet, to our knowledge, dual steady-state labeling has not been used to assess the actual contribution of reserve-derived C and N to postdefoliation regrowth of plants. But, in a study with 3-month-old alfalfa plants, Avice et al. (1996) used a 10-d-long predefoliation labeling of C and N to assess the redistribution of reserve-derived N and carbohydrates. In that study only 7% of the (labeled) mobilized C accumulated in the regrowing shoot biomass. Much of this was likely associated with amino acids, suggesting a low contribution of reserve carbohydrates to shoot regrowth.
Since the recovery of N uptake is retarded relative to the restoration of photosynthetic activity (Richards, 1993), the reserve dependence of shoot growth may be longer for N than for C. In the growth zones of expanding leaves of severely defoliated perennial ryegrass (Lolium perenne L.), the transition from reserve- to current assimilation-supported growth was more rapid for C than for N (de Visser et al., 1997). Similarly, in the study with alfalfa (labeled) reserve-derived N was the main N source for an extended period after defoliation (Avice et al., 1996). Still, it was not clear from these studies whether the influx of C or N was controlling regrowth of the shoot and which roles carbohydrate and N reserves played in alleviating potential limitations in the supplies of C and N.
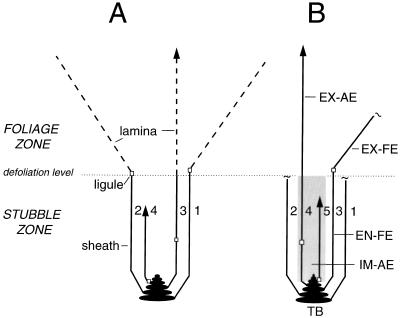
In regrowth studies with grasses a recognition of the functional heterogeneity of the ”stubble” left behind after defoliation is necessary (Davidson and Milthorpe, 1966a; Volenec, 1986; de Visser et al., 1997; Morvan et al., 1997). The stubble includes fully expanded leaf material (mainly leaf sheaths), as well as the (enclosed) basal, immature parts of expanding leaves and leaf primordia formed by the apex at the tiller base (Fig. 1A). The former may serve as a source for mobilized C and N, whereas the latter generate the new foliage and may act as sinks for mobilized C and N. At all times about two leaves are actively expanding in a vegetative grass tiller, with the younger of the two not becoming visible until its tip emerges above the whorl of encircling leaf sheaths. As the older of the two reaches its final size, a new leaf starts to expand, with lamina expansion preceding sheath expansion (Skinner and Nelson, 1994). Thus, at any time during postdefoliation regrowth three functionally distinct categories of leaves may be found in a grass tiller: (a) (the residual parts of) fully expanded leaves that completed growth before defoliation (b) (parts of) fully expanded leaves that completed growth after defoliation, and (c) currently growing leaves (compare with Fig. 1B). The latter (b and c) compose the regrowing part of the tiller (which is hereafter termed the ”regrowing tiller”; Fig. 1) and both include exposed (photosynthetically active) and enclosed (heterotrophic immature or mature) leaf zones.
Figure 1.
Schematic representation of a vegetative perennial ryegrass tiller at defoliation (A) and approximately one leaf appearance interval after severe defoliation (B). The tiller is composed almost entirely of leaf tissue (1–5). Daughter tillers may be present in axils of fully expanded leaves (not shown). The TB includes the apex, nodes, and unelongated internodes of the tiller. The defoliation level marks the boundary between the exposed (grazed/mown) foliage and stubble zones. At defoliation (A) leaves 1 and 2 had reached full expansion, whereas leaves 3 and 4 were expanding (↑). Defoliation removed all lamina tissue of leaves 1 and 2 and (the exposed) part of the lamina of elongating leaf 3. Leaf 4 was not touched, because its tip had not emerged above the defoliation level. Foliage production (exposure of lamina tissue above the defoliation level) during the interval A to B was due to expansion of leaves 3 and 4. At time B leaf 3 had reached its final size. Expansion stopped shortly after the ligule was exposed above the defoliation level. Leaf 4 was still expanding at time B, and leaf 5 had started to expand. Expansion and maturation of cells takes place in a zone (IM-AE) that extends from the base of the expanding leaf to the location where leaf tissue emerges from the encircling sheaths of older leaves (at approximately defoliation level). The sheath was actively expanding in leaf 4 at time B (note position of the ligule). Leaf 5 was still completely enclosed and expanding as a function of lamina growth. At any time during refoliation, the regrowing tiller can be divided into five functionally distinct zones: the TB, the EX-AE, and the IM-AE (leaves 4 and 5 in B [shaded area]) and the EX-FE and EN-FE, which stopped to elongate after defoliation (leaf 3 in B). Leaves 1 and 2 had stopped to expand before defoliation and, hence, do not form part of the regrowing tiller, as defined here.
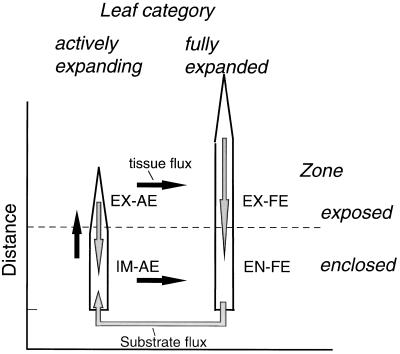
Growth (cell production and expansion) is confined to the (enclosed) immature base of the expanding grass leaf (Volenec and Nelson, 1981; MacAdam et al., 1989; Schnyder et al., 1990), and the tissue that emerges from the enclosing leaf sheaths is (almost) fully differentiated and photosynthetically active (Wilhelm and Nelson, 1978; Boffey et al., 1980; Dean and Leech, 1982; MacAdam and Nelson, 1987; Gastal and Nelson, 1994). Thus, cell production and expansion in the immature zone gives rise to a flux of (almost) fully mature tissue to the exposed zone of expanding leaves (Fig. 2). Furthermore, when a leaf stops to expand, it is laterally displaced to the expanded leaf category (organ flux). During undisturbed growth the tissue-bound efflux of C and N from the immature zone is compensated by concurrent import of the substrate. However, following severe defoliation of perennial ryegrass both the fresh mass (i.e. volume) of the immature zone and the C mass per unit fresh mass of the immature zone of expanding leaves decreased strongly, indicating that axial relative to radial expansion was transiently increased and that C influx was less than was necessary to balance the tissue-bound efflux of C (de Visser et al., 1997). The latter may have been caused by the use of carbohydrates, which were already present in the immature zone at defoliation (Davidson and Milthorpe, 1966a; Volenec, 1986; Morvan et al., 1997). However, reduced synthesis of structural biomass per unit (volumetric) growth could also contribute to a dilution of C in the immature zone. This possibility has not been studied to date. However, because it would contribute to sustaining the efflux (exposure) of photosynthetically active tissue at reduced costs, such a mechanism could facilitate the recovery from defoliation and the transition from reserve- to current assimilation-dependent growth.
Figure 2.
Conceptual model of tissue and substrate (i.e. assimilate) fluxes among functionally distinct zones of a regrowing tiller of perennial ryegrass. Leaf expansion is confined to the basal region of the IM-AE and gives rise to a flux of tissue to the EX-AE. As leaves stop to expand, they are ”displaced” to the expanded leaves category (EX-AE → EX-FE, IM-AE → EN-FE). Tissue flux is mainly in the form of structural biomass. Substrate flux is mainly directed toward the immature zone, where cell production, expansion, and maturation (including synthesis of proteins and secondary cell walls) take place. Substrate fluxes to and from other plant parts are not shown.
The aim of this study was to assess the relative importance of reserve- and current assimilation-derived substrate supply in the regrowing shoot and of putative mechanisms operating within the regrowing shoot itself in controlling postdefoliation regrowth of a defoliation-tolerant species, perennial ryegrass (L. perenne L.). To this end, we used steady-state labeling of all postdefoliation-assimilated C and N, periodic sampling of regrowing tillers, and analysis of WSC and of the mass and isotope composition of C and N in functionally distinct zones of regrowing tillers.
MATERIALS AND METHODS
Plant Material, Growth Conditions, and 13CO2/12CO2 and 15N/14N Labeling
Details of the plant material, growth conditions, and 13CO2/12CO2 and 15N/N labeling were described previously (de Visser et al., 1997). Clonal plants of two genotypes of perennial ryegrass (Lolium perenne L.) were established in a greenhouse in a hydroponic system in Wageningen (The Netherlands). Well-established vegetatively growing plants (n = 100) of both genotypes were transferred to and distributed randomly in two plant growth chambers (model E15, Conviron, Winnipeg, Manitoba, Canada) in Bonn, Germany. A 16-h light period of 400 μmol m−2 s−1 PPFD at plant height was supplied by cool-white fluorescent and incandescent lamps. Temperature was controlled at 20°C/17°C and RH near 70%/85% during the light/dark periods, respectively. Individual plants were held in 1.2-L pots with aerated nutrient solution (de Visser et al., 1997). Nutrient solutions (with nitrate as the sole N source) were replaced when the nitrate concentration, as determined by indicator strips (Merckoquant, 10020, Merck, Darmstadt, Germany), had declined to about 20% (i.e. 1.5 mm). All plants remained vegetative throughout the experiment. After 13 d in the growth chamber the plants were defoliated at 5 cm stubble height and redistributed equally among the two chambers. Defoliation was performed shortly before the start of the light period.
An open-system, steady-state 13CO2/12CO2-labeling technique (Schnyder, 1992; de Visser et al., 1997) was used to label all photosynthate fixed during the 14-d postdefoliation regrowth period. Before defoliation both chambers received ambient air with a CO2 partial pressure of approximately 36 Pa and a δ13C value (δ) of approximately −8.8o/oo. Immediately following defoliation the δ of the CO2 in one of the two chambers was changed to −28.1o/oo (using CO2 of fossil organic origin; Buse, Bad Hönningen, Germany) for the entire regrowth period. Air with a CO2 partial pressure of 36 Pa was supplied to the chamber at a constant 36 m3 h−1. This provided for a near-maximum expression of C isotope discrimination (Δ) during labeling (de Visser et al., 1997), since CO2 was injected at >30 times the rate of photosynthetic CO2 uptake by plants. Also, at the time of defoliation the plants in the labeling chamber were transferred from a standard nutrient solution with 0.3682 atom % 15N to a solution enriched to 1.00 atom % 15N in nitrate. In the other chamber (labeling control), the isotope composition of both the N in the nutrient solution and the CO2 supplied to the plants was kept the same as during the predefoliation period. This was done to allow for a determination of eventual defoliation-, genotype-, time-, and zone-specific effects on C and N isotope discrimination (see below; de Visser et al., 1997). The other growth conditions were kept identical in both growth chambers.
Sampling
Plants were sampled at 0, 1, 2, 5, 8, and 14 d after defoliation. Sampling always started at the beginning of the light period and was completed within 6 h. On each date four plants of each genotype were randomly sampled from each of the growth chambers. Each plant had approximately 50 ”mature” tillers. Mature tillers are defined as having at least one fully expanded leaf at the time of defoliation and usually included one or two daughter tillers in the axils of fully expanded leaves. The daughter tillers had no fully expanded leaves at defoliation. A subsample consisting of eight mature tillers was removed from each plant. Selected mature tillers were divided into (a) leaf tissue that had reached full expansion before defoliation (mainly sheath tissue), (b) daughter tillers, and (c) tissue that experienced growth after defoliation. The latter fraction represented the ”regrowing tiller” and was dissected to yield the functionally distinct zones shown in Figure 1, i.e. (a) the IM-AE (0–5 cm from TB); (b) the EX-AE (tissue above the 5-cm defoliation level); (c) the EN-FE (0–5 cm from TB) and (d) the EX-FE (>5 cm); and (e) the TB, consisting of the regrowing tiller's apex, nodes, and unelongated internodes (Fig. 1B). Actively expanding leaves were defined as having their ligule at ≤2 cm from the leaf base. Leaves with the ligule at >2 but ≤5 cm were arbitrarily assigned to the fully expanded leaves fraction (with tissue located between 0 and 5 cm of the TB added to the EN-FE fraction and tissue >5 cm to EX-FE), since expansion was confined to the sheath part of the leaf and the expansion rate was slower and rapidly decreasing with time (compare with Schnyder et al., 1990). All plant material was kept on a cool surface (approximately 0°C) during dissection. Immediately following collection the individual samples were weighed, freeze-dried, weighed again, ground to homogeneity in a ball mill, and then stored at −30 °C until needed.
C, N, and Carbohydrate Analysis
Aliquots of the plant material were analyzed for the content and isotope composition of C and N using an elemental gas chromatograph interfaced to a continuous flow isotope-ratio mass spectrometer (Roboprep TCD-Tracermass, Europa Scientific, Crewe, UK; de Visser et al., 1997). Since differences in C and N concentration (grams per gram dry mass) and isotope composition were very small between replicates (compare with Table I), the elemental and isotope analyses were restricted to two of the four replicates sampled from each genotype and growth chamber (sd averaged 0.3‰ for δ of C in the control and 0.5‰ in the labeling chamber and 0.001 atom % 15N in the control and 0.015 atom % 15N in the labeling chamber).
Table I.
Average coefficients of variation (CV) for dry mass, C and N concentration, and the fraction of predefoliation C and N in regrowing tillers of perennial ryegrass
| Parameter | CV |
|---|---|
| % | |
| Dry mass (g/tiller) | 14.2 |
| C concentration (g/g dry mass) | 1.6 |
| N concentration (g/g dry mass) | 6.1 |
| Fraction of predefoliation C (g C/g C) | 2.9 |
| Fraction of predefoliation N (g N/g N) | 3.4 |
Average CV was calculated from the CVs of sampling date × genotype combinations.
For analysis of WSC, 10 mg of ground dry material was weighed into 2.2-mL capped Eppendorf tubes and 2 mL of water was added. Tubes were sealed immediately and transferred to a 95°C water bath for 10 min, shaken for 30 min, and centrifuged at 10,000g for 10 min, and the supernatants were removed with a pipette. Comparison with two additional extractions demonstrated that ≥98% of the total WSC was dissolved in the first extract. WSC were analyzed with a continuous flow system similar to the one described by Wolf and Ellmore (1975). An aliquot of the WSC extract was hydrolyzed in 0.1 m sulfuric acid for 25 min at 92°C. Reducing power of the hydrolyzed carbohydrates was detected photometrically at 425 nm after reduction of a potassium ferricyanide solution. Analysis of reference Glc, Fru, Suc, and fructan (all biochemical grade from Merck) yielded response factors of the individual carbohydrates, which were proportional to the amount of hexose residues present in 1 g of substrate.
Data Analysis
The C and N isotope data (δ13C and atom % 15N) were used to calculate the weight fractions (fpre and fpost, where fpost = 1 − fpre) of C and N derived from pre- and postdefoliation assimilation. The fraction of predefoliation C (fpre) in a sample was obtained from δP = fpreδPC + (1 − fpre)δPL:
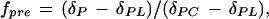 |
where δP is the δ of a given sample harvested from the labeling chamber, δPL is the δ of the C assimilated from the labeling CO2, and δPC is the δ of the parallel (replicate) sample collected from the control chamber. The δPL was not determined experimentally. But, since C isotope discrimination is independent of the isotope composition of CO2 (Deléens et al., 1983; Farquhar and Richards, 1984; Schnyder, 1992), δPL was calculated as:
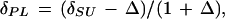 |
where δSU is the δ of the labeling CO2, and Δ is the genotype-, zone-, replicate- and time-specific C isotope discrimination, as determined in the parallel sample collected from the control chamber (de Visser et al., 1997). The mass of predefoliation C in a sample (Cpre, milligrams per tiller) was calculated as:
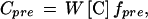 |
where W is the dry mass (milligrams per tiller) and [C] is the C concentration (grams per gram dry mass) in the sample (Cpost = C − Cpre, where C is total C mass per tiller). Cpre and Cpost were estimated for samples collected from both the labeling and control chambers using their replicate-specific fpre estimate (note that fpre was a parameter that was derived from paired samples collected from the labeling and control chambers and included variation in both). Dry mass and C and N mass parameters did not differ among the two chambers (P > 0.05), as was expected since growth conditions were the same. For replicates in which elemental concentrations and isotope composition were not analyzed, estimates of pre- and postdefoliation C mass were obtained from their original dry mass (W) and randomly assigned fpre estimates and elemental concentrations ([C]), as determined in the other replicates.
N isotope data were evaluated accordingly, but N isotope discrimination was neglected because it was insignificant relative to the enrichment of the nitrate by 15N (de Visser et al., 1997). The masses of pre- and postdefoliation C and N in the total regrowing tiller were calculated as the sum of masses present in the component tiller zones (a–e; see above).
Accumulation rates of pre- and postdefoliation C and N (milligrams of C or N per tiller per day) were calculated from the net changes in mass of pre- and postdefoliation C and N in the regrowing tiller as observed in the respective sampling intervals. For calculation of the accumulation rates of C and N and estimation of the associated variation, all replicates within a sampling date × genotype combination were first ranked according to total tiller mass. Accumulation rate was then calculated separately for the two genotypes and data pairs with the same rank number. This procedure removed variation associated with differences in initial tiller mass. Data from the two genotypes were then combined since differences were insignificant for the relationships studied here.
RESULTS
The Regrowing Tiller
Refoliation
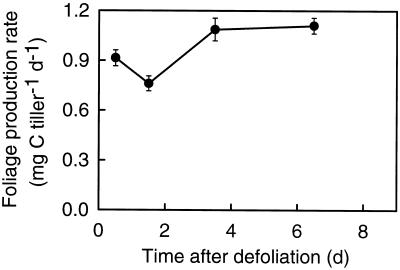
Defoliation was severe, because it removed essentially all lamina tissue and approximately 70% of total shoot biomass (data not shown). Foliage production was assessed by following the total mass of C in leaf tissue exposed above the defoliation level (foliage zone, compare with Fig. 1). Foliage production rate was decreased on d 1 (−18%) and d 2 (−31%) when compared with the rate at 1 week after defoliation, but it increased strongly between d 2 and 5 after defoliation (Fig. 3).
Figure 3.
Foliage production rate in regrowing tillers of perennial ryegrass during the first 8 d of regrowth after severe defoliation. Foliage denotes all leaf (essentially lamina) tissue exposed above the defoliation level. Rates were calculated as the net change over time of C mass in foliage as observed during sampling intervals (see Methods). Vertical bars indicate ±se (n = 16; eight tillers per replicate).
C and N Accumulation Rates
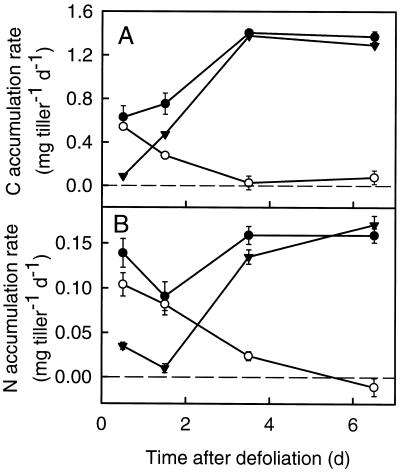
The net C balance of the regrowing tiller was positive during d 1 (Fig. 4A), whereas the whole-plant C balance was negative on that day (R. de Visser and H. Schnyder, unpublished data). C accumulation (i.e. gross C influx less respiration and export) on d 1 (86%) was mainly due to accumulation of reserve-derived C (i.e. of predefoliation C imported from other plant parts [roots and senescing sheaths]). The C accumulation rate of the regrowing tiller increased strongly between d 1 and 5, and this was related to a strong increase in the accumulation of currently (i.e. postdefoliation) fixed C (Fig. 4A). During d 2 postdefoliation fixed C already accounted for 63% of the total (i.e. pre- plus postdefoliation) C accumulation; thereafter, postdefoliation C provided more than 90% in the regrowing tiller.
Figure 4.
Net accumulation rate of total C (•), predefoliation C (○), and postdefoliation C (▾) (A) and total N (•), predefoliation N (○), and postdefoliation N (▾) (B) in regrowing tillers of perennial ryegrass during the first 8 d of regrowth after severe defoliation. Rates were calculated as the net change over time of C or N mass (total, predefoliation, and postdefoliation) as observed during sampling intervals (see Methods). The regrowing tiller included the TB and all of the leaves that experienced growth after defoliation but not the leaves that had stopped to expand before defoliation (compare with Fig. 1). Vertical bars indicate ±se (n = 16).
The N accumulation rate on d 1 was only moderately depressed when compared with the rate at about 1 week after defoliation (Fig. 4B). The high rate of N accumulation on d 1 was related to import of both reserve-derived (predefoliation) and currently absorbed (postdefoliation) N, with reserve-derived N contributing the bulk (75%) of the accumulation.
Accumulation of N in the regrowing tiller was transiently depressed on d 2, and this was due to decreased accumulation of both pre- and postdefoliation-absorbed N. In relative terms the decrease was stronger for post- than for predefoliation-absorbed N. Thus, the contribution of current N uptake to N accumulation in the regrowing shoot was only 10% on d 2. After d 2 the accumulation rate of N increased, which was mainly due to increased accumulation of postdefoliation-absorbed N, which became the dominant N source about 3 d after defoliation (Fig. 4B).
C:N Ratio in Reserve- and Current Uptake-Derived Biomass Accumulating in the Regrowing Tiller
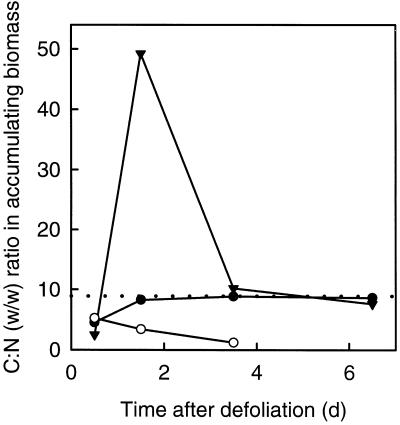
The total (i.e. pre- and postdefoliation) biomass accumulating in the regrowing tiller during d 1 had a C:N ratio of 4.5. However, the biomass accumulating during d 2 closely matched the C:N ratio of the whole-tiller biomass observed at defoliation and of the regrowing tiller biomass at the end of the regrowth period (Fig. 5).
Figure 5.
C:N (w/w) ratio in total biomass (•) and in pre- (○) and postdefoliation biomass (▾) accumulating in regrowing tillers of perennial ryegrass during the first 8 d of regrowth after severe defoliation. Ratios were calculated from the daily net rates of C and N accumulation presented in Figure 4 (compare with Fig. 4 for se). Note that the strong increase in the C:N ratio of postdefoliation biomass accumulating between d 1 and 2 resulted from an increase in the rate of postdefoliation C accumulation and a (concurrent) decrease in the rate of postdefoliation N accumulation. The dotted line indicates the C:N ratio (8.9) of regrowing tiller biomass at 14 d after defoliation, which was the same as in total tiller biomass at defoliation.
Predefoliation biomass accumulating on d 1 had a C:N (w/w) ratio of 5.2 (Fig. 5), which decreased steadily thereafter. Postdefoliation biomass accumulating during d 1 was very rich in N, but on d 2 it contained very little N. After d 2 the C:N ratio in new biomass derived from postdefoliation assimilation rapidly approached an equilibrium value near 9.
The Tiller Zones
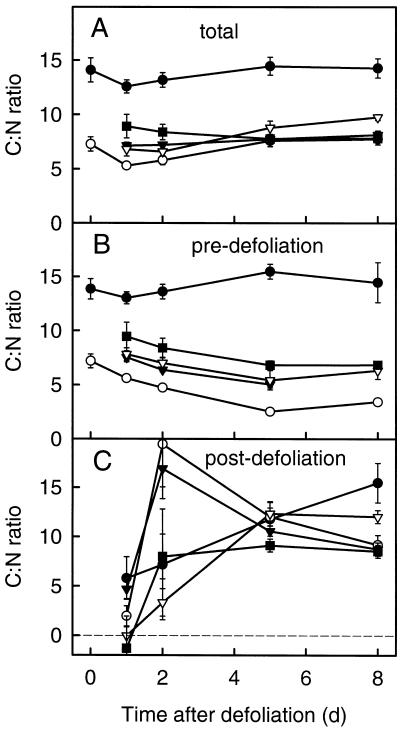
C:N Ratio in Biomass
The biomass in the different zones of the regrowing tiller exhibited large zone-specific differences in the C:N ratio (Fig. 6A) and in the changes over time of the C:N ratio in pre- and postdefoliation biomass (Fig. 6, B and C). In all of the zones the C:N ratio of total biomass decreased transiently after defoliation (Fig. 6A). The C:N ratio in predefoliation biomass was lowest in the immature zone and highest in the TB (Fig. 6B). In all of the zones, except for the TB, the C:N ratio of predefoliation biomass decreased significantly between d 0 and 5 after defoliation. This decrease was strongest in the immature zone.
Figure 6.
C:N ratio in total biomass (A), predefoliation biomass (B), and postdefoliation biomass (C) in functionally distinct zones of regrowing tillers of perennial ryegrass during the first 8 d of regrowth after severe defoliation: IM-AE (○), EX-AE (▾), EN-FE (▿), EX-FE (▪), and TB (•). For definition and designation of zones, see the legends for Figures 1 and 2. Vertical bars indicate ±2 se (n = 16).
Significant zone-specific differences were also evident in the time course of the C:N ratio of postdefoliation-assimilated biomass. In the IM-AE and EX-AE (compare with Fig. 1), the C:N ratio of postdefoliation biomass followed the changes observed for the C:N ratio of postdefoliation biomass accumulating in the entire regrowing tiller (compare Figs. 5 and 6C). This was related to the high turnover of biomass in these zones (generation of new tissue from current substrate [Fig. 5]) and concurrent efflux of mature tissue (compare with Fig. 2; see Discussion). The C:N ratio of the postdefoliation biomass in other zones (TB, EN-FE, and EX-FE) changed more gradually over time (Fig. 6C).
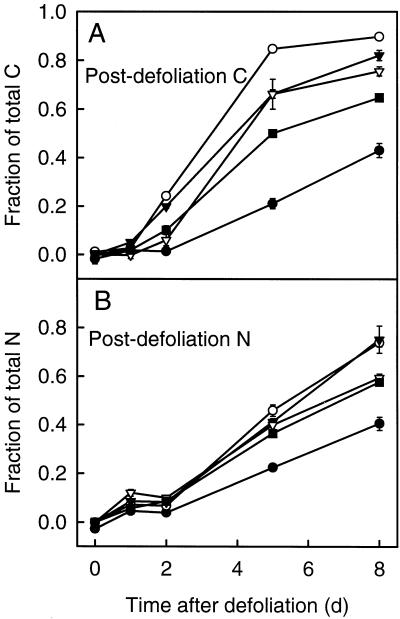
Fraction of Postdefoliation C and N
The rates of increase in the fraction of postdefoliation C and N in biomass differed strongly among the different zones and were different for C and N (Fig. 7). For postdefoliation C the rate decreased in the order IM-AE > EX-AE > EN-FE > EX-FE > TB (Fig. 7A).
Figure 7.
Time course of the fraction of postdefoliation C (A) and postdefoliation N (B) in functionally distinct zones of regrowing tillers of perennial ryegrass during the first 8 d of regrowth after severe defoliation: IM-AE (○), EX-AE (▾), EN-FE (▿), EX-FE (▪), and TB (•). For definition and designation of zones, see legends for Figures 1 and 2. Vertical bars indicate ±sd (n = 4).
The kinetics of postdefoliation N differed from the pattern observed for C: In particular, postdefoliation N always represented a smaller fraction of total N (Fig. 7B) compared with C (Fig. 7A). This effect resulted from the longer duration of predefoliation N supply than of predefoliation C supply to the regrowing tiller (Fig. 4). Furthermore, there was a significant transient slowing down between d 1 and 2 in the increase of the fraction of postdefoliation N in all of the zones, demonstrating that decreased postdefoliation N accumulation in the regrowing tiller on d 2 (Fig. 4B) affected N supply to all of the zones (compare with Fig. 9C). Also, the kinetics of the increase of postdefoliation N in the exposed zones did not differ from the enclosed zones (Fig. 7B) in either the actively expanding or the fully expanded leaf category.
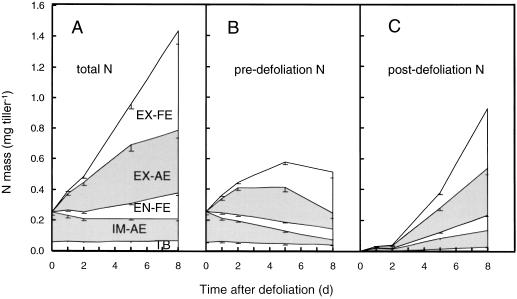
Figure 9.
Masses of total N (A), predefoliation N (B), and postdefoliation N (C) in regrowing tillers of perennial ryegrass and in its component zones during the first 8 d of regrowth after severe defoliation: IM-AE, EX-AE, EN-FE, EX-FE, and TB. Leaves that had stopped to expand before defoliation are not included, because they did not form part of the regrowing tiller as defined here. For definition and designation of zones, see the legends for Figures 1 and 2. Vertical bars indicate ±2 se (n = 16).
Predefoliation Biomass
The zones of the regrowing tiller differed strongly in the temporal changes of predefoliation C and N (Figs. 8B and 9B), as calculated from the total masses of C and N (Figs. 8A and 9A) and the fractions of postdefoliation C and N (Fig. 7). In the TB the masses of predefoliation C and N changed little over time, whereas they decreased rapidly in the immature zone. Rapid loss of predefoliation C and N from the immature zone between d 0 and 2 was associated with rapid accumulation in the exposed zone of expanding leaves. The latter, however, was a transient feature with the masses of predefoliation C and N in the EX-AE decreasing after d 5. Conversely, continuous accumulation of predefoliation C and N was evident in both the EN-FE and EX-FE (as a result of tissue/organ flux, compare with Fig. 2). At d 8 most of the predefoliation C and N present in the regrowing tiller was contained in the expanded leaf zones (Figs. 8B and 9B).
Figure 8.
Masses of total C (A), predefoliation C (B), and postdefoliation C (C) in component zones of regrowing tillers of perennial ryegrass during the first 8 d of regrowth after severe defoliation: IM-AE, EX-AE, EN-FE, EX-FE, and TB. Leaves that had stopped to expand before defoliation are not included, because they did not form part of the regrowing tiller as defined here. For definition and designation of zones, see legends for Figures 1 and 2. Vertical bars indicate ±2 se (n = 16).
Since the immature zone had a strongly negative balance for predefoliation C and N, the accumulation of predefoliation C and N in the whole regrowing tiller between d 0 and 8 was much less than in its component mature zones (EX-AE plus EN-FE plus EX-FE; Figs. 8B and 9B). Accumulation of predefoliation C and N in the composite of mature zones (EX-AE plus EN-FE plus EX-FE) was 2.5 and 1.5 times the accumulation of predefoliation C and N in the entire regrowing tiller because of flux of predefoliation C and N from the immature to the mature zones (Figs. 8B and 9B).
Postdefoliation Biomass
Accumulation of postdefoliation-absorbed C and N was evident in all of the zones, but the distribution changed strongly with time (Figs. 8C and 9C). Initially, the bulk of the postdefoliation C and N was present in the IM-AE and EX-AE. However, because predefoliation C and N in these zones were exchanged by postdefoliation-absorbed C and N and their total masses changed little, the mass of postdefoliation C and N in the IM-AE and EX-AE increased relatively little after d 5. After d 5 the bulk of the postdefoliation C and N accumulation occurred in the zones of expanded leaves, which comprised an increasing proportion of the total regrowing tiller biomass (Figs. 8, A and C, and 9, A and C).
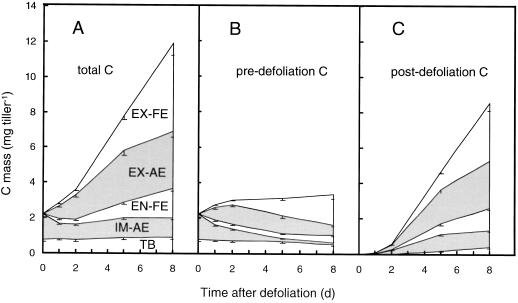
The Fractional Contribution of WSC-C to Tissue C
Except in the immature zone, WSC-C was a minor component of the total C in the different zones (Fig. 10). WSC-C accounted for 14% of the total C in the immature zone at defoliation. During d 1 the WSC fraction in the immature zone decreased rapidly to a level that was only 32% of that at defoliation. Subsequently, the WSC fraction in the immature zone increased gradually to and above the level at defoliation. In the exposed zones (i.e. the foliage) of both actively expanding and fully expanded leaves, WSC-C accounted for less than 8% of total tissue C throughout the 1st week after defoliation. In the EN-FE the fractional contribution of WSC to tissue C was slightly higher and increased substantially after d 5.
Figure 10.
Fractional contribution of WSC-C to the mass of C in functionally distinct zones of regrowing tillers of perennial ryegrass during the first 8 d after severe defoliation: immature zone of actively expanding leaves (IM-AE, ○), exposed zone of actively expanding leaves (EX-AE, ▾), enclosed zone of fully expanded leaves (EN-FE, ▿), and exposed zone of fully expanded leaves (EX-FE, ▪). For definition and designation of zones see Figures 1 and 2. Vertical bars indicate ±2 se (n = 16).
The concentration of starch in the different zones was not determined in the present experiment. However, earlier studies of the two genotypes showed low starch concentrations (<10% of total nonstructural carbohydrates) in leaves. Also, in studies of perennial ryegrass grown under conditions favoring nonstructural carbohydrate accumulation, starch was a minor component of the nonstructural carbohydrates (<15%) in both leaf blades and sheaths (Guerrand, 1997).
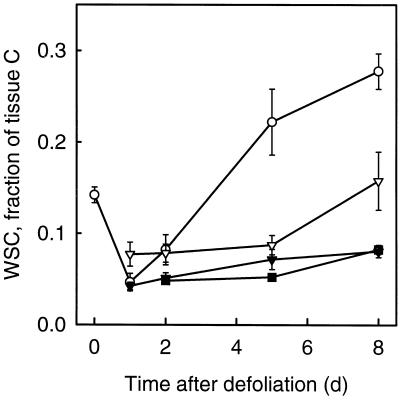
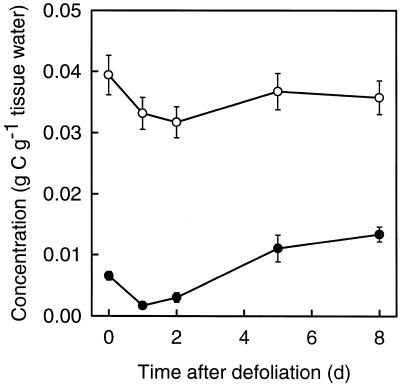
The Concentrations of WSC and Structural Biomass in the Immature Zone
In the immature zone the concentration (expressed as grams of C per gram of tissue water) of both WSC and structural biomass (estimated as total tissue C minus WSC-C, thus including protein C and possibly trace amounts of starch) decreased transiently after defoliation (Fig. 11). The WSC concentration was very low 1 and 2 d after defoliation but increased rapidly thereafter. In absolute terms the initial (0–1 d after defoliation) decrease in the concentration of structural biomass was even larger (−6.3 mg C g−1 tissue water) than the decrease in the WSC concentration (−4.9 mg C g−1 tissue water).
Figure 11.
Concentration of WSC-C (•) and structural C (○) in the IM-AE of actively expanding leaves of perennial ryegrass during the first 8 d after severe defoliation. Vertical bars indicate ±2 se (n = 16).
DISCUSSION
Zonal Heterogeneity in the Kinetics of Pre- and Postdefoliation C and N
Steady-state labeling of postdefoliation-absorbed C and N in combination with sequential sampling and separation of functionally distinct zones of the regrowing tiller demonstrated large zone-specific differences in the change over time of the fraction of postdefoliation C in total tissue C (Fig. 7A). The ranking of zones in terms of the increase over time in the fraction of postdefoliation C (IM-AE > EX-AE > EN-FE > EX-FE > TB) corresponded closely to the gradient of (mean) age of tissue present in the different zones (compare with Skinner and Nelson, 1994). Thus, at all sampling times the fraction of postdefoliation C in total C was lowest in the zones that contained the oldest tissue and highest when the tissue was youngest (immature zone).
Interpretation of these results requires a consideration of the factors determining labeling kinetics, i.e. the specific rates and isotopic composition of ”tissue fluxes” among zones of a tiller (Fig. 2) and ”substrate in- and efflux” (turnover) of the plant part studied. For instance, it is important to take into account that structural C (estimated as total tissue C minus WSC-C) is largely immobile and accounted for more than 90% of the C present in mature tissue zones (except for the enclosed zone of expanded leaves at d 8; compare with Fig. 10), in contrast to structural N (protein), which is highly mobile (turnover rate up to 10% d−1; Bouma et al., 1994). Structural biomass (including protein) is synthesized mainly when the leaf tissue is located in the immature zone (MacAdam and Nelson, 1987; Gastal and Nelson, 1994; Maurice, 1997). Accumulation of structural biomass in the fully expanded leaf category and in the EX-AE thus results from the tissue-bound efflux of structural C from the immature zone. The first tissue to be exposed above the defoliation level was (almost) fully differentiated at the time of defoliation and, hence, its structural biomass was largely synthesized before defoliation. The biomass in the immature zone is rapidly turned over (Schnyder and Nelson, 1989) and was rapidly depleted of predefoliation C (Fig. 7A). Accordingly, the structural biomass formed within and displaced from the immature zone after defoliation was progressively depleted of predefoliation C. Hence, although structural biomass accumulating in the zones of the fully expanded leaf category during the first days was mainly composed of predefoliation C, the structural biomass accumulating at later stages (and as a result of tissue flux mainly) was primarily composed of postdefoliation C, thus causing the apparent dilution of predefoliation C in the fully expanded leaf zones (Fig. 7A).
Another important aspect of zonal heterogeneity is the distinction between exposed leaf zones (foliage) and whole leaves or tillers. The total mass of predefoliation C in foliage (EX-AE plus EX-FE) increased significantly until d 5 after defoliation (Fig. 8B), although (reserve-derived) predefoliation C accumulation in the regrowing tiller was minimal after d 2 (Figs. 4A and 8B). This delayed accumulation of predefoliation C in foliage was related to the time needed for displacement of tissue from the immature zone to the (exposed) foliage zone (up to approximately 12 d; compare with Schnyder et al., 1990). These relationships also explain why investigation of predefoliation C and N accumulation in foliage overestimates both the duration of reserve utilization in regrowth and the contribution of reserves to refoliation: When assessed on the basis of the masses of predefoliation C and N present in foliage at d 8, the reserve-derived C and N accumulation in the entire regrowing tiller was overestimated by 102% and 55%, respectively (Figs. 8B and 9B). The rapid transition to current assimilation-driven growth observed in this study contrasts with findings from other studies, suggesting a major role of reserves for about 1 week (carbohydrates; Alberda, 1957; Gonzalez et al., 1989; Johansson, 1993) up to 2 weeks (N; Ourry et al., 1989; Thornton et al., 1993a, 1993b). In these studies reserve-derived substrate use in regrowth was inferred from the kinetics of reserve mobilization in the stubble (Alberda, 1957) or from the accumulation of predefoliation N (Thornton et al., 1993a, 1993b) or C (Johansson, 1993) in foliage. Mobilization gives no proof of the actual use of mobilized substrate in shoot regrowth, and accumulation of predefoliation C and N in foliage overestimates the duration and contribution of reserve use in regrowth due to neglect of tissue flux.
Differences in turnover rate (in- and efflux) of C and N in zones may also contribute to variation in labeling kinetics. In a steady-state labeling experiment with wheat, Gebbing et al. (1998) found no evidence for turnover of C in structural carbohydrates of fully expanded leaves. But WSC pools (Borland and Farrar, 1988) and proteins may be turned over rapidly by currently assimilated C and N. However, WSC turnover would not have a large effect on the C isotope composition of tissue in mature zones (EX-AE, EX-FE, EN-FE), since WSC was a minor component of tissue C (Fig. 10). However, the fraction of postdefoliation N in total N of the exposed and enclosed zones of leaves increased at very similar rates, although the tissue in these zones was formed at different times. This phenomenon was the same in the actively expanding and fully expanded leaf categories and cannot be explained only by tissue-bound efflux of N from the immature zone. The relatively high rates of protein turnover (Bouma et al., 1994), N cycling within plants (Larsson et al., 1991; Laine et al., 1994), and postemergence accumulation of postdefoliation-absorbed N in exposed tissue (Maurice, 1997) likely contributed to this effect. More research is needed to clarify further the relationships among tissue flux, substrate flux, and turnover of the different C and N pools in the functionally distinct zones and organs.
Control of Refoliation and Tiller Regrowth by Substrate Supply
Since photosynthesis and N uptake were severely depressed immediately after defoliation (R. de Visser and H. Schnyder, unpublished data), but all active shoot meristems and leaf growth zones were left behind intact, one should expect that initial refoliation was controlled by substrate supply. Indeed, the foliage production rate on d 1 and d 2 after defoliation was approximately 25% less than 1 week after defoliation (Fig. 3). This effect was related to a decreased substrate supply to expanding leaves as reflected in the decreased C and N accumulation rates in the regrowing tiller (Fig. 4) and in the negative C balance of the immature zone during the first 2 d after defoliation (Fig. 8A). The negative C balance of the immature zone on d 1 must have resulted from a higher tissue-bound efflux of C relative to the concurrent net influx of substrate C in this zone (Fig. 2). Several mechanisms contributed to rapid refoliation by an imbalance between efflux of tissue C and influx of C substrate in the immature zone on d 1: (a) a promotion of longitudinal relative to radial expansion (leading to an 18% decrease in the mass of tissue water contained in the immature zone, de Visser et al., 1997), (b) increased partitioning of imported C to synthesis of structural biomass relative to deposition of WSC, (c) mobilization of carbohydrates stored within the immature zone, and (d) a decreased rate of synthesis of structural biomass relative to growth-associated water influx into expanding tissue. The decreases in the fractional contribution of WSC to tissue C content (Fig. 10) and in the mass of WSC in the immature zone (−0.16 mg WSC-C) on d 1 were likely related to both decreased partitioning of imported carbohydrates toward WSC deposition and mobilization and use of the WSC, which were already present in the immature zone at defoliation. Dilution by growth-associated water uptake in expanding cells and consequent tissue-bound displacement of WSC to other zones (compare with Fig. 2) could also contribute to the decrease in the WSC content of the immature zone. However, the mass of WSC lost from the immature zone on d 1 was 3 times larger than the amount of WSC accumulated in the other leaf zones, demonstrating that mobilization of WSC was the main cause for WSC loss in the immature zone. Notably, however, the net loss of C from the immature zone on d 1 (−0.55 mg) was much larger than could be accounted for by the loss of WSC (−0.16 mg). This discrepancy was partially due to a dilution of structural C in the immature zone (Fig. 11), which resulted from decreased synthesis of structural biomass relative to growth-associated water influx into expanding cells. A similar dilution of structural biomass in growth zones of tall fescue leaves was observed during the dark period of diurnal cycles, when growth-associated water influx into expanding tissue was stimulated more than the synthesis of structural biomass (Schnyder and Nelson, 1988; Schnyder et al., 1988). All of these mechanisms contributed to decrease the C investment for leaf production and foliage exposure when C supply to the regrowing tiller was low immediately after defoliation. Conversely, the concentration of N in fresh mass of the immature zone did not change after defoliation (de Visser et al., 1997) and the total mass of N in the immature zone decreased much less than the mass of C (compare with Figs. 8A and 9A), showing that the balance between substrate import and tissue-bound efflux was much closer for N than for C.
Foliage production rate appeared to be related to C rather than N supply. Foliage production rate was least on d 2, 31% less than 1 week after defoliation (Fig. 3). Also, N (−43%) and C (−45%) accumulation rates in the regrowing tiller were significantly less than 1 week after defoliation (Fig. 4). Moreover, the WSC concentration was very low (Fig. 11), indicating no opportunity for carbohydrate mobilization in the immature zone on d 2. Also, the concentration of structural C was still low (Fig. 11), demonstrating that leaf production continued with reduced investments of C in the synthesis of structural biomass. The increase in foliage production after d 2 (Fig. 3) was associated with strong increases in the net rates of current assimilation-derived C and N accumulation in the regrowing tiller (Fig. 4) and increasing concentrations of structural C and WSC in the immature zone (Fig. 11). Whereas changes in WSC concentration in the immature zone closely paralleled the changes in foliage production rate (Figs. 3 and 11), such parallels did not exist for N (de Visser et al., 1997).
Preferential allocation of current assimilate to growing tiller tissue has repeatedly been indicted as a mechanism contributing to rapid refoliation (Richards, 1993). The present data indicate that such preferential allocation is related to a “priviledged” position of the immature zone relative to the main source of current photosynthate produced during the initial period after defoliation. The exposed parts of the actively expanding leaf category (EX-AE) made up more than 82% of the total foliage generated by the regrowing tillers until 2 d after defoliation (Fig. 8A). Current photosynthate and reduced N produced and exported by this tissue must pass the immature zone (Fig. 2), which is a strong sink for current photosynthate (Allard and Nelson, 1991) and reduced N (Gastal and Nelson, 1994). Rapid turnover of Suc in the immature zone by C fixed after defoliation (Morvan-Bertrand et al., 1999) may be related to this effect.
Control by C or N Substrate Supply?
Whereas the relationships discussed above support the view that refoliation was (at least partially) controlled by substrate supply, a question remaining is whether this control was exerted by C or N or both. Clearly, a balanced supply of carbohydrates and N is required for optimal growth. Extensive studies with a range of C3 species have consistently shown that during early growth stages growth is maximum and colimited by the availabilities of C and N, when the C:N (w/w) ratio in shoot biomass is approximately 8.6 (Lemaire and Gastal, 1997). This ratio is almost identical to the C:N ratio of 8.9 of tillers at defoliation and of the regrowing tillers 14 d after defoliation, indicating an optimal balance between the supplies of C and N during predefoliation undisturbed growth and after recovery from defoliation. The C:N ratio of 4.5 for total (i.e. reserve- plus current assimilation-derived) new biomass accumulating in the regrowing tiller on d 1 (Fig. 5A) suggests a relative shortage of C and/or relative abundance of N as the substrates for shoot growth. However, after d 1 the C:N ratio in total biomass accumulating in the regrowing tiller always ranged between 8.3 and 8.9 (Fig. 5), indicating that, although substrate supply likely controlled growth, the C:N ratio in the substrate available for growth was near optimal balance. Thus, at least after d 1, tiller regrowth appeared to be colimited by the supplies of C and N.
Role of Reserves Versus Current Assimilates
Transition from reserve- to current assimilation-dependent growth was much faster than was suggested in studies in which steady-state labeling was not used to differentiate between the fluxes of reserve- and current assimilation-derived C and N to the regrowing shoot or in which the functional heterogeneity of the grass stubble left behind after defoliation was not recognized (see above). Reserve-derived biomass accumulating in the regrowing tiller had a low C:N ratio (Fig. 5), supporting earlier evidence that much of the reserve-derived C accumulation in regrowing tillers was in the form of amino-C (Avice et al., 1996; de Visser et al., 1997). Using the assumptions that (a) all predefoliation N was imported in the form of amino acids having a mean C:N (w/w) ratio of 2.6 (Fisher and Macnicol, 1986) and (b) that the C associated with the reserve-derived N was all predefoliation C, we estimated that the import of amino-C accounted for 50% and 75% of the reserve-derived C accumulation in the regrowing tiller on d 1 and 2 after defoliation (Table II). Since postdefoliation fixed C was the main C source already on d 2 (Fig. 4A) and amino-C was the main component of reserve-derived C accumulation, it was highly unlikely that reserve-derived carbohydrates were a significant source of substrate for tiller biomass after d 1 (Table II). However, these carbohydrates may have contributed indirectly to tiller regrowth via energy supply in shoot respiration and maintenance of root integrity and function.
Table II.
Estimated contributions of predefoliation reserves to accumulation of carbohydrate- and amino-C in regrowing tillers of perennial ryegrass on d 1 and 2 after severe defoliation
| Parameter | d 1
|
d 2
|
||
|---|---|---|---|---|
| Amino-C | CHO-C | Amino-C | CHO-C | |
| Accumulation rate of predefoliation C (mg tiller−1 d−1) | 0.27 | 0.27 | 0.21 | 0.07 |
| Fraction of predefoliation C accumulation | 0.50 | 0.50 | 0.75 | 0.25 |
| Fraction of total (i.e. pre- plus postdefoliation) CHO-C accumulation | — | 1.01 | — | 0.13 |
Predefoliation amino-C accumulation rate was calculated as accumulation rate of predefoliation N (Fig. 4B) times 2.6 (see text). The accumulation rate of reserve-derived carbohydrate-C (CHO-C) was estimated as the accumulation rate of predefoliation C (Fig. 4A) minus the accumulation rate of predefoliation amino-C, as defined above.
The strong decrease in accumulation of postdefoliation-absorbed N between d 1 and 2 (Fig. 4B) was a significant factor contributing to the increased C:N ratio of postdefoliation-absorbed biomass accumulating in the regrowing tiller during d 2 (Fig. 5). Jarvis and Macduff (1989) and MacDuff et al. (1989) already noted that defoliation entailed an inhibition of N uptake in perennial ryegrass plants, which were well supplied with N before defoliation. However, inhibition of nitrate uptake occurred with some delay, the rate declining over a period of 15 h after defoliation. The present data show that this lag in the response of root function had a direct effect on the supply of postdefoliation-absorbed N to the regrowing tiller. However, as was indicated by the near-optimal C:N ratio of total (reserve- plus current assimilation-derived) new biomass accumulating in the regrowing tiller, decreased supply of current uptake-derived N on d 2 was compensated by the sustained supply of reserve-derived N (Fig. 5).
Reserve-derived N accumulation in the regrowing tiller was sustained over a longer period than was the influx of reserve-derived carbohydrates (Fig. 4; Table II). As a consequence, the C:N ratio in reserve-derived biomass accumulating in the regrowing tiller decreased strongly over time (Fig. 5). This occurred while the C:N ratio in total (i.e. reserve- and current assimilation-derived) biomass accumulating in the regrowing tiller was almost constant at approximately 8.6 after d 1. Thus, although the C:N ratio in reserve- and current assimilation-derived biomass diverged strongly, the two sources were highly complementary in terms of providing a substrate with an optimal C and N composition. To our knowledge, such an effect has not been observed before. It may reflect the operation of a mechanism working at the plant level and balancing the fluxes of reserve- and current assimilation-derived C and N among the regrowing shoot and the root system. Such a mechanism may contribute to minimize trade-offs in resource allocation (root versus shoot growth, maintenance, and function) in the face of limited availability of both substrates. The nature of the putative mechanism is not clear, but one hypothesis is that substrate supply to sinks is demand driven, involving chemical signaling (sugars: Frommer and Sonnewald [1995], Farrar [1996], Koch [1996]; and/or plant growth regulators (hormones): Jackson [1993], Munns and Cramer [1996] and Van der Werf and Nagel [1996]). Also, the vascular architecture of the plant (compare with the position of the immature zone relative to current assimilate supply) may be involved.
In conclusion, the present study yields evidence for an important role of several mechanisms in facilitating rapid refoliation in a defoliation-tolerant grass species: (a) reduced C investment for leaf expansion associated with both ”spatial” (transient promotion of longitudinal relative to radial leaf expansion, de Visser et al., 1997) and ”chemical dilution” (reduced C investment per unit volumetric leaf expansion, i.e. transient dilution of structural biomass in immature zones), (b) utilization in regrowth of WSC, which were already present in the immature zone at defoliation, (c) rapid transition to current photosynthate (carbohydrate)-driven growth (which may be partially related to a priviledged position of the immature zone of expanding leaves relative to the main path of current photosynthate produced during initial refoliation), (d) transient import of mobilized assimilate (mainly N), and (e) rapid reestablishment of a balanced C:N ratio in the new biomass produced in the regrowing tiller resulting from complementarity in the supply of reserve- and current assimilation-derived C and N. Rapid refoliation was likely due to a high sink capacity and strength of the regrowing tiller, since none of the expanding leaf tissue and shoot meristems were removed by defoliation. Further research with a range of species differing in defoliation tolerance and grown in contrasting conditions is needed to assess the relative importance of each of the above mechanisms in conferring or limiting tolerance to defoliation.
ACKNOWLEDGMENTS
We wish to thank Prof. W. Kühbauch (Universität Bonn, Germany) and Siebe van de Geijn (AB-DLO, Wageningen, The Netherlands) for continued support and Thomas Gebbing, Elisabeth Huber-Sannwald, Markus Lötscher, and Rudi Schäufele (all at Technische Universität München, Germany) for valuable suggestions concerning the manuscript. Hanno Vianden, Sandra Beck, Ludwig Schmitz (all at Universität Bonn), Riet de Kock (AB-DLO), and Brigitte Schilling (Technische Universität München) provided invaluable technical assistance.
Abbreviations:
- EN-FE
EX-FE, enclosed and exposed zones of leaves that had reached full expansion by the time of sampling but had experienced growth after defoliation, respectively
- EX-AE
IM-AE, exposed and immature (enclosed) zones of actively expanding leaves, respectively
- TB
tiller base zone
- WSC
water-soluble carbohydrate(s)
Footnotes
This work was supported by the Commission of the European Communities, Directorate General VI for Agriculture, Divison for the Coordination of Agricultural Research, Brussels, Belgium (grant no. GT920078 to R.d.V.), and the Deutsche Forschungsgemeinschaft (grant no. SFB 607).
LITERATURE CITED
- Alberda T. The effect of cutting, light intensity and night temperature on growth and soluble carbohydrate content of Lolium perenne. Plant Soil. 1957;8:199–230. [Google Scholar]
- Allard G, Nelson CJ. Photosynthate partitioning in basal zones of tall fescue leaf blades. Plant Physiol. 1991;95:663–668. doi: 10.1104/pp.95.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avice JC, Ourry A, Lemaire G, Boucaud J. Nitrogen and carbon flows estimated by 15N and 13C pulse-chase labeling during regrowth of alfalfa. Plant Physiol. 1996;112:281–290. doi: 10.1104/pp.112.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot J, Lefevre J, Boucaud J. Changes in the amide and amino acid composition of xylem exudate from perennial ryegrass (Lolium perenne L.) during regrowth after defoliation. Plant Soil. 1991;136:59–64. [Google Scholar]
- Boffey SA, Selldén G, Leech RM. Influence of cell age on chlorophyll formation in light-grown and etiolated wheat seedlings. Plant Physiol. 1980;65:680–684. doi: 10.1104/pp.65.4.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland AM, Farrar JF. Compartmentation and fluxes of carbon in leaf blades and leaf sheaths of Poa annua L. and Poa × jemtlandica (Almq.) Richt. Plant Cell Environ. 1988;11:535–543. [Google Scholar]
- Bouma TJ, de Visser R, Janssen JHJA, De Kock MJ, Van Leeuwen PH, Lambers H. Respiratory energy requirements and rate of protein turnover in vivo determined by the use of an inhibitor of protein synthesis and a probe to assess its effect. Physiol Plant. 1994;92:585–594. [Google Scholar]
- Davidson JL, Milthorpe FL. Leaf growth in Dactylis glomerata following defoliation. Ann Bot. 1966a;30:173–184. [Google Scholar]
- Davidson JL, Milthorpe FL. The effect of defoliation on the carbon balance in Dactylis glomerata. Ann Bot. 1966b;30:185–198. [Google Scholar]
- Dean C, Leech RM. Genome expression during normal leaf development. I. Cellular and chloroplast numbers and DNA, RNA, and protein levels in tissues of different ages within a seven-day-old wheat leaf. Plant Physiol. 1982;69:904–910. doi: 10.1104/pp.69.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deléens E, Pavlidès D, Queiroz O. Application du tracage isotopique naturel par le 13C à la mesure du renouvellement de la matière foliaire chez les plantes en C3. Physiol Veg. 1983;21:723–729. [Google Scholar]
- de Visser R, Vianden H, Schnyder H. Kinetics and relative significance of remobilized and current C and N incorporation in leaf and root growth zones of Lolium perenne after defoliation. Assessment by 13C and 15N steady-state labeling. Plant Cell Environ. 1997;20:37–46. [Google Scholar]
- Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol. 1984;11:539–552. [Google Scholar]
- Farrar JF. Regulation of root weight ratio is mediated by sucrose: opinion. Plant Soil. 1996;185:13–19. [Google Scholar]
- Fisher DB, Macnicol PK. Amino acid composition along the transport pathway during grain filling in wheat. Plant Physiol. 1986;82:1019–1023. doi: 10.1104/pp.82.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer WB, Sonnewald U. Molecular analysis of carbon partitioning in solanaceous species. J Exp Bot. 1995;46:587–607. [Google Scholar]
- Gastal F, Nelson CJ. Nitrogen use within the growing leaf blade of tall fescue. Plant Physiol. 1994;105:191–197. doi: 10.1104/pp.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbing T, Schnyder H, Kühbauch W. Carbon mobilization in shoot parts and roots of wheat during grain filling: assessment by 13C/12C steady-state labeling, growth analysis and balance sheets of reserves. Plant Cell Environ. 1998;21:301–313. [Google Scholar]
- Gonzalez B, Boucaud J, Salette J, Langlois J, Duyme M. Changes in stubble carbohydrate content during regrowth of defoliated perennial ryegrass (Lolium perenne L.) on two nitrogen levels. Grass Forage Sci. 1989;44:411–415. [Google Scholar]
- Guerrand D (1997) Étude des voies de synthèse des fructanes chez Lolium perenne L. Doctoral thesis. University of Caen, France
- Jackson MB. Are plant hormones involved in root to shoot communication? Adv Bot Res. 1993;19:104–187. [Google Scholar]
- Jarvis SC, Macduff JH. Nitrate nutrition of grasses from steady-state supplies in flowing solution culture following nitrate deprivation and/or defoliation. I. Recovery of uptake and growth and their interactions. J Exp Bot. 1989;40:965–975. [Google Scholar]
- Johansson G. Carbon distribution in grass (Festuca pratensis L.) during regrowth after cutting—utilisation of stored and newly assimilated carbon. Plant Soil. 1993;15:11–20. [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Laine P, Bigot J, Ourry A, Boucaud J. Effects of low temperature on nitrate uptake, and xylem and phloem flows of nitrogen, in Secale cereale L. and Brassica napus L. New Phytol. 1994;127:675–683. doi: 10.1111/j.1469-8137.1994.tb02970.x. [DOI] [PubMed] [Google Scholar]
- Larsson CM, Larsson M, Purves JV, Clarkson DT. Translocation and cycling through roots of recently absorbed nitrogen and sulphur in wheat (Triticum aestivum) during vegetative and generative growth. Physiol Plant. 1991;82:345–352. [Google Scholar]
- Lemaire G, Gastal F. N uptake and distribution in plant canopies. In: Lemaire G, editor. Diagnosis of the Nitrogen Status in Crops. Berlin: Springer; 1997. pp. 3–43. [Google Scholar]
- MacAdam JW, Nelson CJ. Specific leaf weight in zones of cell division, elongation and maturation in tall fescue leaf blades. Ann Bot. 1987;59:369–376. [Google Scholar]
- MacAdam JW, Volenec JJ, Nelson CJ. Effects of nitrogen on mesophyll cell division and epidermal cell elongation in tall fescue leaf blades. Plant Physiol. 1989;89:549–556. doi: 10.1104/pp.89.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macduff JH, Jarvis SC, Mosquera A. Nitrate nutrition of grasses from steady-state supplies in flowing solution culture following nitrate deprivation and/or defoliation. II. Assimilation of NO3− and short-term effects on NO3− uptake. J Exp Bot. 1989;40:977–984. [Google Scholar]
- Maurice I (1997) Mise en place du volume et dépôt de composés carbonés et azotés au cours de la croissance de la feuille de fétuque élevée (Festuca arundinacea Schreb.). Doctoral thesis. Institut National Polytechnique de Lorraine, Nancy-Metz, France
- Morvan A, Challe G, Prud'homme MP, Le Saos J, Boucaud J. Rise of fructan exohydrolase activity in stubble of Lolium perenne after defoliation is decreased by uniconazole, an inhibitor of the biosynthesis of gibberellins. New Phytol. 1997;136:81–88. [Google Scholar]
- Morvan-Bertrand A, Pavis N, Boucaud J, Prud'homme MP (1999) Partitioning of reserve and new assimilated carbon in roots and leaf tissues of Lolium perenne during regrowth after defoliation: assessment by steady-state labelling and carbohydrate analysis. Plant Cell Environ (in press)
- Munns RE, Cramer G. Is coordination of leaf and root growth mediated by abscisic acid? Opinion. Plant Soil. 1996;185:33–49. [Google Scholar]
- Ourry A, Bigot J, Boucaud J. Protein mobilization from stubble and roots, and proteolytic activities during post-clipping re-growth of perennial ryegrass. J Plant Physiol. 1989;134:298–303. [Google Scholar]
- Ourry A, Kim TH, Boucaud J. Nitrogen reserve mobilization during regrowth of Medicago sativa L. Relationships between availability and regrowth yield. Plant Physiol. 1994;105:831–837. doi: 10.1104/pp.105.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme MP, Gonzalez B, Billard JP, Boucaud J. Carbohydrate content, fructan and sucrose enzyme activities in roots, stubble and leaves of ryegrass (Lolium perenne L.) as affected by source/sink modification after cutting. J Plant Physiol. 1992;140:282–292. [Google Scholar]
- Richards JH (1993) Physiology of plants recovering from defoliation. In MJ Baker, ed, Grasslands for Our World. SIR Publishing, Wellington, New Zealand, pp 46–54
- Schnyder H. Long-term steady-state labeling of wheat plants by use of natural 13CO2/12CO2 mixtures in an open, rapidly turned-over system. Planta. 1992;187:128–135. doi: 10.1007/BF00201634. [DOI] [PubMed] [Google Scholar]
- Schnyder H, Nelson CJ. Diurnal growth of tall fescue leaf blades. I. Spatial distribution of growth, deposition of water, and assimilate import in the elongation zone. Plant Physiol. 1988;86:1070–1076. doi: 10.1104/pp.86.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder H, Nelson CJ. Growth rates and assimilate partitioning in the elongation zone of tall fescue leaf blades at high and low irradiance. Plant Physiol. 1989;90:1201–1206. doi: 10.1104/pp.90.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder H, Nelson CJ, Spollen WG. Diurnal growth of tall fescue leaf blades. II. Dry matter partitioning and carbohydrate metabolism in the elongation zone and adjacent expanded tissue. Plant Physiol. 1988;86:1077–1083. doi: 10.1104/pp.86.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder H, Seo S, Rademacher IF, Kühbauch W. Spatial distribution of growth rates and of epidermal cell lengths in the elongation zone during leaf development in Lolium perenne L. Planta. 1990;181:423–431. doi: 10.1007/BF00195897. [DOI] [PubMed] [Google Scholar]
- Skinner RH, Nelson CJ. Epidermal cell division and the coordination of leaf and tiller development. Ann Bot. 1994;74:9–15. doi: 10.1093/aob/74.1.9. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sprague VG. Composition of the roots and stubble of perennial rye grass following partial defoliation to 1.5 in. Plant Physiol. 1943;18:656–670. doi: 10.1104/pp.18.4.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton B, Millard P, Duff EI, Buckland ST. The relative contribution of remobilization and root uptake in supplying nitrogen after defoliation for regrowth of laminae in four grass species. New Phytol. 1993a;124:689–694. doi: 10.1111/j.1469-8137.1993.tb03859.x. [DOI] [PubMed] [Google Scholar]
- Thornton B, Millard P, Galloway S. The effects of temperature and form of nitrogen supply on the relative contribution of root uptake and remobilization in supplying nitrogen for laminae regrowth of Lolium perenne L. J Exp Bot. 1993b;44:1601–1606. [Google Scholar]
- Van der Werf A, Nagel OW. Carbon allocation to shoots and roots in relation to nitrogen supply is mediated by cytokinins and sucrose: opinion. Plant Soil. 1996;185:21–32. [Google Scholar]
- Volenec JJ. Nonstructural carbohydrates in stem base components of tall fescue during regrowth. Crop Sci. 1986;26:122–127. [Google Scholar]
- Volenec JJ, Nelson CJ. Cell dynamics in leaf meristems of contrasting tall fescue genotypes. Crop Sci. 1981;21:381–385. [Google Scholar]
- Volenec JJ, Ourry A, Joern BC. A role for nitrogen reserves in forage regrowth and stress tolerance. Physiol Plant. 1996;97:185–193. [Google Scholar]
- Wilhelm WW, Nelson CJ. Growth analysis of tall fescue genotypes differing in yield and leaf photosynthesis. Crop Sci. 1978;18:951–954. [Google Scholar]
- Wolf DD, Ellmore TL. Automated hydrolysis of nonreducing sugars and fructosans from plant tissue. Crop Sci. 1975;15:775–777. [Google Scholar]