Abstract
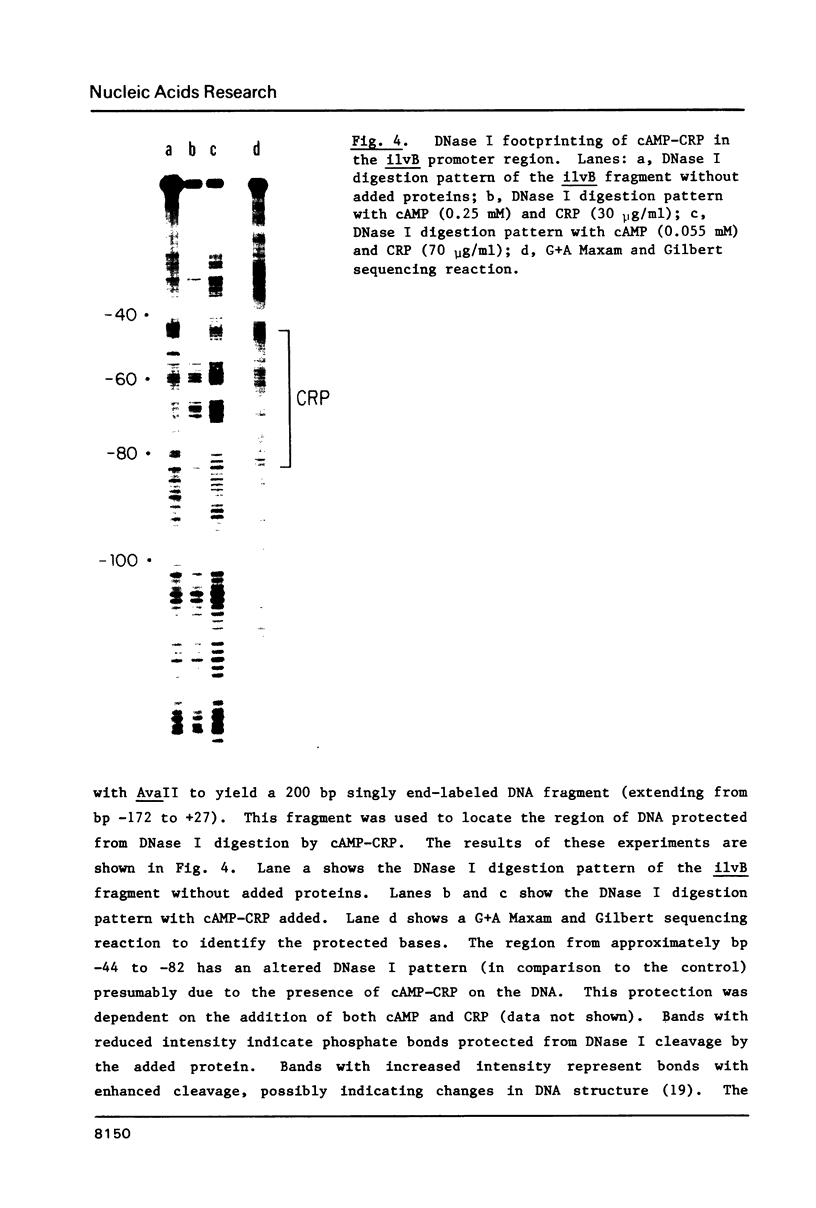
DNase and restriction site protection studies show that cAMP and its receptor protein (CRP) bind to the promoter of the ilvB operon at approximately position -44 to -82. This region contains sequences that are homologous to those found in other CRP-dependent promoters. In vitro transcription from the ilvB promoter was markedly increased by the addition of cAMP and CRP. This stimulation was not found when the ilvB template lacked the proposed CRP binding site. cAMP-CRP did not alter the extent of transcription termination within the ilvB leader suggesting that this regulatory system may be independent of the attenuation mechanism involved in the negative control of this operon. The results of restriction enzyme site protection studies and experiments with altered promoter fragments indicate that the mechanism for CRP stimulation of the ilvB operon may be similar to a model recently proposed for lac.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Garges S. How cyclic AMP and its receptor protein act in Escherichia coli. Cell. 1982 Jun;29(2):287–289. doi: 10.1016/0092-8674(82)90145-3. [DOI] [PubMed] [Google Scholar]
- Chapon C., Kolb A. Action of CAP on the malT promoter in vitro. J Bacteriol. 1983 Dec;156(3):1135–1143. doi: 10.1128/jb.156.3.1135-1143.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs G. J., Ohtsubo H., Ohtsubo E., Sonnenberg F., Freundlich M. Restriction endonuclease mapping of the Escherichia coli K12 chromosome in the vicinity of the ilv genes. J Mol Biol. 1977 Nov 25;117(1):175–193. doi: 10.1016/0022-2836(77)90030-4. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Deeley M. C., Yanofsky C. Transcription initiation at the tryptophanase promoter of Escherichia coli K-12. J Bacteriol. 1982 Aug;151(2):942–951. doi: 10.1128/jb.151.2.942-951.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denney R. M., Yanofsky C. Detection of tryptophan messenger RNA in several bacterial species and examination of the properties of heterologous DNA-RNA hybrids. J Mol Biol. 1972 Mar 14;64(2):319–339. doi: 10.1016/0022-2836(72)90501-3. [DOI] [PubMed] [Google Scholar]
- Freundlich M. Cyclic AMP can replace the relA-dependent requirement for derepression of acetohydroxy acid synthase in E. coli K-12. Cell. 1977 Dec;12(4):1121–1126. doi: 10.1016/0092-8674(77)90174-x. [DOI] [PubMed] [Google Scholar]
- Friden P., Newman T., Freundlich M. Nucleotide sequence of the ilvB promoter-regulatory region: a biosynthetic operon controlled by attenuation and cyclic AMP. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6156–6160. doi: 10.1073/pnas.79.20.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden P., Voelkel K., Sternglanz R., Freundlich M. Reduced expression of the isoleucine and valine enzymes in integration host factor mutants of Escherichia coli. J Mol Biol. 1984 Feb 5;172(4):573–579. doi: 10.1016/s0022-2836(84)80024-8. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C. A., Hatfield G. W. Attenuation of the ilvB operon by amino acids reflecting substrates or products of the ilvB gene product. Proc Natl Acad Sci U S A. 1984 Jan;81(1):76–79. doi: 10.1073/pnas.81.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C. A., Hatfield G. W. Nucleotide sequence of the ilvB multivalent attenuator region of Escherichia coli K12. Nucleic Acids Res. 1983 Jan 11;11(1):127–139. doi: 10.1093/nar/11.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Ogden S., Haggerty D., Stoner C. M., Kolodrubetz D., Schleif R. The Escherichia coli L-arabinose operon: binding sites of the regulatory proteins and a mechanism of positive and negative regulation. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3346–3350. doi: 10.1073/pnas.77.6.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Rosenberg M. A promoter of pBR322 activated by cAMP receptor protein. Nucleic Acids Res. 1981 Jul 24;9(14):3365–3377. doi: 10.1093/nar/9.14.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg G. D., Sarthy A., Larson A. E., Backer M. P., Crabb J. W., Lidstrom M., Hall B. D., Furlong C. E. Xylose isomerase from Escherichia coli. Characterization of the protein and the structural gene. J Biol Chem. 1984 Jun 10;259(11):6826–6832. [PubMed] [Google Scholar]
- Schmitz A. Cyclic AMP receptor proteins interacts with lactose operator DNA. Nucleic Acids Res. 1981 Jan 24;9(2):277–292. doi: 10.1093/nar/9.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Freundlich M. Regulation of cyclic AMP of the ilvB-encoded biosynthetic acetohydroxy acid synthase in Escherichia coli K-12. Mol Gen Genet. 1980 Apr;178(1):179–183. doi: 10.1007/BF00267227. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., O'Neill M., de Crombrugghe B. Interaction site of Escherichia coli cyclic AMP receptor protein on DNA of galactose operon promoters. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5090–5094. doi: 10.1073/pnas.76.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann A., Joseph E., Danchin A. Cyclic AMP as a modulator of polarity in polycistronic transcriptional units. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3194–3197. doi: 10.1073/pnas.76.7.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]