Abstract
WNT signaling has been shown to influence the development of the heart. Although recent data suggested that canonical WNTs promote the emergence and expansion of cardiac progenitors in the pregastrula embryo, it has long been accepted that once gastrulation begins, canonical WNT signaling needs to be suppressed for cardiac development to proceed. Yet, this latter supposition appears to be odds with the expression of multiple canonical WNTs in the developing heart. The present study examining the effect of ectopic canonical WNT signaling on cardiogenesis in the developing frog was designed to test the hypothesis that heart formation is dependent on the inhibition of canonical WNT activity at the onset of gastrulation. Here we report that cardiac differentiation of explanted precardiac tissue from the dorsal marginal zone was not suppressed by exposure to WNT1 protein, although expression of Tbx5, Tbx20, and Nkx2.5 was selectively reduced. Pharmacological activation of WNT signaling in intact embryos using the GSK3 inhibitor SB415286 did not prevent the formation of an anatomically normal and functionally sound heart, with the only defect observed being lower levels of the cardiac transcription factor Nkx2.5. In both the explant and whole embryo studies, expression of muscle genes and proteins was unaffected by ectopic canonical WNT signaling. In contrast, canonical Wnt signaling upregulated expression of the cardiac stem cell marker c-kit and pluripotency genes Oct25 and Oct60. However, this regulatory stimulation of stem cells did not come at the expense of blocking cardiac progenitors from differentiating.
Introduction
WNTs comprise a large family of secreted signaling proteins that regulate pattern formation in the developing embryo and control stem cell behavior [1–7]. WNTs are commonly separated into 2 groups based on their activation of disparate signal transduction pathways. Signaling by canonical WNTs, such as WNT1 and WNT3A, results from their inhibition of GSK3β, which in turns allows for β-catenin-mediated transcriptional activity. Conversely, other WNT proteins, such as WNT5a and WNT11, act primarily through noncanonical pathways that are GSK3β and β-catenin independent.
The importance of WNT signaling for the formation and development of the heart is a topic that has garnered a great deal of research attention [3,4,8–16]. Despite this scientific scrutiny, the role WNT signaling plays in facilitating cardiac development is still unclear. WNTs were initially implicated as regulators of cardiogenesis from studies in Drosophila, which showed that heart formation was dependent on canonical WNT signaling [17]. In vertebrates, the primary WNT protein that plays a similar positive role in promoting the development of the heart is WNT11 [8,9,18], which paradoxically mediates its activity through noncanonical WNT pathways [3,9,19]. In contrast, canonical WNTs have been proposed to play an inhibitory role in the formation of the vertebrate heart [16,20], which is an idea whose primary support arose from in vitro studies with precardiac tissue from the developing frog [21,22]. Yet, WNT regulation of cardiogenesis may not be so clear cut, as investigations with embryonic stem cells have indicated that both canonical and noncanonical WNTs promote cardiac differentiation [23–28]. Recent studies suggested that canonical WNT regulation of cardiac differentiation may be phasic, either promoting or inhibiting cardiogenesis, depending on the stage in development [12,13,16,26,29,30].
Canonical WNTs have also received much interest due to their well-characterized role as stem cell maintenance factors [31,32]. These molecules are able to stimulate stem/progenitor cell proliferation, and preserve and/or enhance expression of pluripotency genes, such as nanog and Oct4, in receptive cell populations [33,34]. The function of canonical WNTs as stem cell regulators has led to the proposal that expansion of cardiac progenitors in the pregastrula embryo requires canonical WNT activity, which subsequently needs to be suppressed to permit cardiomyogenic differentiation at the onset of gastrulation [12,16,23,25,26]. Yet, not all published reports have provided support for this working hypothesis [28,35–37]. For example, we were unable to confirm that canonical WNTs suppress cardiomyogenic differentiation in experimentation using explanted precardiac tissue from the chick embryo [4]. To further examine the functional role of canonical WNT signaling in cardiac development, we chose to use Xenopus laevis as the animal model, since studies with the developing frog have provided the strongest experimental support for the negative role these molecules are thought to play in heart formation [20–22]. In this study, the developing frog embryo was used as a model to examine the impact of canonical WNT regulation on the development of the heart in situ. Specifically, we tested whether (a) ectopic canonical WNT signaling is able to suppress heart formation, (b) canonical WNT inhibition of cardiac development is stage dependent, and (c) there is a corresponding canonical WNT mediated stem cell expansion within the developing heart.
Materials and Methods
Embryo culture and treatments
Frog embryos were obtained using standard procedures [38]. Mature eggs were produced by injection of Xenopus laevis females with 500 U human gonadotropin (Sigma) to induce ovulation. Eggs were fertilized in vitro in 1% modified Barth's solution (MBS), dejellied in 2% cysteine, pH 7.8, and reared in 0.1% MBS. Embryonic stages were classified according to standards established by Nieuwkoop and Faber [39]. Embryos displaying a dorsal blastoporal groove, but not exhibiting cellular involution on the ventral side, were identified as stage 10.25, as previously designated [40,41]. Embryos of this stage were obtained by incubation at room temperature for ∼10 h postfertilization. The GSK3β inhibitor SB415286 and PI3K inhibitor LY294002 were obtained from Tocris Bioscience and Sigma-Aldrich, respectively. Embryos were immersed in the appropriate dose of these chemical reagents in 0.1×MBS for the time of exposure, followed by several rinses in 0.1×MBS and incubated in 0.1×MBS until desired stages were attained. Control embryos were subjected to the same series of washes and media changes without addition of chemical reagents.
Microinjection of synthetic RNA
The Δβ-catenin cDNA expression vector, which generates a constitutively active form of β-catenin, has been described in detail previously [42]. RNA was transcribed with the mMessage mMachine kit (Ambion) following the manufacturer's protocols. RNA was pressure-injected equatorially into the 2 dorsal blastomeres at the 4-cell stage, as described [9].
Microdissection and explant culture
Dorsal marginal zone (DMZ) tissue was isolated from stage 10.25 Xenopus laevis by dissection with an eyelash knife. After dissections in 0.5×MBS, explanted tissue was placed in fresh 0.5×MBS containing 1×penicillin/streptomycin (Sigma) and cultured at room temperature in Nunc 4-well dishes precoated with 2% sterile agarose. DMZ tissue was exposed to WNT1 protein (PeproTech) immediately after harvesting from the embryo. RNA used for examining expression of cardiac transcription factors and muscle proteins, respectively, was isolated from explants harvested on the second or fifth day of culture, which corresponded to stages 30 and 42 of sibling embryos incubated in parallel. For chemical treatments, DMZ tissue was washed several times in 0.5×MBS after time of exposure and then incubated further in fresh media.
Immunofluorescent staining
Immunofluorescent labeling was performed using previously described protocols [4,8,43]. Cultures and whole embryos were methanol fixed, and then exposed to sarcomeric myosin heavy chain-specific antibody (MF20) obtained from the Developmental Studies Hybridoma Bank at The University of Iowa, Iowa City, IA. Immunostaining was observed following incubation with fluorescein-labeled secondary antibody (Jackson ImmunoResearch Laboratories). For whole embryo immunostaining, the ventral dermis overlaying the developing heart was carefully removed before adding primary antibody, which allowed antibody to fully penetrate the tissue.
RNA isolation and polymerase chain reaction amplification
Cultures and excised embryonic tissue were placed in RNAlater (Ambion) immediately after harvesting. Afterward, RNA was isolated using RNeasy kits (Qiagen), and reverse-transcribed using High Capacity cDNA Reverse Transcription Kit (Applied BioSystems). The cDNA was then preamplified with TaqMan PreAmp Master Mix (Applied BioSystems) using 180 nM of forward and reverse gene-specific primers. Comparative quantitative polymerase chain reaction (PCR) analysis was performed with the StepOne plus qPCR system (Applied BioSystems) using TaqMan qPCR Master Mix (Applied BioSystems). Primer pairs and probes used in this study, which are provided in the Supplementary Table S1 (Supplementary Data are available online at www.liebertonline.com/scd), were specific for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), elongation factor 1α (EF1α), siamois, islet1, GATA6, Tbx5, Tbx20, Nkx2.5, cardiac α actin (Actc), cardiac myosin heavy chain-α (cMHCα), cardiac troponin I (cTnI), Oct25, Oct60, and c-Kit. Relative gene expression levels were estimated by ΔΔCt method using 2 housekeeping genes: GAPDH and EF1α. Comparisons between groups of 2 were determined by Student's t-test and multiple groups with Bonferroni-corrected ANOVA testing, as calculated using the InStat statistical application (Graphpad Software). Statistical significance was defined at a value of P≤0.05.
Canonical WNT assays
Analysis of DMZ tissue for expression of the WNT target gene siamois [44,45] and transfected cells for TOPflash TCF reporter activity [46,47] were used as methods for measuring canonical WNT signaling. For the latter experiments, the TCF binding enhancer from the TOPflash and its mutated nonfunctional variant FOPflash plasmids (Upstate Biotechnology) were ligated into the GFP reporter pGlow-TOPO (Invitrogen). Relative canonical WNT activity was examined using QCE6 cells as reporter cell line, which were transfected with TOPflash-GFP and FOPflash-GFP using previously described protocols [18]. Transfected cells were plated into chamber slides and cultured in the absence or presence of WNT1 or SB415286. After 2 days, cells were immunostained with anti-GFP antibody (Invitrogen), counterstained with the nuclear stain DAPI, and imaged at 20×by confocal microscopy. GFP-positive cells and total cell numbers (DAPI-labeled cells) from 2 independent experiments were manually counted using ImageJ software and the Cell Counter plug-in (Kurt De Vos, University of Sheffield; kurt.devos@iop.kcl.ac.uk), to determine percentage of cells that expressed TOPflash-GFP and and FOPflash-GFP reporters.
Results
Ectopic activation of canonical WNT signaling in the early blastula prevents heart formation in the Xenopus embryo
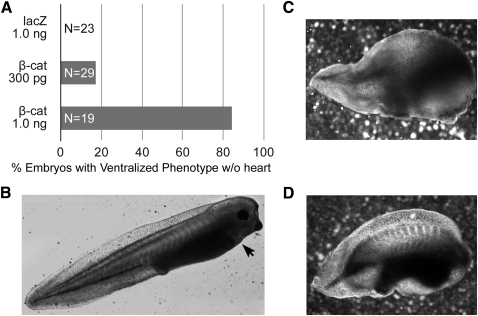
Previous studies examining WNT regulation of cardiac development in Xenopus used a standard assay that follows the differentiation in culture of DMZ tissue, where cardiac progenitors become localized to the mesoderm [9,22,48]. For activating canonical WNT signaling on the dorsal side, embryos were injected with WNT mRNA into the 2 dorsal blastomeres at the 4-cell stage, which decreased prevalence of cardiac gene expression in explants harvested from the embryo at the onset of gastrulation (stage 10.25) [22,48]. In our first series of experiments, we extended those experiments by determining the effect of ectopic canonical WNT signaling on heart formation when embryos were allowed to develop intact. Activation of this pathway was accomplished by microinjecting mRNA encoding for a constitutively active form of β-catenin, which promotes canonical WNT signal transduction independently of the upstream WNT signal [42]. As controls, embryos were injected with lacZ mRNA. The injection of 300 pg and 1 ng of constitutively active β-catenin mRNA produced a severe ventralized phenotype without a heart in >17% and >84% of the embryos, respectively (Fig. 1A). In comparison, control embryos injected with 1 ng lacZ mRNA developed normally (Fig. 1A, B). Although these data could be interpreted as confirming canonical WNT suppression of heart development, it should be noted that a considerable amount of development occurs from the time RNA is introduced into the early blastula to the beginnings of gastrulation when nondifferentiated mesoderm starts to coalesce into heart-forming regions. Since it is obvious that the β-catenin-injected embryos suffered from abnormalities far greater than just the absence of a heart (Fig. 1C, D), these experiments could not exclude the possibility of disrupted cardiac development being a byproduct of the global maldevelopment that resulted from aberrant WNT signal transduction. Accordingly, we readjusted the experimental protocol in the remainder of the study to precisely time ectopic canonical WNT signal transduction to the onset of gastrulation, when cardiac progenitors first localize to the mesoderm.
FIG. 1.
Ectopic activation of canonical WNT signaling in the early blastula. (A) The indicated mRNA was injected into both dorsal blastomeres at the 4-cell stage, and allowed to develop until stage 40 when they were scored for embryonic phenotype and heart formation. Injection of control lacZ RNA did not disrupt development, as normal embryos developed. Approximately 17% and 84% of embryos injected with 300 pg or 1.0 ng of constitutively active β-catenin, respectively, exhibited a ventralized embryonic phenotype without a heart. (B) Representative lacZ-injected embryo, with the arrow showing the position of the contractile heart. (C, D) Examples of β-catenin-injected embryos that displayed a heart-negative phenotype.
Treatment of DMZ explants with WNT protein
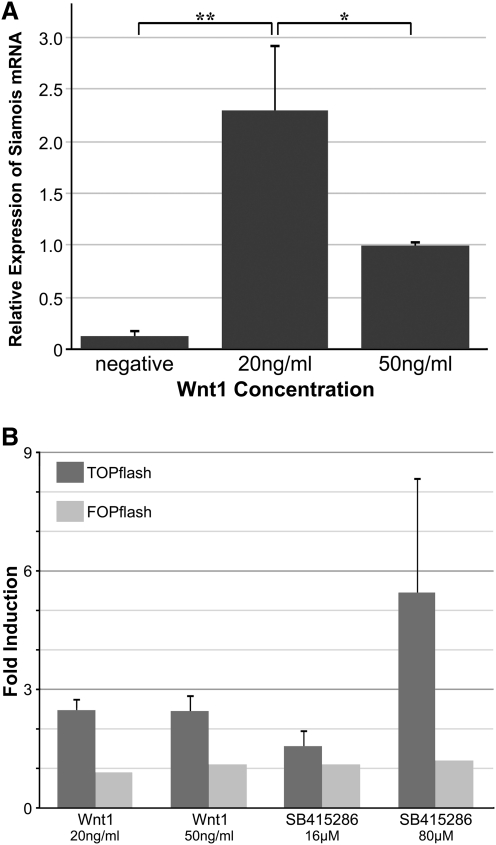
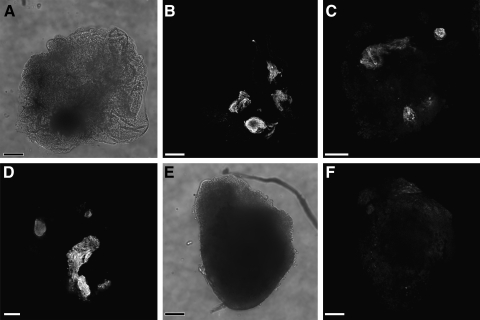
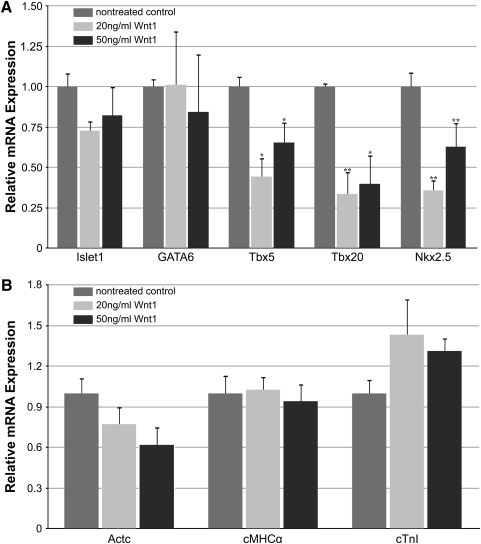
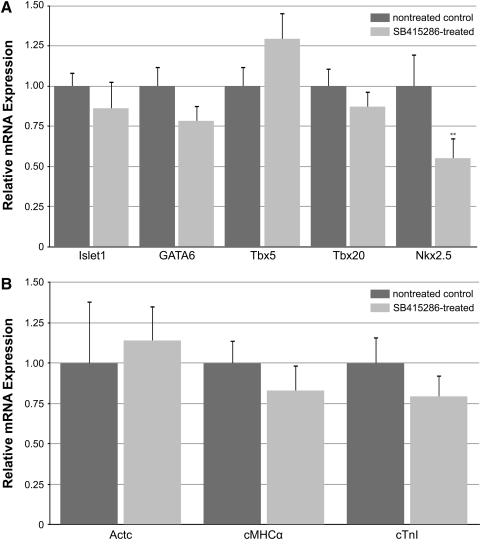
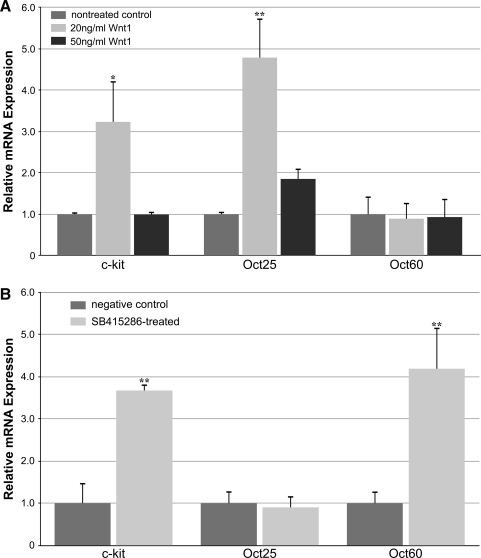
To test directly the effect of canonical WNT signaling on precardiac progenitors, we treated stage 10.25 DMZ explants with WNT1 protein. Concentrations were optimized using the upregulation of the WNT target gene siamois as guideline [44,45,49]. Thus, it was determined that the optimal working concentration of WNT1 was 20 ng/mL, as higher amounts of the protein resulted in a lessened induction of siamois expression (Fig. 2A). Canonical WNT activity of these working concentrations of WNT1 was confirmed using the standard TOPflash reporter assay (Fig. 2B). Differentiation of DMZ tissue treated with 20 ng/mL or 50 ng/mL WNT1 did not appear to differ from nontreated controls, as explants cultured under these various conditions exhibited similar patterns of sarcomeric myosin immunoreactivity (Fig. 3A–D). As a comparison, myocyte differentiation in DMZ explants was blocked by inhibition of phosphatidylinositol 3-kinase (PI3K) (Fig. 3E, F), whose activity is required for myocardial development [50,51]. Cardiomyogenic differentiation of WNT1-treated tissue was confirmed by gene expression analysis (Fig. 4). Transcription of Islet1, which is a cardiac progenitor cell marker that maps to both the primary and secondary heart fields in Xenopus [52], was unaffected by canonical Wnt exposure (Fig. 4A). Levels of the cardiac transcriptional factor GATA6 were likewise unchanged by WNT1 treatment (Fig. 4A). However, expression of the cardiac-associated transcription factors Tbx5, Tbx20, and Nkx2.5 were significantly downregulated in response to WNT1 (Fig. 4A). Yet, surprisingly, RNA levels for the cardiac-specific muscle proteins cardiac α actin (Actc), cardiac myosin heavy chain-α (cMHCα), and cardiac troponin I (cTnI) were equivalent in nontreated and WNT1-treated DMZ tissue (Fig. 4B).
FIG. 2.
Canonical WNT activity. (A) Induction of siamois was measured in dorsal marginal zone (DMZ) tissue cultured in the absence or presence of the indicated WNT1 concentration before RNA isolation and polymerase chain reaction (PCR) analysis during day 2 of culture. Highest levels of the WNT target gene siamois were obtained with 20 ng/mL WNT1. **P values between groups was <0.01. *P values between groups was <0.05. (B) Canonical WNT activity of WNT1 protein and the GSK3 inhibitor SB415286 was tested using QCE6 cells transfected with TOPflash-GFP and FOPflash-GFP reporter plasmids. The results are presented as fold-induction over nontreated control, with error bars corresponding to standard error of the mean.
FIG. 3.
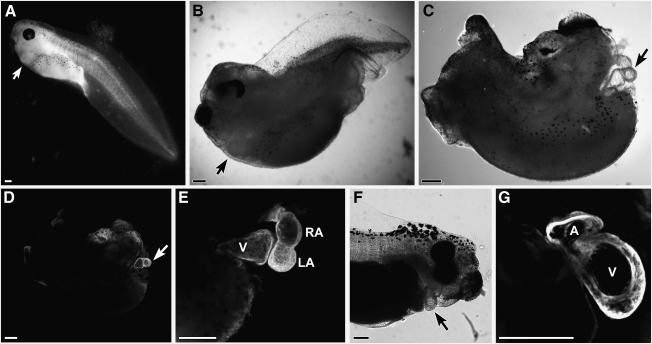
Cardiac differentiation of DMZ tissue. DMZ explants were cultured for 4 days in the (A–C) presence or (D) absence of 20 ng/mL WNT1 and immunostained for sarcomeric myosin heavy chain. (A, B) Brightfield and fluorescent images of an individual WNT1-treated explant that displayed sarcomeric myosin immunoreactivity. (C) Another example of an explant incubated with WNT1 exhibiting cardiac tissue that stained positive for sarcomeric myosin. A comparison of the WNT1-treated tissue with (D) nontreated control DMZ demonstrates that ectopic canonical WNT signaling did not alter the pattern of sarcomeric myosin expression within the tissue. (E, F) In contrast, cardiac differentiation of the DMZ tissue was blocked by exposure of DMZ explants to 50 μM LY294002, which inhibits signaling via phosphatidylinositol 3-kinase, as shown for an individual explant imaged by brightfield and fluorescent microscopy. Scale bars=100 μm.
FIG. 4.
Cardiac gene expression in DMZ tissue. DMZ tissue was cultured in the absence or presence of the indicated WNT1 concentration before RNA isolation and PCR analysis of cardiac (A) transcription factors and (B) muscle genes. (A) WNT1 treatment had little impact on of Islet-1 and GATA6 transcription but decreased expression of Tbx5, Tbx20, and Nkx2.5. (B) RNA levels for the cardiac-specific muscle proteins Actc, cMHCα, and cTnI were not significantly affected by WNT1 exposure. *P<0.05; **P<0.01 between WNT1-treated group and control.
Ectopic activation of canonical WNT signaling during gastrulation
The explant experimentation described above suggests that treatment with canonical WNT proteins does not prevent differentiation of precardiac tissue, although WNT1 exposure did selectively reduce expression of certain cardiac genes. To pursue this issue further, we examined the influence canonical WNT signaling would have on heart formation in situ, if ectopically activated from the onset of gastrulation. To efficiently promote this signaling pathway in the developing frog, stage 10.25 embryos were treated with SB415286, which is a selective GSK3 inhibitor that is used to phenocopy canonical WNT signal transduction [53–57]. The ability of SB415286 to promote canonical WNT signaling was confirmed in cultured cells using the TOPflash reporter (Fig. 2B). Embryos were subjected to a range of SB415286 concentrations for various times of exposure and allowed to develop for 3 additional days, which would correspond to stage 42 for control embryos, before analyzing the developmental outcomes. Despite differences in the time course of the SB415286 experimentation, the resulting phenotypes from all these treatments were remarkably consistent, with the severity of the defect corresponding to the concentrations of the reagent used for each of the exposure times (Table 1). The maximum doses of SB415286 that sustained embryonic survival were 4 mM for 10 min, 2 mM×2 hr, and 500 μM for 24 hrs. Four types of malformations were observed with exposure to SB415286: (a) a mild defect that was exhibited as a bend in the tail (Fig. 5A); (b) a highly anteriorized phenotype, with the embryos exhibiting a severely truncated tail, but fully developed head (Fig. 5B); (c) embryos that exhibited both a truncated tail and highly deformed head (Fig. 5C); and (d) embryos whose tissue remained viable but did not continue to develop following treatments. Remarkably, all the SB415286 embryos, except the few whose development was arrested by the treatment, had functional contractile hearts (Fig. 5). This contrasts to treatment with the PI3K inhibitor LY294002, which prevented cardiac differentiation and heart formation (Table 1). As shown at high magnification, the morphology of the hearts in the SB415286-treated embryos was normal, with well-formed ventricular and atrial chambers (Fig. 5C–G). The low impact of canonical WNT signaling on the development of the heart was confirmed by gene expression analysis, as cardiac tissue from SB415286-treated and nontreated control embryos displayed comparable levels of the cardiac transcription factors islet1, GATA6, Tbx5, and Tbx20 (Fig. 6A), and muscle genes Actc, cMHCα, and cTnI (Fig. 6B). The only cardiac-associated molecule that demonstrated depressed levels in response to SB415286 treatment was Nkx2.5 (Fig. 6A), whose expression was similarly reduced in the WNT1-treated DMZ explants.
Table 1.
Phenotype of Treated and Nontreated Embryos
| Reagent | Time of treatment | Total | Normal | A | B | C | D | Beating | % Contractile |
|---|---|---|---|---|---|---|---|---|---|
| Control | 160 | 150 | 7 | 2 | 0 | 1 | 159 | 99.4 | |
| SB415286 | 4 mM×10 min | 24 | 0 | 1 | 4 | 17 | 2 | 21 | 87.5 |
| SB415286 | 2 mM×30 min | 32 | 0 | 10 | 13 | 8 | 1 | 31 | 96.9 |
| SB415286 | 2 mM×1 h | 35 | 0 | 7 | 20 | 8 | 0 | 35 | 100 |
| SB415286 | 2 mM×2 h | 16 | 0 | 0 | 0 | 15 | 1 | 15 | 93.8 |
| SB415286 | 500 μM×24 h | 47 | 0 | 0 | 22 | 25 | 0 | 47 | 100 |
| LY-294,002 | 100 μM | 17 | 0 | 0 | 0 | 0 | 17 | 0 | 0 |
Embryos scored for the following phenotypes on day 4 of development: normal; type A, mild defect exhibited as a bend in the tail; type B, a highly anteriorized phenotype that was denoted by a severely truncated tail, but fully developed head; type C, severe malformation consisting of both a truncated tail and highly deformed head; and type D, viable but arrested development following treatment. Embryos were also scored for the presence of a beating heart.
FIG. 5.
Ectopic activation of canonical WNT signaling in the early gastrula. This signaling pathway was promoted in the developing frog by treating stage 10.25 embryos with the GSK3 inhibitor SB415286. Types of malformations observed in responses to SB415286 treatment were embryos displaying: (A) mild defects consisting of a bent tail; (B) an anteriorized phenotype that results in a severely truncated tail, but fully developed head; and (C) a compound defect consisting of both a truncated tail and highly deformed head. Despite the differences in the severity of the defects, each of these embryos developed a functional and anatomically normal heart (arrows). Note that the ventral dermis overlaying the developing heart was removed from the latter embryo to better observe the organ's structure. (D) Same embryo as C, showing sarcomeric myosin immunolabeling of the heart (arrow). (E) High magnification of a heart from another SB415286-treated embryo that was incubated to stage 40. The sarcomeric myosin-immunolabeling highlights the developing ventricle (V), left (LA) and right atrium (RA) (F) SB415286-treated embryo that was incubated to stage 46 and exhibited an anatomically normal heart (arrow). (G) Same embryo at higher magnification, with sarcomeric myosin staining showcasing well-formed atrial and ventricular chambers. Scale bars=200 μm.
FIG. 6.
Cardiac gene expression analysis following treatment of the embryo. Cardiac tissue from nontreated or SB415286-treated embryos was isolated and examined for expression of the (A) transcription factors Islet-1, GATA6, Tbx5, Tbx20, and Nkx2.5 and (B) muscle genes Actc, cMHCα, and cTnI by real-time PCR. The only gene whose expression was significantly downregulated by SB415286 exposure was the transcription factor Nkx2.5. **P<0.01 between SB415286-treated group and control.
Canonical WNT signaling promotes stem cell expansion in cardiogenic tissue
In addition to its hypothesized role in regulating the formation of the heart, canonical WNTs have demonstrated their function as stem cell maintenance and expansion factors [31,32]. Accordingly, we examined the capacity of this signal transduction pathway to enhance the display of stem cell phenotypes in cardiogenic tissue, both as a test for canonical WNT signaling in the explants and whole embryos, and to ascertain the regulatory influence this pathway has in modulating the development of the heart. For these experiments, the presence of multiple stem cell markers was assayed in cultured DMZ explants exposed to WNT1 protein, as well as in cardiac tissue obtained from SB415286-treated embryos (Fig. 7). In both the cultures and developing heart, canonical WNT signaling promoted increases in expression of c-kit, which has been shown in mammalian systems to be a marker for cardiac stem cells [58–61]. The expression of pluripotency genes was also examined to determine if WNT signaling stimulated the display of stem cells with a broader differentiation potential. Interestingly, we saw a divergence of expression of Oct25 and Oct60, which are homologs of the mammalian gene Oct3/4 [62–64], among cultured DMZ tissue and the whole embryos. While exposure of DMZ tissue to WNT1 enhanced expression of Oct25, Oct60 expression was unaffected by these treatments. Conversely, subjecting the embryo to SB415286 caused an upregulation of Oct60 in developing cardiac tissue, without an accompanying stimulation of Oct25. Thus, canonical WNT signaling enhanced expression of both cardiac stem cell markers and pluripotency genes within developing heart tissue, which occurred without concomitant suppression of cardiac differentiation. The implications of these findings in regard to WNT regulation of heart formation and stem cell maintenance are discussed below.
FIG. 7.
Stem cell marker expression in response to canonical WNT signaling. (A) PCR analysis of DMZ tissue cultured without or in the presence of 20 ng/mL or 50 ng/mL WNT1. Treatment with 20 ng/mL WNT1 promoted an upregulation of both c-kit and oct25 expression. Expression of oct60 in DMZ explants was not affected by WNT1. (B) PCR analysis of cardiac tissue removed from nontreated and SB415286-treated embryos. SB415286 exposure promoted an upregulation of both c-kit and oct60 expression. In contrast, SB415286 did not stimulate expression of oct25. **P<0.01 between treated group and negative control.
Discussion
WNTs have been shown to have significant impact on embryogenesis, stem cell phenotype, and cancer [2,7,31]. It is also commonly accepted that WNT signaling plays a crucial role in regulating cardiovascular development [3,4,8,12,14,16]. Yet, its importance for the formation of the heart is still undefined. For several years, the prevailing paradigm was that cardiogenesis within anterior lateral mesoderm is initiated in response to the inhibition of canonical WNT signaling. However, newer data revealed that there is a more complicated relationship between this signal transduction pathway and heart development. Studies with ES cells indicated that canonical WNTs could have a positive influence of cardiac tissue formation [23–28]. Recent investigations in the whole embryo suggested that timing during development may determine whether cardiac differentiation is stimulated or suppressed by canonical WNTs, and have prompted a modified hypothesis that these molecules have a biphasic influence on heart development [12,13,16,26,30]. Thus, it has been proposed that exposure to canonical WNT signals before gastrulation is required for cardiac induction and/or expansion of cardiac progenitors, whereas once gastrulation begins, suppression of these same signals is necessary to allow cardiac differentiation to continue. It is not clear how this notion could be consistent with earlier studies showing that cardiac induction can be suppressed by ectopic canonical WNT signaling before gastrulation [21,22], which is a result we confirmed in this study. Nor is it clear that this premise is reconcilable with the complex pattern of various canonical WNTs within the developing myocardium and new evidence that these molecules promote the expansion of myocyte formation within the developing heart [3,12,29,65,66].
Previous investigations in the developing frog have shown that abnormal expression of canonical WNTs at blastula stages inhibited subsequent cardiac differentiation in DMZ tissue [21,22]. In this study, we extended those findings to show that initiating ectopic canonical WNT signaling on the dorsal side in the early blastula will prevent intact embryos from forming a heart. However, those embryos suffered from malformations far more severe then the absence of a heart, which is not surprising since a considerable amount of development occurs from the time RNA is introduced into the early blastula to the beginnings of gastrulation when nondifferentiated mesoderm starts to coalesce into heart-forming regions. Thus, it is difficult to determine from molecular manipulations at blastula-stages whether canonical WNTs are exerting direct or indirect influences on heart development. Accordingly, we delayed treatment until the onset of gastrulation when cardiac progenitors localize to the mesoderm. In 1 set of experiments, explanted DMZ, which is the region that contains cells fated to give rise to the heart, were incubated with WNT1 protein. In a second group of experiments, the cell- and tissue-permeable GSK3 inhibitor SB415286 was used to promote canonical WNT signaling in the whole embryo. In both cases, ectopic activation of canonical WNT signaling did not prevent cardiac tissue formation. While ectopic WNT signaling did decrease expression levels of certain cardiac transcription factors, other signs of impaired heart development were not apparent, as contractile cardiac tissue formed and anatomically normal and functional hearts developed.
An effect that canonical WNT activity did elicit on cardiogenic tissue was its enhancement of stem cell marker expression. Both WNT1 and SB415286 stimulated expression of the c-kit stem cell marker, which suggests that activation of canonical WNT signaling amplified cardiac progenitors within developing heart tissue. In addition, WNT1 and SB415286 promoted an increase in pluripotency gene expression. In the frog, there are multiple homologs of mammalian Oct-4, which is a key molecule that controls pluripotency of embryonic stem cells [67]. Oct25 is a zygotically expressed gene whose normal expression in the embryo persists in the marginal zones of the early gastrula [62–64]. The ability of WNT1 to enhance Oct25 in DMZ tissue may be indicative of an augmentation of this pluripotency gene among stem cells that remain in this tissue. The lack of Oct25 stimulation in cardiogenic tissue from SB415286-treated embryos may reflect that the later staged tissue no longer maintains the potential to express Oct25. Thus, it was a surprise that SB415286 exposure resulted in the upregulation of Oct60, which is a maternally expressed gene that disappears by late blastula stages [62–64]. Although the significance of differential Oct25 and Oct60 expression is not clear, as differences in the functions of these 2 molecules have not been determined, the upregulation of these molecules and c-kit demonstrates that canonical WNTs can act as stem cell enhancement factors in precardiac and cardiac tissue.
The results presented in this study demonstrate that ectopic canonical WNT signaling can prevent heart formation when activated at the earliest stages of development, but that this aberrant cardiac development is associated with global embryonic defects. Delaying ectopic WNT activity to the onset of gastrulation produced no similar gross cardiac defects. Ectopic activation of canonical WNT signaling in the whole embryo from the onset of gastrulation did not prevent the formation of a structurally and functionally sound heart. The 1 cardiac deficit uncovered was the lowered expression of Nkx2.5. That decreases in the levels of this transcription factor were not accompanied by obvious gross structural defects in the heart was not unexpected, as haploinsufficiency of Nkx2.5 in mouse and humans is associated primarily with conduction tissue abnormalities [68,69]. WNT1 treatment targeted to precardiac mesoderm-containing DMZ tissue produced a broader reduction in cardiac gene transcription, as Tbx5 and Tbx20 also showed decreased levels of expression. Yet, similar to the outcomes in the whole embryo, activation of canonical WNT signaling did not inhibit myocardial differentiation of DMZ explants, as muscle gene and sarcomeric protein expression did not differ from nontreated controls. That lowered expression of these Tbx genes did not indicate a block in myocardial differentiation is consistent with previous findings, as haploinsufficiency of Tbx5 and Tbx20 produces cardiac anomalies, although not a block in the formation of a 4 chambered heart [70,71]. Altered levels of Tbx factors may also present a complicated readout for cardiac differentiation, as these latter genes exert both positive and negative effects on heart development in the embryo [71,72]. The gene expression data from this study indicate that canonical WNT signaling can exert some negative influences on heart development. However, these influences are subtle and do not block cardiac differentiation per se. This is not surprising as multiple WNTs, WNT targets, and WNT inhibitors are exhibited in a complex spatial and temporal pattern in the developing heart [3,12,29,30]. Thus WNTs help shape the complex structure of the heart with phasic canonical WNT regulation of cardiac differentiation acting to regulate how, but not whether, cardiogenesis proceeds. WNT regulation of stem cell phenotype may come into play in this regulation, as depending on place and time within the embryonic heart, stem cell expansion may play a beneficial or detrimental role in the remodeling events in the developing heart.
To summarize, canonical WNT signaling is not cardiosuppressive, although continual and global activation of this pathway would not be an optimal regulatory environment for the normal morphological development of the heart. This work confirms that canonical WNTs can act as stem cell factors, as they can expand stem cell expression in cardiogenic tissue. However, this regulatory stimulation of stem cells does not come at the expense of blocking cardiac progenitors from differentiating. While this study is consistent with reports that canonical WNTs have utility for expanding cardiopotent stem cell populations, inhibitors of these molecules are not a prerequisite for coaxing a myocyte phenotype from these stem cells.
Supplementary Material
Acknowledgments
We gratefully acknowledge Dr. Randall Moon (University of Washington) for the kind gift of the Δβ-catenin expression plasmid. This work was supported by NIH RO1HL073190 (L.M.E.) and NIH RO1073270 (A.F.R).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Angers S. Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 2.Cadigan KM. Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol. 2009;1:a002881. doi: 10.1101/cshperspect.a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenberg LM. Eisenberg CA. Wnt signal transduction and the formation of the myocardium. Dev Biol. 2006;293:305–315. doi: 10.1016/j.ydbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg LM. Eisenberg CA. Evaluating the role of Wnt signal transduction in promoting the development of the heart. Sci World J. 2007;7:161–176. doi: 10.1100/tsw.2007.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald BT. Tamai K. He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Amerongen R. Mikels A. Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- 7.van Amerongen R. Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg CA. Eisenberg LM. WNT11 promotes cardiac tissue formation of early mesoderm. Dev Dyn. 1999;216:45–58. doi: 10.1002/(SICI)1097-0177(199909)216:1<45::AID-DVDY7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Pandur P. Läsche M. Eisenberg LM. Kühl M. Wnt-11 activation of a non-canonical Wnt signaling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 10.Alfieri CM. Cheek J. Chakraborty S. Yutzey KE. Wnt signaling in heart valve development and osteogenic gene induction. Dev Biol. 2010;338:127–135. doi: 10.1016/j.ydbio.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty MP. Dawn B. Noncanonical Wnt11 signaling and cardiomyogenic differentiation. Trends Cardiovasc Med. 2008;18:260–268. doi: 10.1016/j.tcm.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gessert S. Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 2010;107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 13.Klaus A. Birchmeier W. Developmental signaling in myocardial progenitor cells: a comprehensive view of Bmp- and Wnt/beta-catenin signaling. Pediatr Cardiol. 2009;30:609–616. doi: 10.1007/s00246-008-9352-7. [DOI] [PubMed] [Google Scholar]
- 14.Martin J. Afouda BA. Hoppler S. Wnt/beta-catenin signalling regulates cardiomyogenesis via GATA transcription factors. J Anat. 2010;216:92–107. doi: 10.1111/j.1469-7580.2009.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy II. Railo A. Rapila R. Hast T. Sormunen R. Tavi P. Rasanen J. Vainio SJ. Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and beta-catenin expression. Cardiovasc Res. 2010;85:100–109. doi: 10.1093/cvr/cvp254. [DOI] [PubMed] [Google Scholar]
- 16.Tian Y. Cohen ED. Morrisey EE. The importance of Wnt signaling in cardiovascular development. Pediatr Cardiol. 2010;31:342–348. doi: 10.1007/s00246-009-9606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park M. Wu X. Golden K. Axelrod JD. Bodmer R. The wingless signaling pathway is directly involved in Drosophila heart development. Dev Biol. 1996;177:104–116. doi: 10.1006/dbio.1996.0149. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg CA. Gourdie RG. Eisenberg LM. Wnt11 is expressed in early avian mesoderm and required for the differentiation of the quail mesoderm cell line QCE6. Development. 1997;124:525–536. doi: 10.1242/dev.124.2.525. [DOI] [PubMed] [Google Scholar]
- 19.Tada M. Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 20.Foley AC. Gupta RW. Guzzo RM. Korol O. Mercola M. Embryonic heart induction. Ann NY Acad Sci. 2006;1080:85–96. doi: 10.1196/annals.1380.008. [DOI] [PubMed] [Google Scholar]
- 21.Marvin MJ. Di Rocco G. Gardiner A. Bush SM. Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider VA. Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon C. Cordes KR. Srivastava D. Wnt/beta-catenin signaling acts at multiple developmental stages to promote mammalian cardiogenesis. Cell Cycle. 2008;7:3815–3818. doi: 10.4161/cc.7.24.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsley RC. Gill JG. Kyba M. Murphy TL. Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 25.Naito AT. Shiojima I. Akazawa H. Hidaka K. Morisaki T. Kikuchi A. Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Nat Acad Sci USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno S. Weidinger G. Osugi T. Kohn AD. Golob JL. Pabon L. Reinecke H. Moon RT. Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Nat Acad Sci USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terami H. Hidaka K. Katsumata T. Iio A. Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Comm. 2004;325:968–975. doi: 10.1016/j.bbrc.2004.10.103. [DOI] [PubMed] [Google Scholar]
- 28.Paige SL. Osugi T. Afanasiev OK. Pabon L. Reinecke H. Murry CE. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian Y. Yuan L. Goss AM. Wang T. Yang J. Lepore JJ. Zhou D. Schwartz RJ. Patel V. Cohen ED. Morrisey EE. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev Cell. 2010;18:275–287. doi: 10.1016/j.devcel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavery DL. Martin J. Turnbull YD. Hoppler S. Wnt6 signaling regulates heart muscle development during organogenesis. Dev Biol. 2008;323:177–188. doi: 10.1016/j.ydbio.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 32.Katoh M. WNT signaling in stem cell biology and regenerative medicine. Curr Drug Targets. 2008;9:565–570. doi: 10.2174/138945008784911750. [DOI] [PubMed] [Google Scholar]
- 33.Marson A. Foreman R. Chevalier B. Bilodeau S. Kahn M. Young RA. Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato N. Meijer L. Skaltsounis L. Greengard P. Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 35.Ai D. Fu X. Wang J. Lu MF. Chen L. Baldini A. Klein WH. Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci USA. 2007;104:9319–24. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura T. Sano M. Songyang Z. Schneider MD. A Wnt- and beta -catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci USA. 2003;100:5834–5839. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal R. Khanna A. Role of smad- and wnt-dependent pathways in embryonic cardiac development. Stem Cells Dev. 2006;15:29–39. doi: 10.1089/scd.2006.15.29. [DOI] [PubMed] [Google Scholar]
- 38.Sive HL. Grainger RM. Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- 39.Nieuwkoop PD. Faber J. Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis. Garland; New York, NY: 1994. [Google Scholar]
- 40.Hausen P. Riebesell M. The Early Development of Xenopus laevis: An Atlas of the Histology. Springer-Verlag; Berlin: 1991. [Google Scholar]
- 41.Heasman J. Patterning the early Xenopus embryo. Development. 2006;133:1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- 42.Yost C. Torres M. Miller JR. Huang E. Kimelman D. Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 43.Ramsdell AF. Bernanke JM. Trusk TC. Left-right lineage analysis of the embryonic Xenopus heart reveals a novel framework linking congenital cardiac defects and laterality disease. Development. 2006;133:1399–1410. doi: 10.1242/dev.02292. [DOI] [PubMed] [Google Scholar]
- 44.Brannon M. Kimelman D. Activation of Siamois by the Wnt pathway. Dev Biol. 1996;180:344–347. doi: 10.1006/dbio.1996.0306. [DOI] [PubMed] [Google Scholar]
- 45.Carnac G. Kodjabachian L. Gurdon JB. Lemaire P. The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm. Development. 1996;122:3055–3065. doi: 10.1242/dev.122.10.3055. [DOI] [PubMed] [Google Scholar]
- 46.Korinek V. Barker N. Morin PJ. van Wichen D. de Weger R. Kinzler KW. Vogelstein B. Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 47.Barolo S. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene. 2006;25:7505–7511. doi: 10.1038/sj.onc.1210057. [DOI] [PubMed] [Google Scholar]
- 48.Afouda BA. Hoppler S. Xenopus explants as an experimental model system for studying heart development. Trends Cardiovasc Med. 2009;19:220–226. doi: 10.1016/j.tcm.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Houston DW. Kofron M. Resnik E. Langland R. Destree O. Wylie C. Heasman J. Repression of organizer genes in dorsal and ventral Xenopus cells mediated by maternal XTcf3. Development. 2002;129:4015–4025. doi: 10.1242/dev.129.17.4015. [DOI] [PubMed] [Google Scholar]
- 50.Klinz F. Bloch W. Addicks K. Hescheler J. Inhibition of phosphatidylinositol-3-kinase blocks development of functional embryonic cardiomyocytes. Exp Cell Res. 1999;247:79–83. doi: 10.1006/excr.1998.4309. [DOI] [PubMed] [Google Scholar]
- 51.Naito AT. Tominaga A. Oyamada M. Oyamada Y. Shiraishi I. Monzen K. Komuro I. Takamatsu T. Early stage-specific inhibitions of cardiomyocyte differentiation and expression of Csx/Nkx-2.5 and GATA-4 by phosphatidylinositol 3-kinase inhibitor LY294002. Exp Cell Res. 2003;291:56–69. doi: 10.1016/s0014-4827(03)00378-1. [DOI] [PubMed] [Google Scholar]
- 52.Brade T. Gessert S. Kuhl M. Pandur P. The amphibian second heart field: Xenopus islet-1 is required for cardiovascular development. Dev Biol. 2007;311:297–310. doi: 10.1016/j.ydbio.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Coghlan MP. Culbert AA. Cross DA. Corcoran SL. Yates JW. Pearce NJ. Rausch OL. Murphy GJ. Carter PS. Roxbee Cox L. Mills D. Brown MJ. Haigh D. Ward RW. Smith DG. Murray KJ. Reith AD. Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 54.Fuentealba LC. Eivers E. Ikeda A. Hurtado C. Kuroda H. Pera EM. De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manisastry SM. Han M. Linask KK. Early temporal-specific responses and differential sensitivity to lithium and Wnt-3A exposure during heart development. Dev Dyn. 2006;235:2160–2174. doi: 10.1002/dvdy.20878. [DOI] [PubMed] [Google Scholar]
- 56.Sen B. Styner M. Xie Z. Case N. Rubin CT. Rubin J. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem. 2009;284:34607–34617. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H. McKnight NC. Zhang T. Lu ML. Balk SP. Yuan X. SOX9 is expressed in normal prostate basal cells and regulates androgen receptor expression in prostate cancer cells. Cancer Res. 2007;67:528–536. doi: 10.1158/0008-5472.CAN-06-1672. [DOI] [PubMed] [Google Scholar]
- 58.Beltrami AP. Barlucchi L. Torella D. Baker M. Limana F. Chimenti S. Kasahara H. Rota M. Musso E. Urbanek K. Leri A. Kajstura J. Nadal-Ginard B. Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 59.Miyamoto S. Kawaguchi N. Ellison GM. Matsuoka R. Shin'oka T. Kurosawa H. Characterization of long-term cultured c-kit+ cardiac stem cells derived from adult rat hearts. Stem Cells Dev. 2010;19:105–116. doi: 10.1089/scd.2009.0041. [DOI] [PubMed] [Google Scholar]
- 60.Tallini YN. Greene KS. Craven M. Spealman A. Breitbach M. Smith J. Fisher PJ. Steffey M. Hesse M. Doran RM. Woods A. Singh B. Yen A. Fleischmann BK. Kotlikoff MI. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA. 2009;106:1808–1813. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kao KR. Bernstein A. Expression of Xkl-1, a Xenopus gene related to mammalian c-kit, in dorsal embryonic tissue. Mech Dev. 1995;50:57–69. doi: 10.1016/0925-4773(94)00325-h. [DOI] [PubMed] [Google Scholar]
- 62.Cao Y. Oswald F. Wacker SA. Bundschu K. Knochel W. Reversal of Xenopus Oct25 function by disruption of the POU domain structure. J Biol Chem. 2010;285:8408–8421. doi: 10.1074/jbc.M109.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hinkley CS. Martin JF. Leibham D. Perry M. Sequential expression of multiple POU proteins during amphibian early development. Mol Cell Biol. 1992;12:638–649. doi: 10.1128/mcb.12.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morrison GM. Brickman JM. Conserved roles for Oct4 homologues in maintaining multipotency during early vertebrate development. Development. 2006;133:2011–2022. doi: 10.1242/dev.02362. [DOI] [PubMed] [Google Scholar]
- 65.Klaus A. Saga Y. Taketo MM. Tzahor E. Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Nat Acad Sci USA. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwon C. Arnold J. Hsiao EC. Taketo MM. Conklin BR. Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Nat Acad Sci USA. 2007;104:10894–10899. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loh YH. Wu Q. Chew JL. Vega VB. Zhang W. Chen X. Bourque G. George J. Leong B. Liu J. Wong KY. Sung KW. Lee CW. Zhao XD. Chiu KP. Lipovich L. Kuznetsov VA. Robson P. Stanton LW. Wei CL. Ruan Y. Lim B. Ng HH. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 68.Jay PY. Harris BS. Maguire CT. Buerger A. Wakimoto H. Tanaka M. Kupershmidt S. Roden DM. Schultheiss TM. O'Brien TX. Gourdie RG. Berul CI. Izumo S. Nkx2-5 mutation causes anatomic hypoplasia of the cardiac conduction system. J Clin Invest. 2004;113:1130–1137. doi: 10.1172/JCI19846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris BS. Spruill L. Edmonson AM. Rackley MS. Benson DW. O'Brien TX. Gourdie RG. Differentiation of cardiac Purkinje fibers requires precise spatiotemporal regulation of Nkx2-5 expression. Dev Dyn. 2006;235:38–49. doi: 10.1002/dvdy.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moskowitz IP. Pizard A. Patel VV. Bruneau BG. Kim JB. Kupershmidt S. Roden D. Berul CI. Seidman CE. Seidman JG. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development. 2004;131:4107–4116. doi: 10.1242/dev.01265. [DOI] [PubMed] [Google Scholar]
- 71.Stennard FA. Costa MW. Lai D. Biben C. Furtado MB. Solloway MJ. McCulley DJ. Leimena C. Preis JI. Dunwoodie SL. Elliott DE. Prall OW. Black BL. Fatkin D. Harvey RP. Murine T-box transcription factor Tbx20 acts as a repressor during heart development, and is essential for adult heart integrity, function and adaptation. Development. 2005;132:2451–2462. doi: 10.1242/dev.01799. [DOI] [PubMed] [Google Scholar]
- 72.Liberatore CM. Searcy-Schrick RD. Yutzey KE. Ventricular expression of tbx5 inhibits normal heart chamber development. Dev Biol. 2000;223:169–180. doi: 10.1006/dbio.2000.9748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.