Abstract
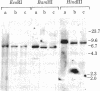
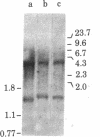
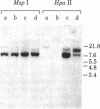
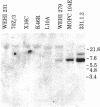
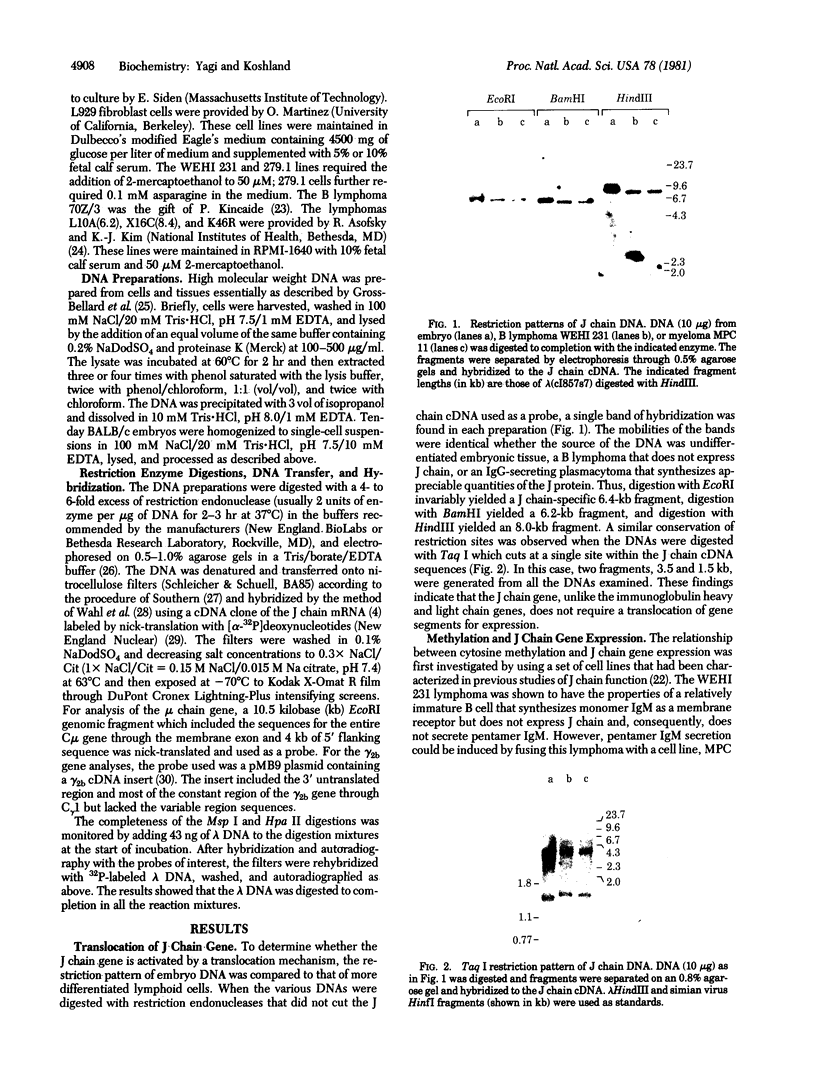
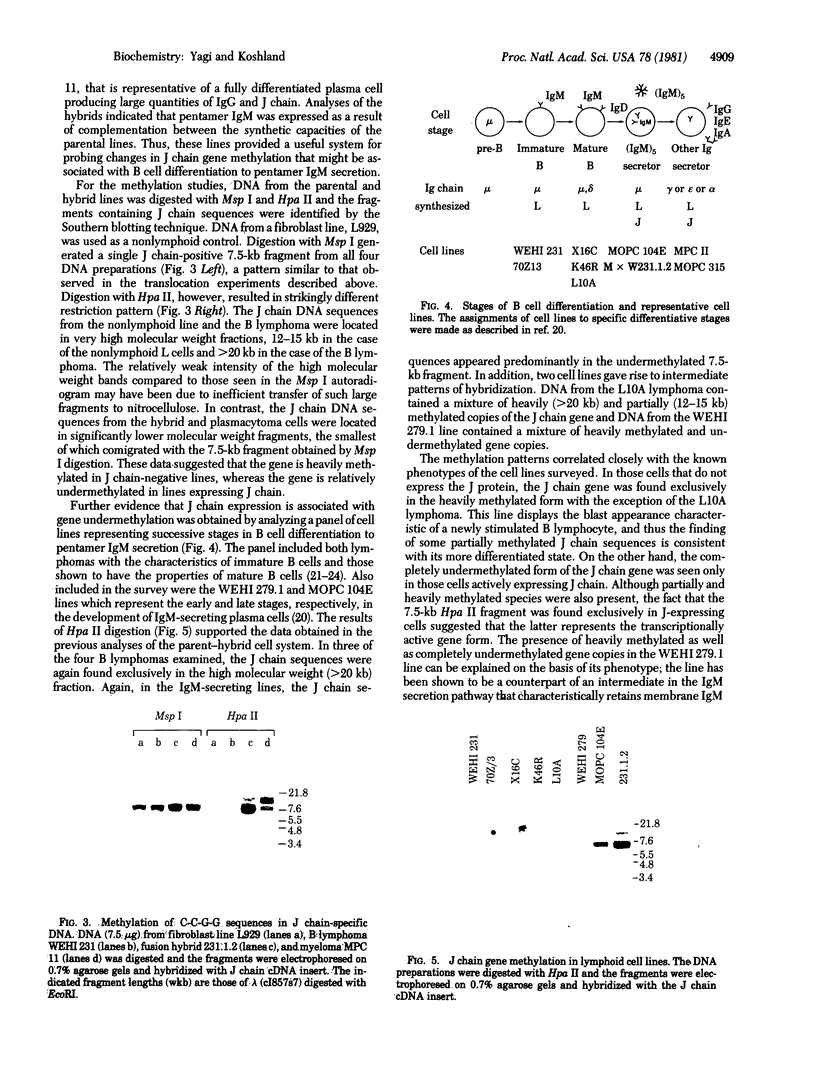
During B cell differentiation, transcription of the J chain gene is initiated. To determine the regulatory mechanism involved, we have analyzed the structure of the J chain gene in cell lines representing successive stages in B cell development. Comparison of restriction sites showed that the J chain gene does not require a rearrangement of DNA for expression; cleavage sites present in embryonic J chain DNA were preserved through all the subsequent differentiative steps. However, comparison of 5-methylcytosine contents showed that J chain gene expression correlates with a loss of methyl groups. The J chain gene was heavily methylated in cell types not expressing J chain (i.e., embryo and lymphomas representative of immature and mature B cells) and significantly less methylated in cell lines representative of antigen-stimulated lymphocytes synthesizing J chain. These changes in J chain gene methylation represent a specific differentiation-induced response. Analyses of the heavy chain C mu and C gamma 2b genes, which are expressed at earlier and later stages than the J chain gene, showed that the C mu sequences were undermethylated in all cell types examined whereas the C gamma 2b sequences were undermethylated only in cell lines expressing the IgG2b protein. The primary encounter of a B cell with antigen must therefore trigger events that effect J chain gene transcription through a mechanism involving loss of methyl groups from cytosine.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. P., Southern E. M. Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis. J Mol Biol. 1978 Jan 5;118(1):27–47. doi: 10.1016/0022-2836(78)90242-5. [DOI] [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Price P., Pedrinan L., Acs G. Correlation between hypomethylation of DNA and expression of globin genes in Friend erythroleukemia cells. Eur J Biochem. 1977 Nov 15;81(1):53–61. doi: 10.1111/j.1432-1033.1977.tb11926.x. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Deletions are associated with somatic rearrangement of immunoglobulin heavy chain genes. Cell. 1980 Jan;19(1):37–51. doi: 10.1016/0092-8674(80)90386-4. [DOI] [PubMed] [Google Scholar]
- Early P. W., Davis M. M., Kaback D. B., Davidson N., Hood L. Immunoglobulin heavy chain gene organization in mice: analysis of a myeloma genomic clone containing variable and alpha constant regions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):857–861. doi: 10.1073/pnas.76.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Honjo T., Kataoka T. Organization of immunoglobulin heavy chain genes and allelic deletion model. Proc Natl Acad Sci U S A. 1978 May;75(5):2140–2144. doi: 10.1073/pnas.75.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Kawakami T., Takahashi N., Honjo T. Complete nucleotide sequence of mouse immunoglobulin mu gene and comparison with other immunoglobulin heavy chain genes. Nucleic Acids Res. 1980 Sep 11;8(17):3933–3945. doi: 10.1093/nar/8.17.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Koshland M. E. Structure and function of the J chain. Adv Immunol. 1975;20:41–69. doi: 10.1016/s0065-2776(08)60206-0. [DOI] [PubMed] [Google Scholar]
- Mather E. L., Alt F. W., Bothwell A. L., Baltimore D., Koshland M. E. Expression of J chain RNA in cell lines representing different stages of B lymphocyte differentiation. Cell. 1981 Feb;23(2):369–378. doi: 10.1016/0092-8674(81)90132-x. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J. IgM in bone marrow-derived lymphocytes. Changes in synthesis, turnover and secretion, and in numbers of molecules on the surface of B cells after mitogenic stimulation. Eur J Immunol. 1974 Mar;4(3):181–188. doi: 10.1002/eji.1830040306. [DOI] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Ralph P. Murine B cell leukemia line with inducible surface immunoglobulin expression. J Immunol. 1978 Aug;121(2):641–647. [PubMed] [Google Scholar]
- Raschke W. C., Mather E. L., Koshland M. E. Assembly and secretion of pentameric IgM in a fusion between a nonsecreting B cell lymphoma and an IgG-secreting plasmacytoma. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3469–3473. doi: 10.1073/pnas.76.7.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. The arrangement and rearrangement of antibody genes. Nature. 1978 Dec 21;276(5690):790–795. doi: 10.1038/276790a0. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. Tissue-specific DNA methylation in a cluster of rabbit beta-like globin genes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6634–6638. doi: 10.1073/pnas.77.11.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P. W., Marcu K. B., Slightom J. L., Blattner F. R. Structure of the constant and 3' untranslated regions of the murine gamma 2b heavy chain messenger RNA. Science. 1979 Dec 14;206(4424):1299–1303. doi: 10.1126/science.117548. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978 Dec;5(12):4631–4634. doi: 10.1093/nar/5.12.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki-Kataoka Y., Kataoka T., Takahashi N., Obata M., Honjo T. Complete nucleotide sequence of immunoglobulin gamma2b chain gene cloned from newborn nouse DNA. Nature. 1980 Feb 21;283(5749):786–789. doi: 10.1038/283786a0. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]