Abstract
Intracellular protein transport between the endoplasmic reticulum (ER) and the Golgi apparatus and within the Golgi apparatus is facilitated by COP (coat protein)-coated vesicles. Their existence in plant cells has not yet been demonstrated, although the GTP-binding proteins required for coat formation have been identified. We have generated antisera against glutathione-S-transferase-fusion proteins prepared with cDNAs encoding the Arabidopsis Sec21p and Sec23p homologs (AtSec21p and AtSec23p, respectively). The former is a constituent of the COPI vesicle coatomer, and the latter is part of the Sec23/24p dimeric complex of the COPII vesicle coat. Cauliflower (Brassica oleracea) inflorescence homogenates were probed with these antibodies and demonstrated the presence of AtSec21p and AtSec23p antigens in both the cytosol and membrane fractions of the cell. The membrane-associated forms of both antigens can be solubilized by treatments typical for extrinsic proteins. The amounts of the cytosolic antigens relative to the membrane-bound forms increase after cold treatment, and the two antigens belong to different protein complexes with molecular sizes comparable to the corresponding nonplant coat proteins. Sucrose-density-gradient centrifugation of microsomal cell membranes from cauliflower suggests that, although AtSec23p seems to be preferentially associated with ER membranes, AtSec21p appears to be bound to both the ER and the Golgi membranes. This could be in agreement with the notion that COPII vesicles are formed at the ER, whereas COPI vesicles can be made by both Golgi and ER membranes. Both AtSec21p and AtSec23p antigens were detected on membranes equilibrating at sucrose densities equivalent to those typical for in vitro-induced COP vesicles from animal and yeast systems. Therefore, a further purification of the putative plant COP vesicles was undertaken.
Protein transport between the various organelles of the endomembrane system and the plasma membrane is facilitated by vesicles, many if not all of which possess a protein coat at their cytosolic surface. The first type of coated vesicle to be described was the CCV, which mediates transport between the plasma membrane and endosomes and between the trans-Golgi network and the lytic compartments of the cell (Schmid and Damke, 1995; Brodsky, 1997). A different kind of coated vesicle, discovered in the 1980s as a result of in vitro studies of the intra-Golgi transport of VSV-G protein and immunologically distinct from CCV, was the COPI (coat protein I)-coated vesicle (Malhotra et al., 1989). The components of the coat were later shown to be ARF, a GTP-binding protein (Serafini et al., 1991), and a heptameric protein complex called the coatomer (Waters et al., 1991). COPII-coated vesicles were obtained when yeast ER was incubated in the presence of a set of five proteins (the GTP-binding protein Sar1p and two heterodimeric complexes, Sec23/24p and Sec13/31p), GTP, and an ATP-regenerating system (Barlowe et al., 1994).
CCVs have been recognized in plants for some time. The components of their coat and their location and function appear to be similar to those of their counterparts in other eukaryotic cells (Beevers, 1996; Robinson et al., 1998). In contrast, the evidence for COP-coated vesicles in plants has remained more conjectural (Staehelin and Moore, 1995; Andreeva et al., 1998b). Except for some algae, such as Chlamydomonas reinhardtii, in which the ER and the Golgi apparatus are close together (e.g. Luykx et al., 1997), vesicle-budding events at the ER in plants are in general difficult to visualize with the electron microscope (Hawes et al., 1996). Nevertheless, attempts at generating and isolating ER-derived transport vesicles from higher plants have been carried out (e.g. Hellgren et al., 1993), albeit without reference to possible coat proteins. In addition to CCV at the trans pole, several different types of Golgi-derived vesicles have been described in higher plants. Some are large, apparently smooth surfaced, and sometimes with structured contents, e.g. the slime-containing vesicles of root-cap cells (Mollenhauer and Morré, 1991) and the storage-protein-containing “dense” vesicles in developing seed tissues (Robinson et al., 1997, 1998). Others are small (≤70 nm in diameter) and are usually found at the peripheries of cis and medial cisternae. When properly fixed and stained, these small vesicles appear to be coated (see Fig. 2 in Hawes et al., 1996), but without the appropriate immunogold-labeling data it remains unclear whether they might represent COP-coated vesicles.
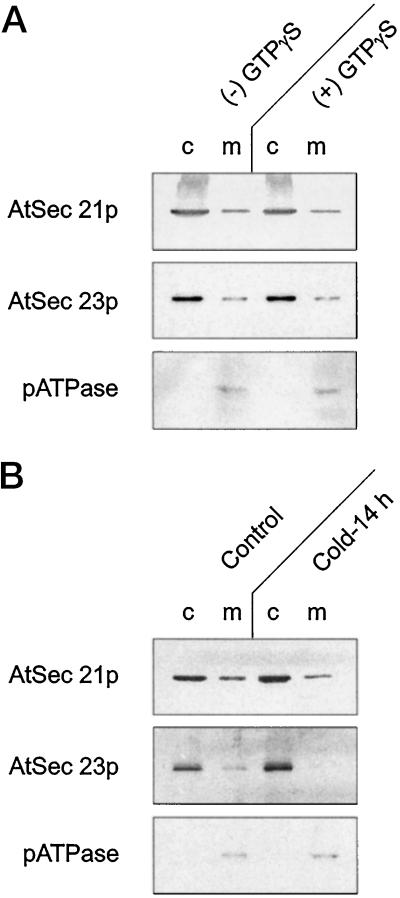
Figure 2.
A, GTPγS does not redistribute membrane-bound and cytosolic AtSec21p and AtSec23p antigens. Equal amounts of cauliflower inflorescence (10 g) were homogenized in 10 mL of HDKE 10 buffer in the presence or absence of 50 μm GTPγS. Microsomal membranes (m) and cytosolic fractions (c) were isolated as described in Methods. The pATPase was used as a standard intrinsic membrane antigen (20 μg of protein per lane). B, Cold temperature causes a displacement of AtSec21p and AtSec23p antigens from the membrane to the cytosolic compartment of the cell. Whole cauliflower plants were held in a cold room for 14 h before microsomal membranes and cytosolic fractions were extracted from the inflorescence (20 μg of protein per lane).
Two indirect lines of evidence support the existence of COP-coated vesicles in plants. First, the fungal metabolite brefeldin A is known to prevent ARF binding in mammalian cells (Dascher and Balch, 1994), thereby preventing coatomer assembly and the formation of COP-coated vesicles (Orci et al., 1991). As a consequence, the cisternae of the Golgi apparatus fuse with one another, tubularize, and are absorbed into the ER (Klausner et al., 1992). Brefeldin A also targets the Golgi apparatus in plants, with similar but not identical morphological effects (Satiat-Jeunemaitre et al., 1996). Second, genes homologous to ARF and Sar1 have been identified from a number of higher plants (d'Enfert et al., 1992; Regad et al., 1993; Bar-Peled and Raikhel, 1997). cDNAs corresponding to other COP coat proteins were identified in a search of the expressed sequence tag database (Andreeva et al., 1998b). Finally, AtSec12p, a Sec12p homolog in Arabidopsis that is an integral ER protein required for COPII-coated vesicle production in yeast (Nakano et al., 1988), has been described (d'Enfert et al., 1992; Bar-Peled and Raikhel, 1997). Thus, at the gene level and in terms of sensitivity toward brefeldin A, plants seem to possess the capacity for COP-coated vesicle production.
To explore this possibility further, we generated antisera against AtSec21p, the Arabidopsis homolog to Sec21p (γ-COP, a 100-kD subunit of the coatomer), and AtSec23p, the Arabidopsis homolog to Sec23p (an 85-kD component of the COPII coat), and have probed subcellular fractions with them. In addition to the binding of AtSec21p and AtSec23p to Golgi and ER fractions, we report a high-density fraction that may contain a population of endogenous COP-coated vesicles. Our experimental organism is the growing inflorescence of cauliflower (Brassica oleracea), which is genetically closely related to Arabidopsis (Dozolme et al., 1995), is readily available in large quantities, and possesses relatively low levels of endogenous proteases, which is advantageous for the detection and characterization of cytosolic coat proteins.
MATERIALS AND METHODS
Materials
Cauliflower (Brassica oleracea L. var botrytis) was grown in a greenhouse. Inflorescences, roughly 10 to 15 cm in diameter, were excised and the woody stalk tissue was removed. Whole plants were used for all experiments, even for cold treatments overnight in a refrigerated room at 4°C. Arabidopsis cell-suspension cultures were established from leaf calli derived from seedlings grown under sterile conditions and maintained in the log phase by weekly subculturing into fresh Gamborg's B5 medium (3.86 g L−1; Sigma G5893) containing 20 g L−1 Glc, 0.5 g L−1 Mes, 0.5 mg L−1 2,4-D, and 50 μg L−1 kinetin. The cultures were maintained at 24°C on an orbital shaker (110 rpm). Bakers' yeast (Saccharomyces cerevisiae cv Vital Gold) was purchased locally (Deutsche Hefewerke, Hamburg, Germany) and taken into suspension culture at 28°C in Sabouraud's Glc broth (Campbell, 1988). Porcine brain from freshly slaughtered animals was obtained from a local slaughterhouse and brought to the laboratory under refrigerated conditions.
Generation and Purification of GST-Fusion Proteins and Preparation of Antisera
Arabidopsis cDNAs homologous to SEC21 (accession no. T75984) and SEC23 (accession no. T04245) were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus) and were cloned by standard procedures into the SalΙ-NotΙ site of the pGEX-4T-3 vector (Pharmacia). These cDNAs have greater than 50% homology to their yeast counterparts in the first 200 bp (Robinson et al., 1998). The Sec21 cDNA clone used here corresponds exactly to a fragment of the recently described full-length sequence for an Arabidopsis genomic sequence homologous to SEC21 (accession no. AL023094). Escherichia coli (strain BL21) was transformed with the ligation product, and cultures containing 100 μg mL−1 ampicillin in Luria-Bertani medium were grown at 37°C until an A600 of 0.8 was reached.
Expression of the GST-fusion constructs was then induced by adding isopropylthio-β-d-galactopyranoside (final concentration, 0.2 mm). The temperature was reduced to 28°C, and after 3 h the cells were collected by centrifugation and resuspended in cold PBST buffer (16 mm Na2HPO4, 4 mm NaH2PO4, 150 mm NaCl, and 1% [v/v] Triton X-100, pH 7.3) containing 0.1% mercaptoethanol and 0.1 mm PMSF. The cells were lysed in a sonifier (Branson Ultrasonics, Danbury, CT) operating at 300 W four times for 30 s each, and the resulting homogenate was centrifuged at 12,000g for 30 min. The supernatants were mixed with 50% glutathione-Sepharose beads (Pharmacia) that had been equilibrated with PBST and shaken at 4°C for 30 min. The beads were sedimented and washed three times with PBST and twice with 50 mm Tris-HCl, pH 8.0. The fusion proteins were eluted from the beads with 50 mm Tris-HCl containing 10 mm GSH and were separated by SDS-PAGE. After electroelution and dialysis three times for 12 h each against 10 mm Tris-HCl, pH 7.5, the proteins were lyophilized. Polyclonal antibodies in rabbits were prepared commercially (Eurogentec, Seraing, Belgium; Biogentec, Berlin, Germany).
Preparation of Microsomal Membrane and Cytosol Fractions
Equal weights of cauliflower floret tissue, Arabidopsis cells, pig brain tissue, and HDKE 10 buffer (40 mm Hepes-KOH, pH 8.0, 1 mm DTT, 10 mm KCl, and 3 mm EDTA) containing 0.32 m Suc and protease inhibitors (2 μg mL−1 aprotinin, 0.5 μg mL−1 leupeptin, 2 μm pepstatin, 2 mm o-phenanthroline, and 1 μg mL−1 trans-epoxysuccinyl-l-leucylamido-[4-guanidino]butane) were placed in a blender (Waring) and homogenized three times in 15-s bursts, and the slurry was passed through a single layer of Miracloth (Calbiochem). After precentrifugation at 18,000g for 20 min, microsomal membranes were sedimented by centrifugation at 100,000g for 1 h. Yeast cells were homogenized in HDKE 10 buffer, pH 7.5, with Suc and protease inhibitors in a cell mill (Vibrogen, Bühler, Tübingen, Germany). Cytosol was prepared by passing an aliquot of the 100,000g supernatant through a Sephadex G-25 column, equilibrating with HDKE 10 buffer, and collecting the fractions constituting the void volume.
Fractionation of Cauliflower Cytosol
Floret tissue (25 g) was homogenized in a blender in 25 mL of TDKE 500 buffer (25 mm Tris-HCl, pH 8.0, 1 mm DTT, 500 mm KCl, and 3 mm EDTA) containing a cocktail of protease inhibitors (see above). After passing through a single layer of Miracloth, the homogenate was precentrifuged at 18,000g for 20 min and then centrifuged at 100,000g for 60 min. The protein concentration in the supernatant (cytosol) was adjusted to 5 mg mL−1 by appropriate dilution with TDKE 500 buffer, and (NH4)2SO4 (final concentration, 40% saturated) was added. After being stirred for 30 min, the precipitated proteins were recovered by centrifugation at 20,000g for 20 min and redissolved in 2 mL of TDKE 150 buffer (150 mm KCl) before being applied to a Sepharose CL-6B gel-filtration column (1 × 110 cm) and eluted with TDKE 150 buffer at a speed of 0.1 mL min−1. Two-milliliter fractions were collected, from which 100-μL aliquots were taken, and the proteins were precipitated with 8% (w/v) TCA and washed twice with absolute ethanol.
Neomycin Precipitation of Cytosolic Proteins
A 40% (NH4)2SO4 precipitate of cauliflower cytosol (see above) was redissolved in 25 mm Tris-HCl, pH 7.4, containing 10 mm KCl, to give a protein concentration of 1.5 mg mL−1. To this, neomycin was added at final concentrations up to 10 mm, followed by centrifugation at 100,000g for 20 min. Equal aliquots of the supernatants were precipitated with 8% (w/v) TCA, and the proteins were separated by 10% SDS-PAGE.
Subcellular Fractionation
Gel Filtration
Microsomal membranes (100,000g pellet) obtained from 20 g of cauliflower inflorescence were resuspended in 2 mL of TDKE 10 buffer (10 mm KCl), pH 7.0, and 10 mg was applied to a Sephacryl S-1000 column (100 mL, precoated with lecithin and equilibrated with TDKE 10 buffer) and eluted with TDKE 10 buffer. Three-milliliter fractions were collected, and the proteins in 200-μL aliquots were precipitated with 8% (w/v) TCA.
Suc-Density-Gradient Centrifugation
Microsomal membranes obtained from 5 g of cauliflower inflorescence homogenized in TDKE 10 buffer, pH 7.0 or in TDKE 10 buffer in which the EDTA was replaced with 3 mm MgCl2 were recovered on the interface of a 55% (w/w) Suc solution (dissolved in TDKE 10 buffer), and then loaded onto a linear 20% to 55% (w/w) Suc gradient. After centrifugation at 150,000g in a vertical rotor for 3 h, 1.5-mL fractions were harvested, and the proteins in 100-μL aliquots from each fraction were precipitated with 8% (w/v) TCA.
Nycodenz-Density-Gradient Centrifugation
Microsomal membranes prepared under low-Mg2+ conditions as described above were loaded onto a discontinuous Suc-density gradient (35%, 40%, and 55% [w/w]) and then centrifuged isopycnically as described above. The membranes collecting at the 40%/50% interface were diluted 1:1 with 70% (w/w) Nycodenz (Sigma) dissolved in TDKE 10 buffer and layered under three Nycodenz solutions of decreasing density (30%, 25%, and 15% [w/w]). After centrifugation at 240,000g for 3 h, the interfaces were removed, and the proteins were precipitated with 8% (w/v) TCA.
Membrane Treatments
To determine the nature of the binding of AtSec21/23p to microsomal membranes, 5-mg aliquots of total membrane pellets were resuspended in HDKE 10 buffer containing either 0.1% or 1% Triton X-100, 0.5 or 2 m urea, 0.1 m Na2CO3 at pH 11, and 0.25, 0.5, or 1 m NaCl and shaken for 1 h at 4°C before being centrifuged at 100,000g for 1 h. Microsomal membranes (200 μg) were also subjected to proteolysis with papain (2 μg in TDKE 10 buffer, pH 7.0, with or without 0.1% Triton X-100) and incubated on ice for various times. At each time indicated, 10 μg of leupeptin and 50 μL of hot, 3-fold-concentrated SDS sample buffer were added, and the mixture was boiled for an additional 5 min. Solubilized and digested proteins, and residual proteins in extracted membranes, were separated by SDS-PAGE and probed with antibodies as described below.
Protein Determinations and Enzyme Assays
Protein concentration was determined by dye binding (Bradford, 1976). Latent IDPase was determined as described by Robinson et al. (1994).
SDS-PAGE and Immunoblotting
Membrane and cytosolic proteins, as well as TCA-precipitated proteins, were separated by standard SDS-PAGE procedures (Holstein et al., 1994) using 10% separating gels. Polypeptides were electrophoretically transferred to nitrocellulose filters using a semidry apparatus operating at 2 mA cm−2 for 90 min. Immunoreactive proteins were detected using an ECL kit (Amersham) according to the manufacturer's instructions. Marker antibodies and their sources and dilutions were as follows: BiP (1:8,000), Dr. J. Denecke, University of York, UK; RGP (1:20,000), Dr. K. Dhugga, Pioneer Hi-Bred International, Johnston, IA; pATPase (1:1,000), Dr. W. Michalke, University of Freiburg, Germany; and β-adaptin (B1/M6; 1:500), Dr. M.S. Robinson, University of Cambridge, UK. AtSec21p and AtSec23p antisera were used at a primary dilution of 1:2,000.
RESULTS
AtSec21p and AtSec23p Are Both Membrane and Cytosol Located
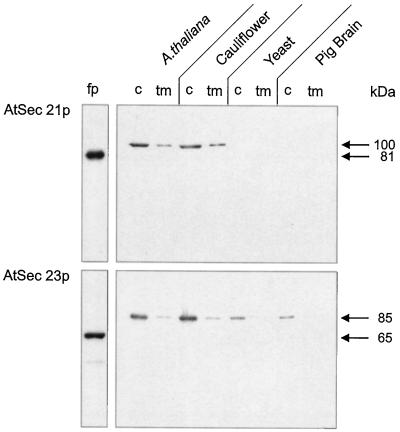
Antibodies prepared against the 81-kD GST-fusion protein of AtSec21p specifically recognized a 100-kD protein in homogenates of both Arabidopsis and cauliflower inflorescences (Fig. 1). Similarly, antibodies directed against the 65-kD AtSec23p GST-fusion protein cross-reacted with an 85-kD protein in both plant extracts. For both plants, relatively more immunoreactive protein was present in the cytosol (per milligram) than was associated with the total membrane fractions. Whereas AtSec21p antibodies failed to recognize any protein in yeast or pig brain homogenates, AtSec23p antibodies cross-reacted with an 85-kD polypeptide in these two nonplant organisms. Again, this antigen was more prominent in the cytosol than in the total membrane fraction on a relative-protein basis.
Figure 1.
Cross-reactivities of antisera generated against GST-fusion proteins (fp) prepared from cDNA clones for AtSec21p and AtSec23p. Antibodies prepared against the 81-kD AtSec21p fusion protein recognized a 100-kD protein in the cytosolic fractions (c) and microsomal membranes (tm) of suspension-cultured Arabidopsis and cauliflower inflorescences, but not in yeast or pig brain. By contrast, antibodies against the 65-kD AtSec23p fusion protein recognized an 85-kD protein in the cytosol of all four organisms. Using equal amounts of protein (20 μg per lane), the signal for the cytosolic fractions was always stronger.
Because the isolation of COP-coated vesicles from membrane fractions in vitro involves the use of nonhydrolyzable GTP analogs, which stabilize the vesicle coats, we investigated the effect of including GTPγS in the homogenizing medium on the relative amounts of membrane-bound to cytosol-located AtSec21p and AtSec23p antigens in cauliflower extracts. As shown in Figure 2A, the presence of 50 μm GTPγS did not lead to a significant redistribution of the two antigens.
It is well known that vesicle-mediated intracellular protein transport is inhibited at low temperatures (Tartakoff, 1987). As with anoxia, prolonged exposure to low temperatures leads to an accumulation of coat-protein complexes in the cytosol of mammalian cells (Goud et al., 1985; Merisko et al., 1986). In plants, prolonged cold treatment causes dramatic changes in the endomembrane system (Mollenhauer et al., 1975), also presumably a consequence of the prevention of vesicle traffic. When cauliflower plants were held overnight at 4°C, the distribution of both AtSec21p and AtSec23p antigens was altered; compared with a true intrinsic membrane protein such as the pATPase (Villalba et al., 1991), the levels of the membrane-bound forms of the AtSec21p and AtSec23p antigens were reduced considerably (Fig. 2B). By contrast, the relative amounts of the two antigens in the cytosol appeared to increase. This result is similar to that obtained by Bar-Peled and Raikhel (1997) for AtSar1p with Arabidopsis cell cultures.
Cytosolic AtSec21p and AtSec23p Belong to Different Protein Complexes
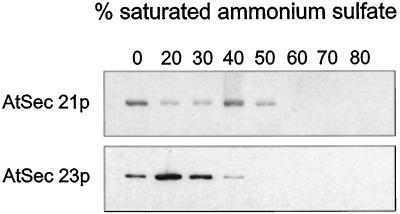
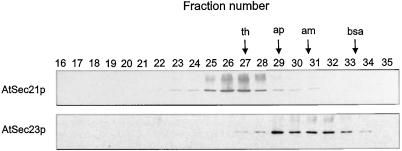
Cytosol from cauliflower inflorescence was subjected to sequential protein precipitation with increasing concentrations of (NH4)2SO4, and the fractions were probed with AtSec21p and AtSec23p antisera. Whereas the AtSec21p antigen was principally found in the 40% (NH4)2SO4 fraction, the majority of the AtSec23p antigen was precipitated by 20% to 30% (NH4)2SO4 (Fig. 3). When a 40% (NH4)2SO4 precipitate was subjected to gel filtration on Sepharose CL-6B, the AtSec21p antigen eluted in fractions earlier than the 669-kD calibrating protein thyroglobulin, indicating a molecular mass somewhat greater than 700 kD. In contrast, the AtSec23p antigen had an elution profile similar to that of β-amylase, i.e. a molecular mass of around 200 kD (Fig. 4). These values correspond well to those given for the COPI coatomer of mammalian cells (Waters et al., 1991) and the COPII Sec23/24 dimer of yeast cells (Hicke et al., 1992).
Figure 3.
AtSec21p and AtSec23p antigens precipitate differently with respect to (NH4)2SO4. Cytosol extracted from fresh cauliflower inflorescence was subjected to sequential protein precipitation with increasing concentrations of (NH4)2SO4 (20 μg of protein per lane).
Figure 4.
Sepharose CL-6B column chromatography of 40% ammonium-sulfate-precipitated cytosolic proteins obtained from cauliflower inflorescence. Calibrating proteins are thyroglobulin (th; 669 kD), apoferritin (ap; 443 kD), β-amylase (am; 200 kD), and BSA (bsa; 66 kD).
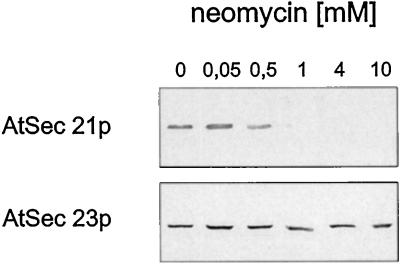
A further indication that the AtSec21p antigen in cauliflower cytosol is part of a plant coatomer complex was found in data obtained with the aminoglycoside antibiotic neomycin (Fig. 5). Coatomer from mammalian cells is known to interact with a dilysine (KKXX) motif present in the cytoplasmic domain of membrane proteins known to travel from the Golgi to the ER (Letourneur et al., 1994; for review, see Lowe and Kreis, 1998). Neomycin contains paired amino groups at three separate locations in the molecule, and it has been suggested by Hudson and Draper (1997) that coatomer has a binding site that accommodates two amino groups such as those present in neomycin. As a result, neomycin effectively cross-links coatomer into large, sedimentable aggregates. We treated cauliflower cytosol with increasing concentrations of neomycin and found that, in contrast to the AtSec23p antigen, the cytosol was effectively depleted of the AtSec21p antigen with 4 mm neomycin (Fig. 5).
Figure 5.
Neomycin selectively precipitates AtSec21p antigen from cauliflower cytosol. Increasing concentrations of neomycin were added to the cytosol proteins, and the sedimentable proteins were removed by centrifugation at 100,000g (see Methods). Aliquots of the soluble supernatant were then subjected to SDS-PAGE, followed by western blotting with AtSec21p and AtSec23p antisera.
Membrane-Associated AtSec21p and AtSec23p Are Not Integral Membrane Proteins
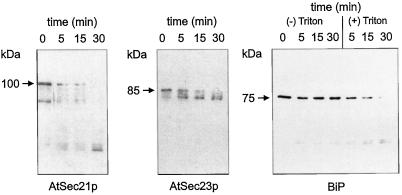
Microsomal membranes from cauliflower cytosol were subjected to proteolysis with the acid protease papain (Fig. 6). In the absence of detergent, papain effectively digested AtSec21p and AtSec23p within 15 min, whereas the ER lumenal protein BiP was only marginally affected by this treatment (probably indicating a small number of unsealed membrane vesicles). In the presence of detergent, BiP was fully degraded after 30 min. These results indicate that AtSec21p and AtSec23p are located at the surface rather than in the lumen of membranes in cell homogenates.
Figure 6.
AtSec21p and AtSec23p antigens are exposed at the surface of membranes. Microsomal membranes from cauliflower inflorescence were resuspended in HDKE 10 buffer and subjected to proteolysis at 1°C with papain in the presence or absence of detergent (see Methods). The membrane suspensions were taken up in sample buffer and subjected to SDS-PAGE followed by western blotting.
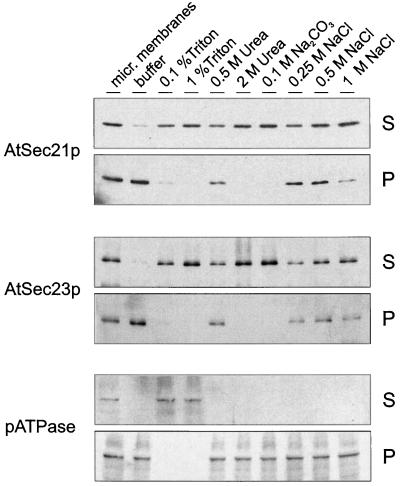
To determine the nature of the membrane association of the AtSec21p and AtSec23p antigens, we subjected cauliflower microsomal membranes to a variety of extraction conditions and analyzed the resulting pellets and supernatants for the presence of the antigens (Fig. 7). As a standard, we recorded the response of an intrinsic membrane protein, pATPase, to these treatments. Resuspension in salt-free buffer alone led to the release of small amounts of the AtSec21p and AtSec23p antigens. NaCl treatment solubilized additional quantities of the two antigens, but even with 1 m NaCl, significant amounts remained membrane bound. On the other hand, 2 m urea, 0.1 m Na2CO3, and 1% Triton X-100 effectively removed AtSec21p and AtSec23p from the membranes. By comparison, pATPase was released from the membranes only through detergent treatment. Thus, like AtSar1p (Bar-Peled and Raikhel, 1997), AtSec21p and AtSec23p are tightly associated peripheral membrane proteins.
Figure 7.
AtSec21p and AtSec23p antigens are membrane-extrinsic proteins. Total membrane fractions from cauliflower inflorescence were taken up in HDKE 10 buffer alone or with Triton X-100, urea, Na2CO3, or NaCl at the concentrations given. After shaking for 1 h at 4°C, the membrane suspensions were centrifuged at 100,000g to separate membrane-bound, pelletable (P) from solubilized (S) proteins.
Subcellular Localization of AtSec21p and AtSec23p
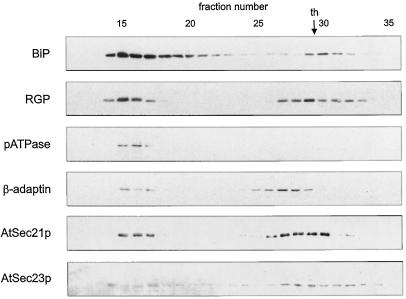
To identify the membranes responsible for binding AtSec21p and AtSec23p, we first attempted to separate organelles by gel filtration on Sephacryl S-1000 (Fig. 8). The elution profiles for AtSec21p and AtSec23p were identical and showed two peaks: one constituting fractions 14 to 18, and the other constituting fractions 25 to 33. Similar profiles were obtained for markers of the ER (BiP), the Golgi apparatus (RGP; Dhugga et al., 1997; Delgado et al., 1998), and the β-adaptin of CCV (B1/M6; Holstein et al., 1994). In contrast, pATPase was found only in fractions 15 to 17. Because the latter is an intrinsic membrane protein (see above), and all of the other markers, including AtSec21p and AtSec23p antigens, are soluble or extrinsic membrane proteins, we assumed that the antigenic proteins detected in fractions 25 to 33 represent dissociated, membrane-free proteins. Huang and Chiang (1997) have also used Sephacryl S-1000 to separate organelles from the total membrane fraction obtained from yeast. Although Sec21p and Sec22p (COPII) coeluted with integral membrane markers for the ER and Golgi, their markers for the plasma membrane (yeast pATPase) and endosomes (Pep12p) had a completely different distribution.
Figure 8.
Sephacryl S-1000 gel filtration of microsomal membranes extracted from cauliflower inflorescence. A microsomal membrane pellet was resuspended in extraction buffer and applied to the column, which was then eluted with the same buffer. Each fraction was monitored by western blotting with antibodies against marker proteins for the ER (BiP), Golgi apparatus (RGP), CCVs (β-adaptin), and plasma membrane (pATPase), as well as with AtSec21p and AtSec23p antibodies. The calibrating soluble protein was thyroglobulin (th).
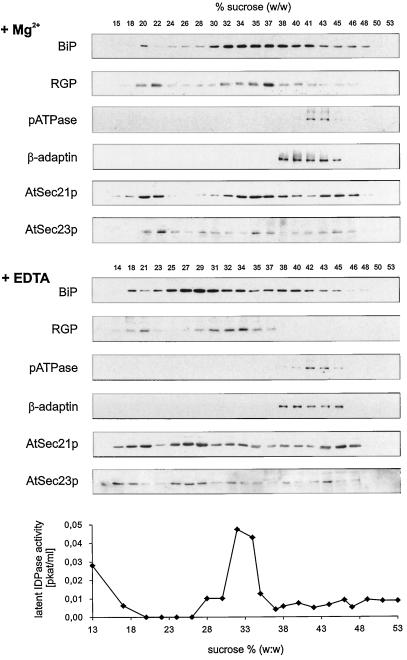
Because no clear membrane separation could be obtained by gel filtration, we performed Suc-density-gradient centrifugation of a cauliflower microsomal membrane fraction prepared under low- (+EDTA) and high- (+MgCl2) Mg2+ conditions (Fig. 9). In both types of gradient, small amounts of BiP, RGP, AtSec21p, and AtSec23p antigens were detected in fractions with densities around 20% (w/w) Suc, representing solubilized proteins. As expected, the major BiP profiles showed a typical shift to a higher density when the ER was retained in its rough, ribosome-attached form. A smaller but still significant shift (from a peak at 34% [w/w] Suc to a peak at 37% [w/w] Suc in high-Mg2+ gradients) was also observed for the Golgi marker RGP, which was confirmed by measurement of latent IDPase activity. Independent of Mg2+, the pATPase equilibrated at densities equivalent to 41% (w/w) Suc. The binding profile for the β-adaptin antibody B1/M6 was very similar and likely represented not only free CCV, but also plasma membrane-bound adaptin, as reported by Drucker et al. (1995). However, it is also possible that clathrin-coated elements of the Golgi apparatus, which are often found detached from the rest of the Golgi stack in situ (Robinson, 1985), tend to become separated from the rest of the cisternae upon homogenization and may also equilibrate at Suc concentrations around 41% (w/w).
Figure 9.
AtSec21p and AtSec23p antigens are bound to ER and Golgi membranes. Isopycnic Suc-density gradients of microsomal membrane fractions from cauliflower inflorescence were prepared under low (+EDTA) or high (+Mg2+) Mg2+ conditions. The fractions were probed by western blotting with the antibodies given in Figure 8 and tested for latent IDPase activity.
Common to the distribution profiles for AtSec21p and AtSec23p, irrespective of the free Mg2+ concentration, were peaks at very high densities (43%–46% [w/w] Suc). However, whereas the other major AtSec23p peak followed the distribution of BiP under both low- and high-Mg2+ conditions, AtSec21p seemed to have a double peak under low Mg2+, one corresponding to the BiP peak at around 28.5% (w/w) Suc and the other coequilibrating with RGP at around 34% (w/w) Suc. These results suggest that AtSec23p might be principally associated with ER membranes, whereas AtSec21p seems to be borne by both ER and Golgi membranes.
Attempted Isolation of COP-Coated Vesicles
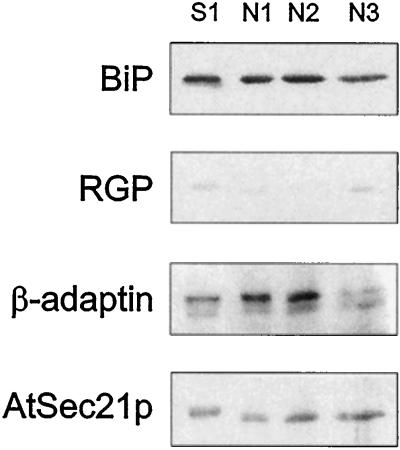
Because COPI-coated vesicles formed in vitro from rabbit liver Golgi also equilibrate at 40% to 45% (w/w) Suc (Malhotra et al., 1989), we surmised that the AtSec21p/23p-containing high-density fractions from cauliflower might contain an endogenous population of COP-coated vesicles. In an attempt to further purify such vesicles, we subjected the membranes present in the high-Suc-density fractions to isopycnic centrifugation on a discontinuous Nycodenz gradient, as was successfully used by Barlowe et al. (1994) for the isolation of yeast COPII vesicles (Fig. 10). Whereas in their experiments the COP vesicles became concentrated at the 15%/25% (w/v) Nycodenz interface, in our case there was no significant enrichment of AtSec21p in any of the gradient interfaces. Moreover, the ER and β-adaptin markers were also equally distributed among the three Nycodenz interfaces.
Figure 10.
Nycodenz-gradient centrifugation of high-density AtSec21p antigen-containing membrane fraction from a Suc gradient. Microsomal membranes from cauliflower inflorescence were layered onto a discontinuous Suc-density gradient and centrifuged isopycnically. The membranes collecting on the 40%/55% (w/w) Suc interface (S1) were layered under a discontinuous Nycodenz gradient (see Methods) and recentrifuged until equilibrium conditions were obtained. TCA-precipitable proteins in the fractions collecting at the three interfaces (N1, 15%/25%; N2, 25%/30%; and N3, 30%/35%) were monitored by western blotting as described above (20 μg of protein per lane).
DISCUSSION
Like the triskelions and adaptors of CCV, the coat subunits of COPI and COPII-coated vesicles are also present in the cytosol as preformed building blocks: the coatomer and the two dimeric complexes, Sec13/31p and Sec23/24p (Barlowe, 1998; Gaynor et al., 1998). The data presented here for AtSec21p and AtSec23p antigens in cauliflower conform with this: Both proteins are part of different cytosolic complexes that are similar in size to their mammalian and yeast counterparts. They associate with membranes, but not when vesicle production is inhibited by low temperature. Moreover, the selective precipitation of the AtSec21p complex with neomycin can be taken as a good indicator that this complex is a plant coatomer.
Anterograde protein transport from the ER in yeast and mammalian cells is accomplished by vesicles that bear a COPII coat (Rowe et al., 1996; Barlowe, 1998) and is mediated by the GTPase Sar1p (Aridor et al., 1995). This has been shown by in vitro vesicle-induction experiments with nuclear or ER membranes, and it has been confirmed in situ by immunogold-localization studies with antisera against Sec13p, Sec23p, and Sar1p that recognized budding profiles on transitional (ribosome-free) ER (Orci et al., 1991, 1994; Shaywitz et al., 1995). On the basis of our subcellular fractionation data, we suggest that the AtSec23p antigen in the cauliflower inflorescence is bound preferentially to ER membranes, suggesting that the principal site of COPII-coated vesicle production in plants is also the ER.
The site of COPI-coated vesicle production is generally assumed to be the Golgi apparatus, whether COPI vesicles are considered to operate in a retrograde direction back to the ER (Letourneur et al., 1994; Sönnichsen et al., 1996), in an anterograde direction across the Golgi stack (for review, see Lowe and Kreis, 1998), or simultaneously in both directions in the same Golgi apparatus (Orci et al., 1997). Indeed, immunocytochemical studies with various coatomer antisera (Duden et al., 1991; Oprins et al., 1993; Griffiths et al., 1995; Orci et al., 1997) invariably reveal a conspicuous labeling of budding vesicles at the periphery of Golgi cisternae. However, a special coatomer-binding domain for the ER has been described in mammalian cells (Oprins et al., 1993; Orci et al., 1994), and both COPI and COPII-coated vesicles can be formed by yeast ER in vitro (Bednarek et al., 1995), although the physiological significance of the latter finding remains unclear (Barlowe, 1998). Our subcellular-fractionation data could also be interpreted as showing the binding of the AtSec21p antigen to both ER and Golgi membranes of the cauliflower inflorescence, but a definitive statement on the site(s) of COPI-coated vesicle formation in plants must await the results of in situ immunogold labeling.
To our knowledge, unlike CCV, COP-coated vesicles have been isolated only after having been induced in vitro (Malhotra et al., 1989; Barlowe et al., 1994). Vesicle production normally requires GTP, which binds to either ARF or Sar1p, but such vesicles are unstable and rapidly lose their coat. However, the nonhydrolyzable analogs GTPγS and GMP-PNP (guanylyl-imidodiphosphate) can also be used to drive vesicle formation, and the resulting COP-coated vesicles retain their stability. In vivo, COP-coated vesicles formed with endogenous GTP are consequently inherently unstable and therefore are difficult to isolate with an intact coat. This explains why the addition of GTPγS to the homogenization medium is without effect, because the GTP-binding sites are already occupied. Nevertheless, our data from Suc-density-gradient centrifugation suggest that an endogenous population of AtSec21/23p-coated vesicles, equilibrating at densities typical for COP-coated vesicles derived from mammals and yeast, survives at least for a short time outside of the plant. Although Golgi membranes are known to be scarce in this fraction, we were unable to remove other membranes (including the ER) by subsequent Nycodenz-density-gradient centrifugation, as was achieved by Barlowe et al. (1994) for COPII-coated vesicles from yeast. Because membrane-bound AtSec21p or AtSec23p cannot be precipitated with our antisera (data not shown), an immunoaffinity purification for plant COP-coated vesicles is not yet possible. Their successful isolation, therefore, will be delayed until an effective and convincing in vitro vesicle-budding system using plant extracts has been established.
Abbreviations:
- BiP
immunoglobulin heavy chain-binding protein cognate
- B1/M6
monoclonal antibody generated against bovine brain β-adaptin
- CCV
clathrin-coated vesicle
- GST
glutathione-S-transferase
- GTPγS
guanosine 5′-O-(3-thiotriphosphate)
- pATPase
plasma membrane-associated ATPase
- RGP
reversibly glycosylated protein
Footnotes
A.M. was the recipient of a scholarship from the Ministry of Culture and Education of the Government of Iran. G.-H.T. was a Research Fellow of the Alexander-von-Humboldt Stiftung (Bonn, Germany). This work was also supported by funds from the Deutsche Forschungsgemeinschaft (grant no. SFB 523).
LITERATURE CITED
- Andreeva AV, Kutuzov MA, Evans DE, Hawes CR. The structure and function of the Golgi apparatus: a hundred years of questions. J Exp Bot. 1998a;49:1281–1291. [Google Scholar]
- Andreeva AV, Kutuzov MA, Evans DE, Hawes CR. Proteins involved in membrane transport between the ER and the Golgi apparatus: 21 putative plant homologues revealed by dbEST searching. Cell Biol Int. 1998b;22:145–160. doi: 10.1006/cbir.1998.0235. [DOI] [PubMed] [Google Scholar]
- Aridor M, Bannykh T, Rowe T, Balch W. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. COPII and selective export from the endoplasmic reticulum. Biochim Biophys Acta. 1998;1404:67–76. doi: 10.1016/s0167-4889(98)00047-0. [DOI] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeong T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherst M, Schekman R. COPII: a membrane coat formed by sec proteins that drives vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bar-Peled M, Raikhel NV. Characterization of AtSec12 and AtSar1. Proteins likely involved in endoplasmic reticulum and Golgi transport. Plant Physiol. 1997;114:315–324. doi: 10.1104/pp.114.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COP I- and COP II-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Beevers L. Clathrin-coated vesicles in plants. Int Rev Cytol. 1996;167:1–35. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brodsky FM. New fashions in vesicle coats. Trends Cell Biol. 1997;7:175–179. doi: 10.1016/S0962-8924(97)01038-6. [DOI] [PubMed] [Google Scholar]
- Campbell I. Standard media for cultivation of yeasts. In: Campbell I, Duffus JH, editors. Yeast: A Practical Approach. Oxford, UK: IRL Press; 1988. p. 277. [Google Scholar]
- Dascher C, Balch WE. Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J Biol Chem. 1994;269:1437–1448. [PubMed] [Google Scholar]
- Delgado IJ, Wang Z, Rocher AD, Keegstra K, Raikhel NV. Cloning and characterization of AtRGP1. A reversibly autoglycosylated Arabidopsis protein implicated in cell wall biosynthesis. Plant Physiol. 1998;116:1339–1349. doi: 10.1104/pp.116.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert C, Geusse M, Gaillardin C. Fission yeast and a plant have functional homologues of the Sar1 and Sec12 proteins involved in ER to Golgi traffic in budding yeast. EMBO J. 1992;11:4205–4210. doi: 10.1002/j.1460-2075.1992.tb05514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhugga KS, Tiwari SC, Ray PM. A reversibly glycosylated polypeptide (RGP1) possibly involved in plant cell wall synthesis: purification, gene cloning and trans Golgi localization. Proc Natl Acad Sci USA. 1997;94:7679–7684. doi: 10.1073/pnas.94.14.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozolme P, Marty-Mazars D, Clémencet M-C, Marty F. Monoclonal antibody TeM 106 reacts with a tonoplast intrinsic protein of 106 kDa from Brassica oleracea L. J Cell Sci. 1995;108:1509–1517. doi: 10.1242/jcs.108.4.1509. [DOI] [PubMed] [Google Scholar]
- Drucker M, Herkt B, Robinson DG. Demonstration of a β-adaptin at the plasma membrane. Cell Biol Int. 1995;19:191–201. [Google Scholar]
- Duden R, Griffiths G, Franke R, Argos P, Kreis TE. β-COP, a 110 kDa protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to β-adaptin. Cell. 1991;64:649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Gaynor EC, Graham TR, Emr SD. COPI in ER/Golgi and intra-Golgi transport: do yeast COPI mutants point the way? Biochim Biophys Acta. 1998;1404:33–51. doi: 10.1016/s0167-4889(98)00045-7. [DOI] [PubMed] [Google Scholar]
- Goud B, Huet C, Louvard D. Assembled and unassembled pools of clathrin: a quantitative study using an enzyme immunoassay. J Cell Biol. 1985;100:521–527. doi: 10.1083/jcb.100.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Pepperkok R, Krijnse-Locker J, Kreis TE. Immunocytochemical localization of β-COP to the ER-Golgi boundary and the TGN. J Cell Sci. 1995;108:2839–2856. doi: 10.1242/jcs.108.8.2839. [DOI] [PubMed] [Google Scholar]
- Hawes CR, Faye L, Satiat-Jeunemaitre B (1966) The Golgi apparatus and pathways of vesicle trafficking. In M Smallwood, JP Knox, DJ Bowles, eds, Membranes: Specialized Functions in Plants. BIOS Publishers, Oxford, UK, pp 337–365
- Hellgren L, Morré DJ, Selldén G, Sandelius AS. Isolation of a putative vesicular intermediate in the cell-free transfer of membrane from transitional endoplasmic reticulum to the Golgi apparatus of etiolated seedlings of garden pea. J Exp Bot. 1993;44:197–205. [Google Scholar]
- Hicke L, Yoshiga T, Schekman R. Sec23p and a novel 105 kDa protein function as a multimeric complex to promote vesicle budding and protein transport from the ER. Mol Biol Cell. 1992;3:667–676. doi: 10.1091/mbc.3.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein SEH, Drucker M, Robinson DG. Identification of a β-type adaptin in plant clathrin-coated vesicles. J Cell Sci. 1994;107:945–953. doi: 10.1242/jcs.107.4.945. [DOI] [PubMed] [Google Scholar]
- Huang P-H, Chiang H-L. Identification of novel vesicles in the cytosol to vacuole protein degradation pathway. J Cell Biol. 1997;136:803–810. doi: 10.1083/jcb.136.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson RT, Draper RK. Interaction of coatomer with aminoglycoside antibiotics: evidence that coatomer has at least two dilysine binding sites. Mol Biol Cell. 1997;8:1901–1910. doi: 10.1091/mbc.8.10.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lowe M, Kreis TE. Regulation of membrane traffic in animal cells by COPI. Biochim Biophys Acta. 1998;1404:53–66. doi: 10.1016/s0167-4889(98)00046-9. [DOI] [PubMed] [Google Scholar]
- Luykx P, Hoppenrath M, Robinson DG. Osmoregulatory mutants that affect the function of the contractile vacuole in Chlamydomonas reinhardtii. Protoplasma. 1997;200:99–111. [Google Scholar]
- Malhotra V, Serafini T, Orci L, Shepherd JC, Rothman JE. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- Merisko EM, Fletcher MA, Palade GE. The reorganization of the Golgi complex in anoxic pancreatic acinar cells. Pancreas. 1986;1:95–108. doi: 10.1097/00006676-198603000-00001. [DOI] [PubMed] [Google Scholar]
- Mollenhauer HH, Morré DJ. Perspectives on Golgi apparatus form and function. J Electron Microscopy Techniques. 1991;17:2–14. doi: 10.1002/jemt.1060170103. [DOI] [PubMed] [Google Scholar]
- Mollenhauer HH, Morré DJ, Vanderwoude WJ. Endoplasmic reticulum-Golgi apparatus associations in maize root tips. Mikroskopie. 1975;31:257–272. [PubMed] [Google Scholar]
- Nakano A, Brada D, Schekman R. A membrane glycoprotein, Sec12p, required for transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol. 1988;107:851–863. doi: 10.1083/jcb.107.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprins A, Duden R, Kreis TE, Geuze HJ, Slot JW. Beta-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J Cell Biol. 1993;121:49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Perrelet A, Ravazzola M, Amherdt M, Rothman JE, Schekman R. Coatomer-rich endoplasmic reticulum. Proc Natl Acad Sci USA. 1994;91:11924–11928. doi: 10.1073/pnas.91.25.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Meda P, Holcomb C, Moore H-P, Hicke L, Schekman R. Mammalian Sec23p homolog is restricted to the endoplasmic reticulum transitional cytoplasm. Proc Natl Acad Sci USA. 1991;88:8611–8615. doi: 10.1073/pnas.88.19.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Söllner TH, Rothman JE. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Regad F, Bardet C, Tremousaygue D, Moisan A, Lescure B, Axelos M. cDNA cloning and expression of an Arabidopsis GTP-binding protein of the ARF family. FEBS Lett. 1993;316:133–136. doi: 10.1016/0014-5793(93)81201-a. [DOI] [PubMed] [Google Scholar]
- Robinson DG. Plant Membranes. New York: John Wiley & Sons; 1985. [Google Scholar]
- Robinson DG, Bäumer M, Hinz G, Hohl I. Ultrastructure of pea cotyledon Golgi apparatus: origin of dense vesicles and the action of brefeldin A. Protoplasma. 1997;200:198–209. [Google Scholar]
- Robinson DG, Hinz G, Holstein SEH. The molecular characterization of transport vesicles. Plant Mol Biol. 1998;38:49–76. [PubMed] [Google Scholar]
- Robinson DG, Hinz G, Oberbeck K. The isolation of plant endo- and plasma membranes. In: Harris N, Oparka K, editors. Plant Cell Biology: A Practical Approach. Oxford, UK: IRL Press; 1994. pp. 245–272. [Google Scholar]
- Rowe T, Aridor M, McCaffery JM, Plutner H, Nuoffer C, Balch WE. COPII vesicles derived from mammalian endoplasmic reticulum microsomes recruit COPI. J Cell Biol. 1996;135:895–911. doi: 10.1083/jcb.135.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satiat-Jeunemaitre B, Cole L, Bourett T, Howard R, Hawes C. Brefeldin A effects in plant and fungal cells: something new about vesicle trafficking? J Microsc. 1996;181:162–177. doi: 10.1046/j.1365-2818.1996.112393.x. [DOI] [PubMed] [Google Scholar]
- Schmid SL, Damke H. Coated vesicles: a diversity of form and function. FASEB J. 1995;9:1445–1453. doi: 10.1096/fasebj.9.14.7589986. [DOI] [PubMed] [Google Scholar]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Shaywitz L, Orci M, Ravazzola M, Swaroop C, Kaiser C. Human Sec13Rp functions in yeast and is located on transport vesicles budding from the endoplasmic reticulum J Cell Biol. 1995;128:769–777. doi: 10.1083/jcb.128.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B, Watson R, Clausen H, Misteli T, Warren G. Sorting by COPI-coated vesicles under interphase and mitotic conditions. J Cell Biol. 1996;134:1411–1425. doi: 10.1083/jcb.134.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin A, Moore I. The plant Golgi apparatus: structure, functional organization, and trafficking mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:261–288. [Google Scholar]
- Tartakoff AM. The Secretory and Endocytic Paths: Mechanism and Specificity of Vesicular Traffic in the Cell Cytoplasm. New York: John Wiley & Sons; 1987. [Google Scholar]
- Villalba JM, Lützelschwab M, Serrano R. Immunocytolocalization of plasma membrane H+-ATPase in maize coleoptiles and enclosed leaves. Planta. 1991;185:458–461. doi: 10.1007/BF00202953. [DOI] [PubMed] [Google Scholar]
- Waters MG, Serafini T, Rothman JE. “Coatomer”: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi-transport vesicles. Nature. 1991;349:248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]