Abstract
To assess myosin heavy chain (MHC) plasticity in aging skeletal muscle with aerobic exercise training, MHC composition was measured at the messenger RNA (mRNA) level and protein level in mixed-muscle homogenates and single myofibers. Muscle samples were obtained from eight nonexercising women (70 ± 2 years) before and after 12 weeks of training (20–45 minutes of cycle exercise per session at 60%–80% heart rate reserve, three to four sessions per week). Training elevated MHC I mRNA (p < .10) and protein (p < .05) in mixed-muscle (54% ± 4% to 61% ± 2%) and single myofibers (42% ± 4% to 52% ± 3%). The increase in MHC I protein was positively correlated (p < .05) with improvements in whole muscle power. Training resulted in a general downregulation of MHC IIa and IIx at the mRNA and protein levels. The training-induced increase in MHC I protein and mRNA demonstrates the maintenance of skeletal muscle plasticity with aging. Furthermore, these data suggest that a shift toward an oxidative MHC phenotype may be beneficial for metabolic and functional health in older individuals.
Keywords: Endurance training, Myofiber, Physical activity, Elderly, Sarcopenia
SARCOPENIA, the age-related loss of skeletal muscle mass, is associated with an increase in disability and a decrease in independent living (1,2). This loss of skeletal muscle mass is mediated by a reduction in total number of myofibers (3,4), decreased size of fast-twitch myosin heavy chain (MHC) IIa fibers (3–5), and altered MHC morphology (6,7). These maladaptations create negative metabolic and functional implications within aging skeletal muscle that impede healthy aging.
Previous investigations have shown that myofibers from skeletal muscle of young individuals are capable of altering their MHC profile in response to various perturbations, such as prolonged unloading (8,9), resistance (10,11), and aerobic exercise training (12,13). Conversely, research focusing on the plasticity of aging skeletal muscle in response to exercise training is heterogeneous (14–18). These equivocal findings may be a result of the techniques that have been implemented to assess the MHC distribution in aging skeletal muscle and/or the characteristics of the aging population examined. For example, after an aerobic exercise program consisting of walking and jogging for 9–12 months, older participants (age 64 ± 3 years) demonstrated a shift toward a more oxidative fiber type profile when analyzed with mATPase histochemistry (16). However, when utilizing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) to determine MHC composition from mixed-muscle homogenates of older (age 60–87 years) men and women, Short and colleagues (17) reported no change in the proportion of MHC protein isoforms despite alterations at the messenger RNA (mRNA) level, which may suggest a distinct translational regulation. The variety of participant population and methodology used to detect MHC composition most likely accounts for the ambiguity after aerobic exercise training in aging skeletal muscle.
To our knowledge, no investigation has examined the MHC distribution of individual myofibers from aging skeletal muscle (>65 years) after an aerobic exercise training program. Therefore, the primary focus of the present study was to provide a profile of MHC composition at the mRNA, mixed-muscle homogenate, and single muscle fiber levels after a 12-week progressive aerobic exercise training program. We have previously reported that this aerobic training program induced hypertrophy of the quadriceps femoris group (11%) and MHC I myofibers (16%) as well as augmented MHC I myofiber power (21%) and improved aerobic capacity (30%) of older women (19,20). Therefore, we hypothesized that after the aerobic training program, MHC I mRNA expression and the percentage of MHC I protein at the mixed-muscle homogenate and single-fiber level would be elevated in a similar cohort of older women (70 ± 2 years).
METHODS
Eight older women (Table 1) volunteered to participate in this investigation. All participants were cleared to participate by the study physician following a thorough physical examination and remained weight stable for the duration of the investigation. Volunteers were excluded based on the following criteria: body mass index ≥28 kg/m2; type 1 or type 2 diabetes; uncontrolled hypertension; active cancer, cancer in remission, or having received treatment for any form of cancer in the previous five years; coronary artery disease; cardiovascular disease (eg, peripheral artery disease, peripheral vascular disease); abnormal thyroid function; nonsedentary defined as one who has completed regular aerobic or resistance exercise more than one time per week for 20 minutes or longer during the previous year; chronic and/or regular non-steriodal anti-inflammatory drug consumption, and any condition that presents a limitation to exercise training (eg, severe arthritis, chronic obstructive pulmonary disease, neuromuscular disorder, moderate or severe cognitive impairment, dizziness) (19). Written informed consent, approved by the Institutional Review Boards of Ball State University and Ball Memorial Hospital, was obtained from each participant prior to study participation.
Table 3.
Summary of mRNA Primers Used for Real-Time Polymerase Chain Reaction
| Protein Isoform | mRNA | Sequence | Gene Bank No. | Amplicon Size (bp) | mRNA Region (bp) | |
| MHC I | MYH7 | Sense | 5′–TGGGGCTGATGCGCCTATTGAGAA–3′ | NM_000257 | 129 | 1,953–2,081 |
| Antisense | 5′–TGGGGATGGGTGGAGCGCAAGTT–3′ | |||||
| MHC IIa | MYH2 | Sense | 5′–GGAGGCTGAGGAACAATCCAACACCAA–3′ | NM_017534 | 129 | 5,795–5,923 |
| Antisense | 5′–TCCCGGCTCTTCACCCGCAGTTT–3′ | |||||
| MHC IIx | MYH1 | Sense | 5′–AGTTGCTGACAAGGCAGCCTATCTCCAA–3′ | NM_005963 | 97 | 1,241–1,337 |
| Antisense | 5′–GACATACTCATTGCCGACCTTGACCCTA–3′ | |||||
Note: bp = base pairs; MHC = myosin heavy chain; mRNA = messenger RNA.
Table 1.
Participant Characteristics
| Pre | Post | |
| Age (years) | 70 ± 2 | |
| Weight (kg) | 66.6 ± 4.6 | 66.3 ± 4.4 |
| VO2max (ml·min−1·kg−1) | 15.5 ± 0.9 | 19.8 ± 0.6* |
| Knee extensor power (watts) | 265 ± 63 | 326 ± 65* |
Experimental Design and Methodology
Each participant completed the experimental protocol over a period of approximately 15 weeks consisting of several visits to the laboratory for baseline measurements of aerobic capacity, whole muscle function, a muscle biopsy, and 42 exercise training sessions (19). Baseline measurements were repeated after the 12-week training protocol.
Aerobic Exercise Training Protocol
Participants performed 12 weeks of aerobic training on a cycle ergometer with 100% exercise adherence (Stairmaster Stratus 3300 CE) as previously described in detail (19). A total of 42 exercise sessions were performed (Table 2). Duration (20–45 minutes), intensity (60%–80% heart rate reserve [HRR]), and frequency (three or four sessions per week) of exercise were progressively increased throughout the 12 weeks. The last 5 weeks consisted of four 45-minute sessions at 80% intensity per week.
Table 2.
Outline of the Aerobic Exercise Training Program
| Week | Duration (min) | Intensity (% HRR) | Frequency (sessions per week) |
| 1 | 20 | 60 | 3 |
| 2 | 30 | 60 | 3 |
| 3 | 40 | 60 | 3 |
| 4 | 40 | 70 | 3 |
| 5 | 40 | 75 | 3 |
| 6 | 40 | 75 | 3 |
| 7 | 40 | 75 | 4 |
| 8 | 45 | 80 | 4 |
| 9 | 45 | 80 | 4 |
| 10 | 45 | 80 | 4 |
| 11 | 45 | 80 | 4 |
| 12 | 45 | 80 | 4 |
Note: HRR = heart rate reserve.
Aerobic Capacity
Participants performed a physician-supervised graded exercise test for the assessment of VO2max before and after the 12-week aerobic training intervention as previously described (19). During the test, participant's heart rate, blood pressure, rating of perceived exertion, and electrocardiogram were monitored, and ventilation and expired air samples were measured by a metabolic cart (TrueOne 2400 Metabolic System; ParvoMedics, Inc.) for the determination of VO2. Participants resting and maximum heart rate were used to determine proper exercise intensity (% HRR) during the training protocol.
Whole Muscle Function
Peak power of the knee extensor muscle group was assessed before and after the 12-week aerobic training intervention using an inertial ergometer (Inertial Technology, Sweden) connected to a strain gauge load cell and potentiometer interfaced with a personal computer (Gateway E-4200). Following multiple orientation sessions with the knee extensor device, participants performed three identical sessions separated by at least 2 days. All tests were bilateral. Prior to any testing, participants performed a 10-minute warm-up on a stationary bicycle followed by small loads on the resistance apparatus. Participants completed three submaximal repetitions followed by three maximal attempts with 3-minute rest between sets. The concentric power output was recorded throughout the full range of motion. Whole muscle power data are presented with an N = 7.
Skeletal Muscle Biopsy
Before and after the 12-week aerobic training intervention, a muscle biopsy was obtained from the vastus lateralis of each participant. The posttraining biopsy sample occurred ∼48 hours after the last exercise session to avoid any transient alterations from the last training session. Tissue was obtained following local anesthetic (Lidocaine HCl 1%) using a 5-mm Bergstrom needle with suction (21). One ∼15 mg piece was placed in RNAlater and stored at −20°C until RNA extraction, one ∼15 mg bundle was placed in cold skinning solution and stored at −20°C until fiber isolation, whereas the remaining sample was immediately frozen and stored in liquid nitrogen.
Gene Expression
Total RNA extraction and RNA quality check.—
Total RNA was extracted in TRI reagent (Molecular Research Center, Cincinnati, OH). The quality and integrity (RNA integrity number of 8.4 ± 0.1) of extracted RNA (158.1 ± 17.2 ng/μL) was evaluated using an RNA 6000 Nano LabChip kit on Agilent 2100 Bioanalyzer.
Reverse transcription and real-time polymerase chain reaction.—
Oligo-primed first-strand complementary DNA was synthesized (150 ng total RNA) using SuperScript II RT (Invitrogen, Carlsbad, CA). Quantification of mRNA transcription (in duplicate) was performed in a 72-well Rotor-Gene 3,000 Centrifugal Real-Time Cycler (Corbett Research, Mortlake, NSW, Australia). GAPDH was used as a reference gene (22). The validation of GAPDH was performed to ensure that its expression was unaffected by the experimental treatment as we have previously described (23,24). All primers used in this study were mRNA specific (on different exons and over an intron) and were designed for SYBR Green chemistry using Vector NTI Advance 9 software (Invitrogen). Each primer sequence is provided in Table 3. A melting curve analysis was generated validating that only one product was present. The details regarding reverse transcription and polymerase chain reaction parameters have been reported previously (22,24).
Relative quantitative polymerase chain reaction data analysis.—
Relative gene expression analysis comparing the expression of a gene of interest in relation to a reference gene is based on the distinct cycle (CT) differences. The method (25) was used in this study as described in detail previously (22,26).
Mixed-Muscle Homogenate MHC Composition
MHC composition was determined from mixed-muscle fiber homogenates by sodium dodecyl sulfate–polyacrylamide gel electrophoresis as described previously (27). Briefly, 100 ng of protein were run overnight at 4°C on a Hoeffer SE 600 gel electrophoresis unit utilizing a 3.5% acrylamide stackinggel with a 5% separating gel. After electrophoresis, gels were silver stained according to Giulian and colleagues (28). MHC isoforms (I, IIa, IIx) were identified according to migration rate, and the relative proportion of each MHC isoform was determined by densitometry (FluorChem SP, Alpha Innotech).
Single Muscle Fiber MHC Distribution
The MHC isoform profile for each fiber was determined by isolating individual fibers under a microscope and performing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SE 600 series; Hoefer, San Francisco, CA) analysis (15). Individual muscle fibers were dissected within 4 weeks of muscle biopsy collection. Following isolation, fibers were solubilized in 80 μL of 1% sodium dodecyl sulfate sample buffer (10% sodium dodecyl sulfate, 6 mg/mL ethylenediaminetetraacetic acid, 0.06 M Tris [pH 6.8], 2 mg/mL bromphenol blue, 15% glycerol, and 5% β-mercaptoethanol). These samples were stored at −80°C until analyzed for MHC content. Samples were loaded on a 3.5% loading and a 5% separating gel and ran 12 hours at 4°C. The gels were silver stained (28) allowing for the MHC isoform profile (I, I/IIa, I/IIa/IIx, IIa, IIa/IIx, and IIx) for each individual fiber to be determined. The fibers that qualified for each respective profile were taken relative to the total number of fibers per participant to determine the percentage of single-fiber MHC isoforms.
Statistical Analysis
Pre- and posttraining values for all variables were analyzed using a paired two-tailed student's t test. Pearson product–moment correlation coefficients were utilized to examine relationships between selected variables. Significance was accepted as p < .05. All values are presented as mean ± standard error.
RESULTS
MHC—mRNA
The mRNA expression for MHC IIx was reduced (p < .05) after aerobic training (Figure 1A). MHC I mRNA demonstrated a trend to increase (p < .10), whereas the MHC IIa mRNA expression was unaltered after 12 weeks of aerobic exercise training.
Figure 1.
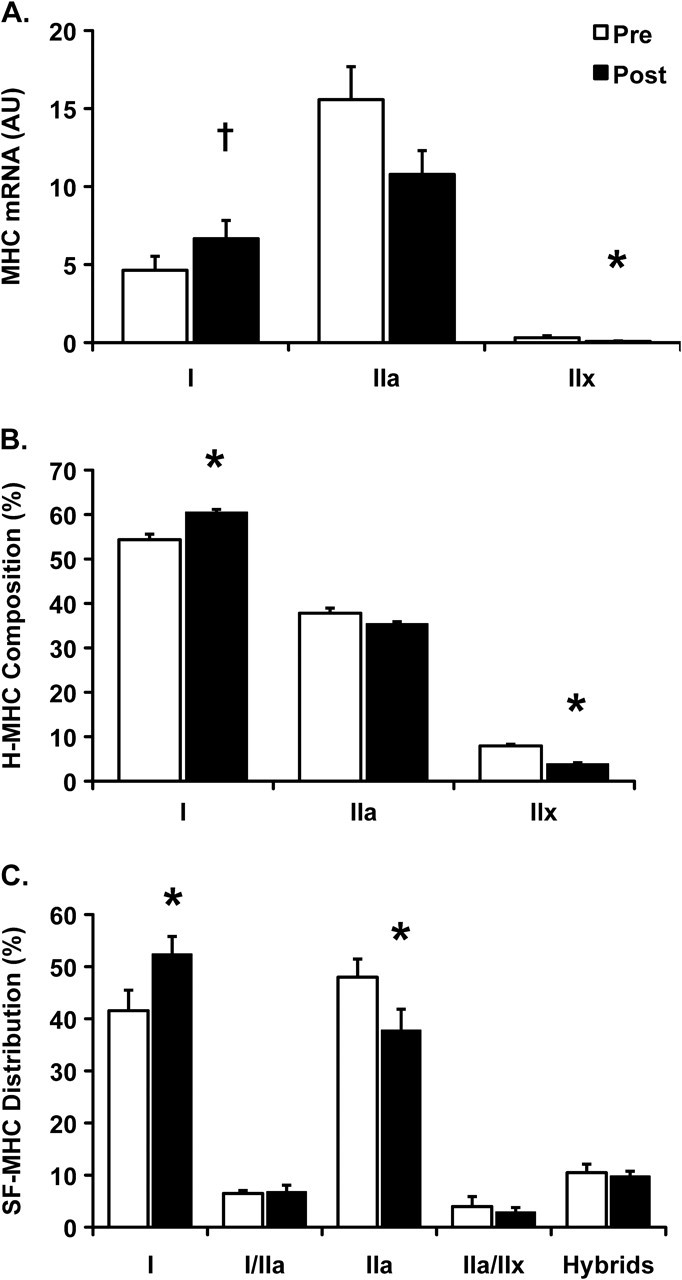
(A) MHC mRNA, (B) mixed-muscle homogenate composition (H-MHC), and (C) single-fiber distribution (SF-MHC) within skeletal muscle of older women pre- and postaerobic exercise training. Hybrids in (C) signify the collective representation of all hybrid fibers (I/IIa and IIa/IIx). Data are presented in mean ± SE. *p < .05, †p < .10 compared with Pre.
MHC—Mixed-Muscle Homogenate
Examining MHC composition in the mixed-muscle homogenate revealed an increase (p < .05) in MHC I protein from pre- (54% ± 4%) to posttraining (61% ± 2%; Figure 1B). The percentage of MHC IIx protein decreased (p < .05) after training (4% ± 1%) compared with pretraining (8% ± 1%), whereas the MHC IIa protein proportion was unaffected (pre: 38% ± 4% and post: 35% ± 2%). Furthermore, the increase in MHC I proportion from a mixed-muscle homogenate was correlated (R2 = .78, p < .05) to the improvements in whole muscle power after aerobic exercise training in older women (Figure 2A).
Figure 2.
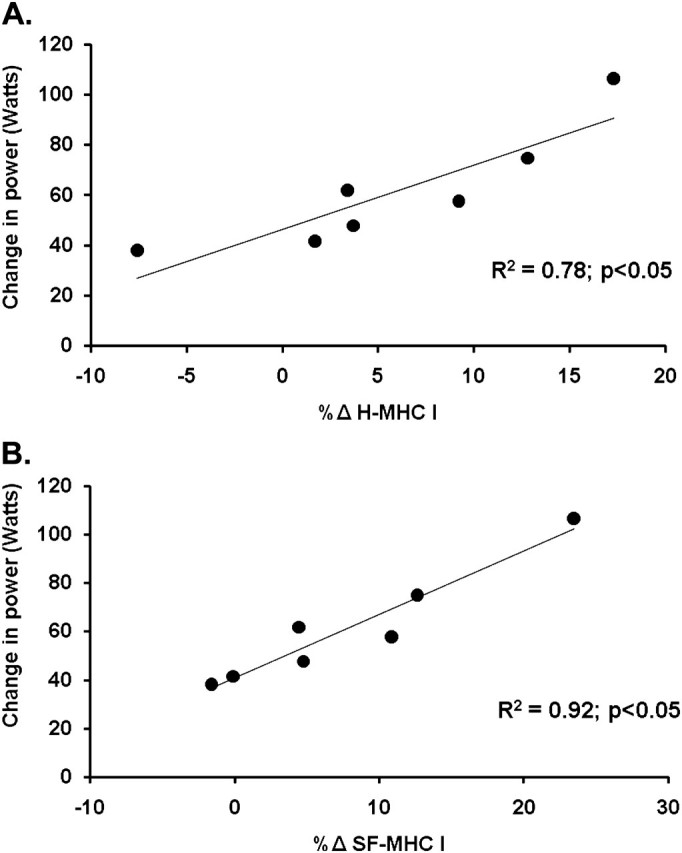
Correlation between the absolute change in whole muscle knee extensor power with the change of myosin heavy chain (MHC) I content in (A) the mixed-muscle homogenate and (B) individual myofibers, with aerobic exercise training in older women (R2 = .78 and .92 respectively, p < .05).
MHC—Single Muscle Fiber
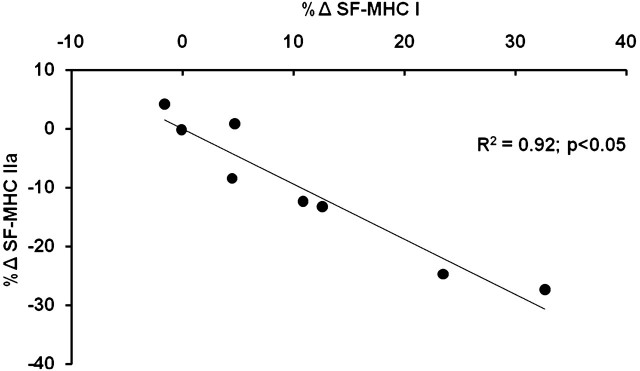
A total of 1,921 single muscle fibers were isolated for MHC analysis. On average, 120 single muscle fibers were examined for myofiber distribution before and after training for each participant. Aerobic training was associated with an increased (p < .05) MHC I fiber distribution (42% ± 4% to 52% ± 3%) and a reduction (p < .05) in MHC IIa fiber distribution (48% ± 3% to 38% ± 4%; Figure 1C). The increase in MHC I fibers and concomitant decrease in MHC IIa fibers were highly correlated (R2 = .92, p < .05; Figure 3). There were no changes in the percentages of any hybrid isoform profiles, and no pure MHC IIx single fibers were identified. Moreover, there was an association (R2 = 0.92, p < .05) between the increase in MHC I fiber distribution and the improvement in whole muscle power after aerobic exercise training (Figure 2B).
Figure 3.
The inverse relationship between the percent change of myosin heavy chain (MHC) I and MHC IIa single-fiber distribution (SF-MHC) after aerobic exercise training in older women (R2 = .92, p < .05).
DISCUSSION
The global purpose of this investigation was to examine the skeletal muscle response to aerobic exercise training in older women. Specifically, we used a multifaceted approach to examine alterations in MHC distribution after an aerobic exercise training program. The main findings from this study were that older women increased MHC I and decreased MHC IIx protein composition, which was accompanied by a trend to upregulate MHC I mRNA and reduce MHC IIx mRNA expression. Additionally, aerobic training evoked an increase in the percentage of individual muscle fibers containing MHC I with a concomitant reduction in the percentage of myofibers containing MHC IIa isoforms. Collectively, reduced fast MHC isoforms (IIa or IIx) with concomitantly increased MHC I isoforms support the notion that aerobic exercise creates an oxidative phenotype in aging skeletal muscle. The shift toward greater relative amounts of MHC I isoforms demonstrates the maintenance of skeletal muscle plasticity with aging and may also indicate a muscle phenotype that is beneficial for metabolic health and functionality in the aging population.
Numerous investigations have suggested that the loss of skeletal muscle mass with age is characterized by a decline in the total number of muscle fibers and specific atrophy of MHC IIa muscle fibers (3,4,29,30). We have previously reported that aerobic exercise training can improve whole muscle size and function in older women (19,20). Interestingly, these improvements occur primarily due to adaptations of MHC I fiber size and contractile function with marginal adaptations in MHC IIa fibers. Therefore, to fully identify the myocellular adaptations to aerobic exercise, it is critical to understand the regulation and distribution of MHC isoforms in aging skeletal muscle.
There have been several factors identified in the regulation of MHC protein composition (31,32), including MHC gene expression (33–35). Therefore, measuring the MHC transcripts is important to recognize the basis for MHC composition in aging skeletal muscle. Aerobic exercise training reduced MHC IIx mRNA expression and tended to elevate MHC I mRNA expression in older women. Additionally, the translated product of these transcripts demonstrated an associated reduction in MHC IIx and increased MHC I protein isoform composition in muscle homogenates. These data support evidence that link the MHC gene family in regulating the relative MHC protein composition, even after 3 months of exercise training in aging skeletal muscle. Previous reports have suggested that aging skeletal muscle contains an inability to synthesize new proteins from mRNA at rest (36) and/or after exercise training (17,37). This may be due to a possible decline of skeletal muscle protein synthesis with age, although collectively these data are ambiguous (38–40). Additionally, after a 16-week aerobic exercise training protocol conducted by Short and colleagues (17), MHC protein composition at the homogenate level did not change nor reflect alterations in MHC mRNA in older individuals. A principal difference between Short and colleagues (17) and the current study is that our protocol induced MHC I myofiber hypertrophy (19). This is relevant because the electrophoretic assessment of MHC protein composition of a mixed-muscle homogenate has been correlated to percent fiber area of the muscle (41). Thus, when utilizing this index as a reflection of the proportion of myosin isoforms within the whole muscle, it is fundamental to note the influence of myofiber size. It is therefore likely that the considerable increase in the proportion of MHC I protein isoform is due to both an increase in MHC I myofiber size and distribution.
To our knowledge, this is the first investigation to report single-fiber MHC distribution after aerobic exercise training in aging skeletal muscle. Utilizing individually dissected single muscle fibers provides an accurate representation of the fiber type diversity in a given muscle (15,28). Employing either mATPase histochemistry or examining mixed-muscle homogenates to determine percent fiber type area provides difficulties in properly evaluating hybrid fibers that coexpress two or more MHC isoforms, which are common in aging skeletal muscle (7,15). The prevalence of hybrid fibers in aging skeletal muscle is most likely due to physical inactivity and/or denervation, which may render these fibers responsive to exercise training (15).
As hypothesized, we observed an increase in the percentage of MHC I and a concomitant reduction in MHC IIa single fibers, which is similar to observations in younger individuals (13). Given the greater power production of MHC IIa fibers relative to MHC I, this shift to a slow-twitch or oxidative fiber type profile may appear less than optimal for maximal improvements in whole muscle function. However, we have previously demonstrated that this training regimen has provided robust enhancements in whole muscle power (20) accompanied with an increased MHC I myofiber power (19). Therefore, within aging skeletal muscle, the transition to greater MHC I fiber composition appears to create a myocellular milieu that is positive for improvements in whole muscle function after aerobic exercise training. In support of this notion, there was a direct correlation between the increase in MHC I content (both homogenate and single fiber) and whole muscle power with aerobic training in the current study (Figure 2). This relationship is intriguing as increased MHC IIa myofiber function would likely translate into more robust improvements in whole muscle function because MHC IIa fibers are functionally superior (five to six greater power production) to MHC I fibers (5). However, our laboratory has observed greater plasticity in MHC I fibers in response to resistance (18) and aerobic training (19), which is potentially an age-related adaptation to exercise training in skeletal muscle of older individuals (age 74 ± 2 and 70 ± 2 years, respectively). This response to exercise training in older individuals would most likely result in a more efficient skeletal muscle profile to withstand the exercise training and activities of daily living (42). These observations are supported by Coggan and colleagues (16) who observed a shift toward a slower more oxidative fiber type utilizing mATPase histochemistry methodology in older individuals (age 64 ± 3 years). These data suggest that aging skeletal muscle remains malleable and modifies its phenotype to sustain the functional demands of an aerobic training program.
In conclusion, the beneficial cardiovascular and metabolic health implications of aerobic exercise have been well characterized in young and old individuals. Interestingly, from the comprehensive assessment of MHC distribution after aerobic training, we continue to provide support for the improvement in health of older individuals. After aerobic training, older women demonstrate a greater proportion of slow-twitch MHC I proteins/fibers with a reciprocal decrease in fast-twitch MHC IIx protein and IIa fibers. This shift has potential clinical relevance as high amounts of MHC IIx isoforms or low proportions of MHC I isoforms have been associated with conditions, such as poor insulin sensitivity (43), diabetes (44), obesity (45), and cardiac heart failure (46). Additionally, a large percentage of MHC I fibers correlates with greater oxidative enzyme capacity (47), satellite cell content (48), weight loss (45), and fatigue resistance (42), which are factors that contribute to greater functionality. Moreover, possessing a greater quantity of MHC I fibers may be advantageous because they are responsive to both aerobic and resistance exercise training in older adults (<80 years). Collectively, these data provide evidence that aerobic exercise training creates a MHC profile within skeletal muscle that may be conducive to the overall health in older adults.
FUNDING
This investigation was supported by the National Institute of Aging grant AG-32127 to M.P.H.
Acknowledgments
The authors would like to thank Matthew Douglass and Paul Reidy for their assistance during data collection and analysis for this study and Kiril Minchev for technical assistance.
References
- 1.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 2.Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 3.Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6(8):588–595. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- 4.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84(2–3):275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 5.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552(Pt 1):47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103(1):31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 7.Andersen JL, Terzis G, Kryger A. Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve. 1999;22(4):449–454. doi: 10.1002/(sici)1097-4598(199904)22:4<449::aid-mus4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol. 2004;557(Pt 2):501–513. doi: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher P, Trappe S, Harber M, et al. Effects of 84-days of bedrest and resistance training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiol Scand. 2005;185(1):61–69. doi: 10.1111/j.1365-201X.2005.01457.x. [DOI] [PubMed] [Google Scholar]
- 10.Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage? Exerc Sport Sci Rev. 2009;37(1):3–9. doi: 10.1097/JES.0b013e318190ea2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raue U, Terpstra B, Williamson DL, Gallagher PM, Trappe SW. Effects of short-term concentric vs. eccentric resistance training on single muscle fiber MHC distribution in humans. Int J Sports Med. 2005;26(5):339–343. doi: 10.1055/s-2004-821041. [DOI] [PubMed] [Google Scholar]
- 12.Harber MP, Gallagher PM, Creer AR, Minchev KM, Trappe SW. Single muscle fiber contractile properties during a competitive season in male runners. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1124–R1131. doi: 10.1152/ajpregu.00686.2003. [DOI] [PubMed] [Google Scholar]
- 13.Trappe S, Harber M, Creer A, et al. Single muscle fiber adaptations with marathon training. J Appl Physiol. 2006;101(3):721–727. doi: 10.1152/japplphysiol.01595.2005. [DOI] [PubMed] [Google Scholar]
- 14.Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R273–R280. doi: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson DL, Godard MP, Porter DA, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain coexpression in single muscle fibers from older men. J Appl Physiol. 2000;88(2):627–633. doi: 10.1152/jappl.2000.88.2.627. [DOI] [PubMed] [Google Scholar]
- 16.Coggan AR, Spina RJ, King DS, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72(5):1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 17.Short KR, Vittone JL, Bigelow ML, et al. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol. 2005;99(1):95–102. doi: 10.1152/japplphysiol.00129.2005. [DOI] [PubMed] [Google Scholar]
- 18.Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol. 2001;281(2):C398–C406. doi: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- 19.Harber MP, Konopka AR, Douglass MD, et al. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1452–R1459. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konopka AR, Douglass MD, Kaminsky LA, et al. Molecular adaptations to aerobic exercise training in skeletal muscle of older women. J Gerontol A Biol Sci Med Sci. 2010;65(11):1201–1207. doi: 10.1093/gerona/glq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergstrom J. Muscle electrolytes in man. Scand J Clin Lab Invest Suppl. 1962;68:1–110. [Google Scholar]
- 22.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18-30 yr) and old (80-89 yr) women. J Appl Physiol. 2006;101(1):53–59. doi: 10.1152/japplphysiol.01616.2005. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol. 2005;98(5):1745–1752. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- 24.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 2007;103(5):1744–1751. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci. 2007;62(12):1407–1412. doi: 10.1093/gerona/62.12.1407. [DOI] [PubMed] [Google Scholar]
- 27.Harber MP, Fry AC, Rubin MR, Smith JC, Weiss LW. Skeletal muscle and hormonal adaptations to circuit weight training in untrained men. Scand J Med Sci Sports. 2004;14(3):176–185. doi: 10.1111/j.1600-0838.2003.371.x. [DOI] [PubMed] [Google Scholar]
- 28.Giulian GG, Moss RL, Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- 29.Lexell J, Downham D. What is the effect of ageing on type 2 muscle fibres? J Neurol Sci. 1992;107(2):250–251. doi: 10.1016/0022-510x(92)90297-x. [DOI] [PubMed] [Google Scholar]
- 30.Grimby G. Muscle performance and structure in the elderly as studied cross-sectionally and longitudinally. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):17–22. doi: 10.1093/gerona/50a.special_issue.17. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy JJ, Esser KA. MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J Appl Physiol. 2007;102(1):306–313. doi: 10.1152/japplphysiol.00932.2006. [DOI] [PubMed] [Google Scholar]
- 32.van Rooij E, Quiat D, Johnson BA, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17(5):662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izumo S, Nadal-Ginard B, Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- 34.Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- 35.Baldwin KM, Haddad F. Skeletal muscle plasticity: cellular and molecular responses to altered physical activity paradigms. Am J Phys Med Rehabil. 2002;81(11 suppl):S40–S51. doi: 10.1097/01.PHM.0000029723.36419.0D. [DOI] [PubMed] [Google Scholar]
- 36.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997;273(4 Pt 1):E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 37.Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab. 2001;280(2):E203–E208. doi: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- 38.Marx JO, Kraemer WJ, Nindl BC, Larsson L. Effects of aging on human skeletal muscle myosin heavy-chain mRNA content and protein isoform expression. J Gerontol A Biol Sci. 2002;57(6):B232–B238. doi: 10.1093/gerona/57.6.b232. [DOI] [PubMed] [Google Scholar]
- 39.Welle S, Bhatt K, Thornton C. Polyadenylated RNA, actin mRNA, and myosin heavy chain mRNA in young and old human skeletal muscle. Am J Physiol. 1996;270(2 Pt 1):E224–E229. doi: 10.1152/ajpendo.1996.270.2.E224. [DOI] [PubMed] [Google Scholar]
- 40.Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol Genomics. 2003;14(2):149–159. doi: 10.1152/physiolgenomics.00049.2003. [DOI] [PubMed] [Google Scholar]
- 41.Fry AC, Allemeier CA, Staron RS. Correlation between percentage fiber type area and myosin heavy chain content in human skeletal muscle. Eur J Appl Physiol Occup Physiol. 1994;68(3):246–251. doi: 10.1007/BF00376773. [DOI] [PubMed] [Google Scholar]
- 42.Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage? Exerc Sport Sci Rev. 2009;37(1):3–9. doi: 10.1097/JES.0b013e318190ea2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hickey MS, Weidner MD, Gavigan KE, Zheng D, Tyndall GL, Houmard JA. The insulin action-fiber type relationship in humans is muscle group specific. Am J Physiol. 1995;269(1 Pt 1):E150–E154. doi: 10.1152/ajpendo.1995.269.1.E150. [DOI] [PubMed] [Google Scholar]
- 44.Hickey MS, Carey JO, Azevedo JL, et al. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am J Physiol. 1995;268(3 Pt 1):E453–E457. doi: 10.1152/ajpendo.1995.268.3.E453. [DOI] [PubMed] [Google Scholar]
- 45.Tanner CJ, Barakat HA, Dohm GL, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282(6):E1191–E1196. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan MJ, Duscha BD, Klitgaard H, Kraus WE, Cobb FR, Saltin B. Altered expression of myosin heavy chain in human skeletal muscle in chronic heart failure. Med Sci Sports Exerc. 1997;29(7):860–866. doi: 10.1097/00005768-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Henriksson J, Reitman JS. Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta Physiol Scand. 1976;97(3):392–397. doi: 10.1111/j.1748-1716.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- 48.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292(1):E151–E157. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]