Abstract
We exposed groups of adult male green treefrogs, Hyla cinerea, to acoustic stimuli (natural chorus or random tones) for seven consecutive nights at three time points during their natural breeding season (May, July, and September) and assessed seasonal changes in plasma androgen levels and number of arginine vasotocin (AVT) immunoreactive cells in the brain over this time period. We also tested whether social cues altered either androgens or AVT-ir cell number or size at each time point. Finally, we analyzed how these factors related to calling behavior. Data were collected over two breeding seasons. Call rate (calls/h) was assessed during the stimulus time (i.e. ‘evoked calling’) and during the remainder of the day (‘spontaneous calling’). Plasma hormone levels were measured at the end of the acoustic treatment when brains were collected for immunocytochemistry. Circulating androgen levels declined over the breeding season. Males exposed to chorus sounds, however, had higher androgen levels than males exposed to tones. AVT-ir cell number increased across the breeding season in the nucleus accumbens but not the amygdala, anterior preoptic area, or magnocellular preoptic area, and soma size decreased in the nucleus accumbens as cell number increased. Social stimulation had no significant influence on either AVT-ir cell measure. Evoked call rate was higher in males exposed to natural chorus sounds compared to those exposed to random tones, but did not change during the season. In contrast, spontaneous call rate was higher at the beginning of the breeding season compared to the end, and unlike evoked calling was correlated with circulating androgen levels across all treatments and time points. AVT-ir soma size was positively correlated with both evoked and spontaneous calling. These results suggest that social exposure can prolong the elevation of gonadal hormones in the bloodstream, thus mitigating or slowing the seasonal decline of such hormones. In contrast, social exposure does not affect the seasonal pattern of AVT-ir cell number or soma size. The reciprocal relationship between social cues and hormones and the subsequent effect on behavior may provide hidden benefits to animals engaging in social interactions. However, unlike steroid hormone levels, the seasonal change in AVT-ir cell number and size is not counteracted by social stimulation.
Key Words: Amphibian, Androgens, Arginine vasotocin, Communication, Seasonality
Introduction
Courtship behaviors in many vertebrates vary seasonally and are gated by circulating gonadal steroids that may be regulated by environmental cues or intrinsic processes. They are also influenced by brain neuromodulators that may themselves be regulated by these same cues as well as by circulating steroid hormones. Seasonal patterns in gonadal steroid hormones have been the subject of numerous studies in many vertebrates. Animals that restrict their breeding patterns to certain times of the year have higher reproductive hormones at that time compared to the nonbreeding period [Smith et al., 1997; Tokarz et al., 1998], though other examples of hormone cycles during the breeding period exist [Woolley et al., 2004]. Evidence exists across numerous vertebrate taxa that in males, androgens – specifically testosterone – fluctuate on a circannual basis in seasonally breeding animals [Licht, 1971; Licht et al., 1983; Zoeller and Moore, 1985; Gobretti et al., 1991; Herman, 1992; Smith et al., 1997; Tokarz et al., 1998; O'Bryant and Wade, 1999, 2002; Holmes and Wade, 2004], presumably stimulating seasonally relevant social behaviors. The spike of testosterone in plasma follows the start of spermatogenesis and gonadal recrudescence [Licht, 1967, 1971; Delgado et al., 1992], and typically precedes androgen-dependent reproductive behaviors [Crews, 1980].
Another important exogenous cue which affects reproductive hormones is the perception of social signals. Although the relationship between circulating gonadal hormones and the production of social behavior is well documented [Arnold, 1975; Crews et al., 1978; Adkins and Schlesinger, 1979; Lindzey and Crews, 1986; Rudd et al., 1996; Stacey and Kobayashi, 1996; Riters et al., 1998; Tokarz et al., 2002], the reciprocal idea that social cues can affect gonadal hormones is also true [Oliveira et al., 1996; Soma et al., 1996; Borges et al., 1998]. In anurans, perceiving conspecific auditory signals leads to a rise in gonadal steroid hormones in both males [Burmeister and Wilczynski, 2000; Chu and Wilczynski, 2001] and females [Lynch and Wilczynski, 2006]. A similar effect is seen in birds [Tramontin et al., 1999]. Engaging in other types of social interactions can also produce increases in gonadal and stress hormones in anurans [Orchinik et al., 1988]. How social cues are transduced in the brain and interact with the hormonal machinery has received much attention over the years. Numerous electrical stimulation and hormone implant studies have implicated the preoptic area (POA) of the forebrain in the control of male courtship behavior including auditory processing of vocalizations in many anurans [Schmidt, 1968; Wada and Gorbman, 1977a, b]. Retrograde tracer injections into the POA of Hyla cinerea demonstrate that projections from auditory thalamic nuclei innervate the POA [Allison and Wilczynski, 1991]. The ventral hypothalamus is also involved in auditory processing [Wilczynski and Allison, 1989; Allison and Wilczynski, 1991], and receives input from thalamic regions as well as from the POA, the lateral amygdala, and the suprachiasmatic nucleus, among others [Allison and Wilczynski, 1994]. These results from H. cinerea suggest that input from auditory nuclei to the basal forebrain structures that concentrate sex hormones [Kelley et al., 1975, 1978] might be a way in which hormonal state, social signals, and environmental cues combine to influence reproductive behaviors [see Schmidt, 1983; Wilczynski et al., 1993].
Arginine vasotocin (AVT), and its mammalian homolog vasopressin (AVP), is a nonapeptide that acts as a brain neuromodulator in addition to a peripherally acting hormone. AVT/AVP populations in the accumbens and amygdala areas of vertebrates have been implicated in the regulation of social behavior, particularly in males, where AVT/AVP stimulates male signaling and has complicated species- and context-dependent effects on social aggression [Goodson and Bass, 2001; Goodson, 2008; Kabelik et al., 2008]. In anuran amphibians, elevating AVT levels increases calling in males [Boyd, 1994; Marler et al., 1995; Chu et al., 1998; Klomberg and Marler, 2000; Burmeister et al., 2001; Trainor et al., 2003; Kime et al., 2007; Hattori and Wilczynski, 2009]. Compared to gonadal steroids, there is relatively little work done on circannual changes or other seasonal changes in brain AVT/AVP measures. Work on AVT in fish suggests that AVT expression and/or activity co-varies with the spawning season, although different measures in different species give different results. HPLC measurements of AVT content in salmon neural tissue show AVT levels to be lower in the non-breeding winter period compared to summer [Gozdowska et al., 2006]. Maruska et al. [2007] assayed AVT cell number and size and reported instead that both number and size in females, and size in males, was larger in the nonspawning period than the spawning period in halfspotted gobies. In males across many species, brain AVT/AVP levels increase with testosterone [Goodson and Bass, 2001; DeVries and Panzica, 2006; Kabelik et al., 2008], although exceptions can be found [Semsar and Godwin, 2003; Kabelik et al., 2010].
In this study, we examined the effects of social stimulation across the natural breeding season of the green treefrog, H. cinerea. This species belongs to the anuran order of amphibians (frogs and toads) and populates the southeastern United States. The breeding period lasts about 2–4 months, and their mating system resembles a lek like that seen in many frogs as well as some birds and mammals. Males aggregate in choruses and emit vocalizations, advertisement calls, to attract females [see Gerhardt and Bee, 2007, for a review of behavior in this and other anurans]. The advertisement call also functions in male-to-male interactions to trigger antiphonal calling as well as regulate some aspects of chorus spatial organization such as inter-male spacing [Wilczynski and Brenowitz, 1988; Brenowitz, 1989]. Females, on the other hand, do not call but rather approach and contact calling males. Their mate choice is dependent on reception of the male's call. Because male calling in this species is seasonal and modulated by both gonadal steroids and AVT, we investigated how both androgens and AVT vary across the breeding season.
Materials and Methods
Animals
Adult male green treefrogs (H. cinerea) were purchased from Charles Sullivan, Co. (Nashville, Tenn., USA) and NASCO (Fort Atkinson, Wisc., USA) in 2003 and 2004. Prior to experimental treatment animals were housed in 10-gallon aquaria with an average of six frogs per cage, containing a 3 × 5 inch (approx. 7.5 × 12.5 cm) Tupperware container filled with treated water, and artificial foliage. The amount of time animals were kept in the lab prior to treatment varied from 2–9 weeks, but not between acoustic treatment group or time of breeding season in which groups were compared. Animals were fed live crickets dusted with Repti Calcium (Fluker Farms) twice a week. The light cycle (14 h light, 10 h dark) and temperature (23–30°C) were kept constant throughout the experiment. Behavioral treatment began 2–9 weeks after arrival in the laboratory. All procedures were approved by the University of Texas Institutional Animal Care and Use Committee.
Acoustic Treatment
Adult males were exposed to acoustic stimuli during three time points: two points during the breeding season, early (May) and middle (July), and one period soon after the breeding period normally ends (September). Males were removed from the lab colony and housed individually in an acoustically sealed chamber which contained an upper and lower compartment separated by a screen (internal dimensions of entire chamber = 14 × 14 × 20 cm). The upper portion held a speaker (Radioshack 277-1008C), a small microphone, and a 1.4-W fluorescent light (Super Lite ML-100 UEI2). The lower portion of the chamber, in which the frog was placed, contained a small Tupperware container filled with water, small rocks, and artificial foliage. The experimental animals were fed crickets inside the chamber twice a week.
Animals were exposed to either a recording of a natural chorus or random pure tones as a positive control. Acoustic exposure occurred between 21:30 h and 02:30 h (typical of a breeding chorus) on 7 consecutive nights. The natural chorus stimulus consisted of a 12-min recording of a natural breeding population of male H. cinerea. The stimulus was played on a continuous tape loop throughout the stimulus period. The positive control stimulus was generated using computer software (SoundEdit 16, Macromedia, San Francisco, Calif., USA) to replace each frog call with a single pure tone that matched the replaced frog call in duration and approximate amplitude. The frequencies assigned to the pure tone were selected using a list of randomized values chosen from 100–500, 1,000–2,600, and 3,800–5,000 Hz, which are within the hearing range of the frogs but exclude the specific frequencies used for communication in this species [Gerhardt, 1974]. The signal emitted from the speaker was calibrated at 86 dB from the center of the lower portion of each chamber, which is consistent with other playback studies of this type [Burmeister and Wilczynski, 2000].
On the day the experiment began, snout-vent length and weight measurements were taken and animals were placed inside the chamber approximately 8 h prior to stimulus onset in order to acclimate to the chamber. Throughout the time in the chamber, calls for each individual were counted using custom software. Number of calls produced while the acoustic stimulus was on (‘evoked calling’) were recorded separately from calls produced at all other times (‘spontaneous calling’). We calculated the calling rate (calls/h) for each call type and averaged this value across the duration of the experiment and for each individual. On the day after the final night of acoustic treatment, animals were rapidly decapitated and trunk blood was taken and centrifuged. Plasma was stored at −20°C until it was processed. The brains were removed and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer overnight and cryoprotected in 20% sucrose in 0.1 M phosphate buffer. Gonads were inspected to confirm the sex of the animal.
Plasma Processing
Enzyme immunoassay procedures followed that of a previous study [Lynch and Wilczynski, 2005]. We measured testosterone in plasma using a commercial enzyme immunoassay kit (Cayman Scientific, No. 582701), which has a cross-reactivity of 27.4 and 18.9% with 5α-dihydrotestosterone and 5β-dihydrotestosterone, respectively. Results from this analysis will therefore be referred to as ‘androgens’ throughout the rest of the report. This kit was validated using pooled frog plasma prior to the onset of the current study [see Lynch and Wilczynski, 2005, for validation procedure].
Twenty microliters of plasma was used for measuring androgens. Plasma samples were mixed with 20 μl tritiated testosterone and extracted using 3 ml of diethyl ether (Fisher Scientific). The extraction procedure resulted in a mean recovery of 72% for androgens. We assayed each sample in triplicate. Intra-assay variation was 6.6%, and inter-assay variation was 8.5%.
AVT Immunocytochemistry
Brains were sectioned into four series at 40 μm onto subbed slides and stored at −20°C until processing. All washes were done three times, for 5 min each at room temperature. Slides were taken out of −20°C and warmed to room temperature. Slide edges were marked with a Super PAP Pen HT (Research Products International) for prevention of leakage. Sections were placed in 0.1% sodium borohydride in PBS for 15 min to unmask antigens for improved antibody penetration and washed with PBS. Sections were incubated in 1% hydrogen peroxide in PBS for 15 min and rinsed with PBS to eliminate endogenous peroxidase activity. Sections were incubated with polyclonal rabbit anti-AVP (ICN; 1:5,000 diluted with 5% normal goat serum in 0.1 M PBS with 0.3% Triton X-100) overnight at room temperature in a humid chamber. Excess primary antibody was washed away with PBS and the tissue was exposed to biotinylated goat-anti-rabbit secondary antibody using an Elite ABC peroxidase kit (Vector Laboratories) for 1 h at room temperature. An avidin-biotin blocking kit (Vector Laboratories; 2 drops/ml) and normal goat serum was used to reduce nonspecific labeling. Antibody binding was visualized using DAB as the chromogen (Vector Laboratories). Tissue was then washed with phosphate buffer and dehydrated in an alcohol series and coverslipped with Permount straight out of xylenes. Omission of the primary antibody eliminated all staining.
Cell Counting and Soma Size Measurements
The number of AVT-ir cells was quantified in four regions: the nucleus accumbens (NAcc), the amygdala, anterior preoptic area, and the magnocellular region of the preoptic area (MPOA). No other appreciable cell groups were identified. Slides were coded prior to quantification so that all measurements were taken blind. All AVT-ir cells in each population in each animal were counted. Note that these cells represent cell numbers in every fourth section through these nuclei, so that the actual cell AVT-ir cell number per animal is estimated to be four times the number reported. The actual number of counted cells was used for statistical analysis. The size of AVT-ir cells was quantified in the same four regions. One photomicrograph was taken for each section in each area in which cells appeared. All cells in these photos were measured by assessing the cross-sectional area in microns squared using ImageJ (NIH). Measurements were taken from both sides of the brain and, as they did not significantly differ from each other, were averaged for each individual for each brain area.
Statistics
Statistical analysis was performed in SysStat 3.5 and SigmaStat 3.5. We assessed group differences in androgen levels separately using two-way ANOVA, followed by LSD post-hoc tests as appropriate. ANOVAs were performed on log-transformed testosterone values, as the raw data were not normally distributed. Group differences in AVT cell number and cell size were also assessed by using separate two-way ANOVAs for each brain area, as was evoked calling and spontaneous calling behavior. Due to the large variation in calling within and between groups, calls were log-transformed prior to any statistical analysis, and correlations between calling and hormone levels were performed using log-transformed hormone data to maintain normality and equal variance in all data sets. In each case, data from 2003 and 2004 were combined. Pearson's correlation was used to detect significant relationships between variables. We used partial correlation or step-wise regression to assess the relationship between AVT and androgens independent of seasonal time point. We used partial correlation to assess the effects of AVT on calling behavior independent of androgens. Statistical significance was determined at the p = 0.05 level. The number of animals per group is shown in table 1.
Table 1.
Androgen levels [mean ng/ml plasma (SEM)] after acoustic treatment for male Hyla cinerea exposed to chorus sounds (Calls) or random tones (Tones) at three time points during a normal breeding period of the year
| May |
July |
September |
||||
|---|---|---|---|---|---|---|
| Calls | Tones | Calls | Tones | Calls | Tones | |
| Number of animals in group | 8 | 9 | 7 | 8 | 9 | 9 |
| Androgen, mean ± SEM | 8.38±1.95 | 6.99±1.18 | 4.69±1.42 | 0.53±0.19 | 2.25±1.23 | 0.64±0.10 |
Results
Hormones
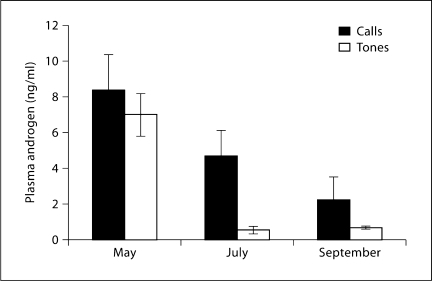
Mean hormone values (±SEM) for each experimental group are shown in table 1. Androgen values vary significantly both with treatment and with season. Androgen levels are highest early in the season and then decline (fig. 1). Analysis using log-transformed androgen levels yielded differences for both the main effects of season and acoustic treatment (F2 = 27.90, F1 = 13.89, respectively, both p < 0.001). The interaction was also significant (F2 = 4.257, p = 0.02). Inspection of the data indicates that the effect of acoustic treatment was most obvious in males sampled from the middle of the breeding season. A Tukey post-hoc test on the log-transformed data shows a strong, significant difference in July (p < 0.001), the difference in September just reaching significance (p = 0.043), while no difference was apparent between call and tone groups in May (p = 0.90)
Fig. 1.
Mean (±SEM) plasma androgens, measured in ng/ml, in each experimental group (call vs. tone stimulation) at each of three time points over a breeding season. ANOVA indicated significant main effects for stimulus condition and seasonal time point, with no significant interaction. See text for details.
AVT-ir Cell Number and Size
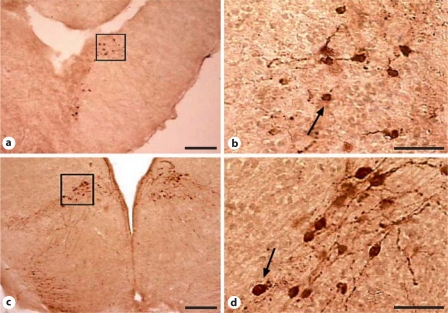
AVT-ir staining was apparent in four populations, the NAcc, amygdala, anterior preoptic area, and MPOA. Figure 2 shows example staining in the NAcc and MPOA.
Fig. 2.
Examples of AVT staining in NAcc (a, b) and MPOA (c, d) from socially stimulated male Hyla cinerea sampled during July. Scale bar = 20 μm.
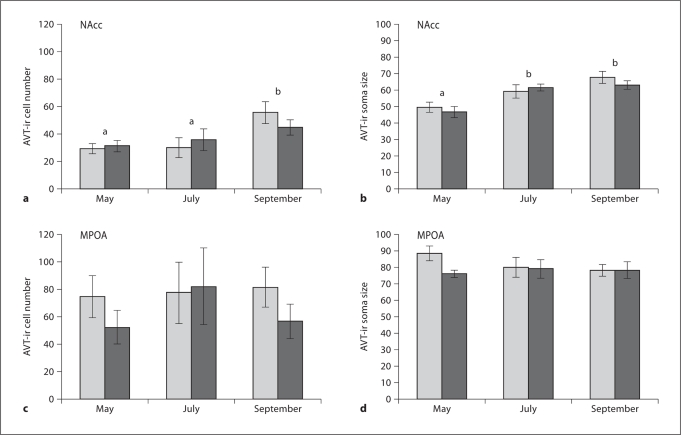
AVT-ir cell number in the NAcc was significantly different across the season (F2= 5.90, p =0.006). Post-hoc tests showed it to be significantly higher in the fall compared to the spring and summer (p < 0.01). AVT-ir cell number did not differ in this region across acoustic treatment groups (p =0.47). No significant interaction existed (F2= 0.56, p =0.57). Similarly, AVT-ir cell size in the NAcc significantly differed during the year (F2 = 18.44, p < 0.0001), but did not differ in this region across acoustic treatment groups (F1 = 0.316, p = 0.577). Post-hoc tests indicate that cell size was larger in July and September compared to May.
Neither AVT-ir cell number nor size differed significantly in any other brain area by treatment groups or time in breeding season. The differences in amygdala cell number with acoustic treatment was closest to significance (F1 = 3.194, p = 0.083). All other group differences were above F < 1.32, p > 0.26 and there was no discernable trend in the data. Figure 3 shows the effects of seasonal and social treatments for both AVT-ir cell number and size in the NAcc. Data from MPOA are also shown as an example of the nonsignificant results obtained from other areas.
Fig. 3.
a, b Seasonal variation in AVT-ir cell number and size (area, in μ2) in the NAcc. Light bars stand for males hearing a conspecific chorus, dark bars stand for males hearing random tones. Letters indicate statistically significant differences among time points within the breeding season from post-hoc pairwise comparisons; stimulus conditions within a time point were not significant. c, d Show similar data for the MPOA, where there were no seasonal or stimulus-condition differences.
Relationship of Androgens to AVT-ir Cell Number and Size
AVT cell number did not correlate significantly with androgen levels across groups. The situation with AVT cell size is more complicated. Affects were assessed for the NAcc. As AVT-ir soma size in the NAcc increases and androgens decline during the year, they are negatively correlated (r2 = 0.159, F44 change = 8.332, p = 0.006) across all groups. However, the relationship between the variables changes when season is factored into the multiple regression to correct for these correlated changes. The result is that when season is accounted for in the multiple regression, the relationship between soma size and androgen level is a positive one (r2 = 0.539, r2 = 0.38, F40 change = 8.258, p < 0.0001). Analyzing the relationship within each time point, however, shows that the positive relationship between AVT cell size and androgen level is only significant in May.
Calling Behavior
We assessed two types of calling behavior: calling in response to social cues (evoked calls) and spontaneous calling (non-evoked calls). As with hormone levels, we assessed differences in calling rate in both 2003 and 2004 combined.
As expected, evoked call rate was higher in males exposed to the chorus compared to males exposed to random tones (main effect of treatment: F1 = 5.29, p = 0.0264). Evoked call rate did not significantly vary across the breeding season (main effect of season: F2 = 1.39, p = 0.261). There was no significant interaction. These data suggest that evoked calling is mainly sensitive to the stimulus that evokes it, and continues throughout the course of the breeding season at an equivalent level.
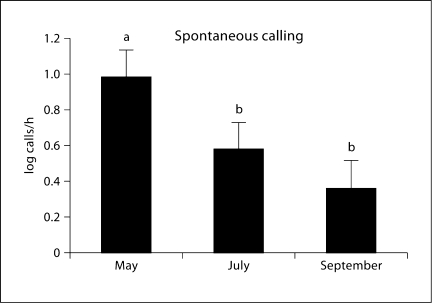
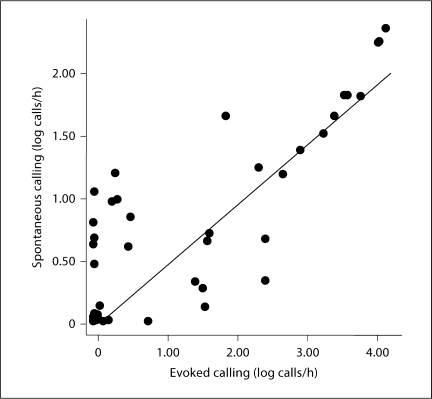
Spontaneous call rate was not affected by acoustic stimulus (main effect of treatment: F1 = 0.03, p = 0.864). That is, animals that heard calls during the acoustic treatment and animals that heard tones during this period did not differ in their calling during the nonstimulus period of the day. However, spontaneous call rate was significantly altered by season such that males at the beginning of the breeding season had significantly higher rates of spontaneous (non-evoked) calling compared to the middle and the end of the breeding season (main effect of season: F2 = 3.55, p = 0.037; fig. 4). No significant interaction between the two factors existed. This result suggests that spontaneous call rate is not altered by previous acoustic treatment, but is sensitive to seasonal cues of some kind. Spontaneous and evoked call rates were significantly correlated with each other within animals across all seasonal periods (r = 0.840, n = 50, p < 0.0001; fig. 5).
Fig. 4.
Mean (± SEM) spontaneous calling at each of three time points during the breeding season. Spontaneous calling is defined as all calls produced outside of the acoustic stimulus period each day. There were no social stimulation effects on spontaneous calling, hence data from those groups are combined. Mean difference across seasonal time points is significant (p = 0.037). Letters indicate significant differences among groups.
Fig. 5.
Scatterplot showing relationship between rate of spontaneous calling and rate of evoked calling (both in log calls/h) across all conditions and seasonal time points. A significant correlation exists between spontaneous and evoked calling (r = 0.840, p < 0.0001).
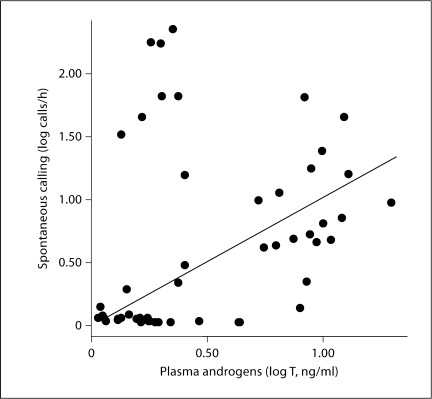
Spontaneous call rate was also significantly correlated with plasma androgen level when all animals were combined (r = 0.306, n = 50, p = 0.031; fig. 6), suggesting that variation in androgen can explain variation in spontaneous calling behavior across all groups. Androgen level did not correlate with evoked calling. AVT-ir soma size in the NAcc is correlated with calling behavior, and this relationship is independent of androgen. By doing a partial correlation to control for androgen levels, a significant positive relationship with AVT-ir soma size exists for both evoked (r2 = 0.291, p < 0.0001) and spontaneous (r2 = 0.224, p = 0.001) calling behavior. We did not see a similar relationship with AVT-ir cell number.
Fig. 6.
Scatterplot showing correlation between spontaneous calling (in log calls/h) and log plasma androgen levels (ng/ml). A significant correlation existed between rate of spontaneous calling and plasma androgens across all groups (r = 0.306, p = 0.031).
Discussion
This study investigated whether time within the breeding season affected social modulation of circulating steroid levels in male anurans and whether seasonal or social factors also influenced AVT-ir cell numbers or soma size. We exposed males to either conspecific cues or a positive control for seven consecutive nights at three points throughout the breeding season and examined plasma androgen levels. As shown previously in our lab [Burmeister and Wilczynski, 2000], we found that males exposed to conspecific cues had higher levels of androgens. We also determined that androgen levels decline throughout the course of the breeding season. Interestingly, it appears that males exposed to conspecific cues maintained higher levels of androgens compared to males exposed to a positive control as the breeding season progresses, suggesting that social communication might serve an important function to maintain reproductive hormone levels at an appropriately high level during the breeding season to facilitate reproductive behavior for a longer period of time. AVT cell number and size also changed over the breeding season, but in contrast to the findings for androgens this change was not modulated by social stimulation. Thus, social stimulation cannot mitigate all seasonal changes in systems responsible for regulating social communication.
Seasonal and Social Changes in Androgens and AVT
Gonadal androgens changed over the course of the breeding season, even though the animals were kept in constant environmental conditions in the lab. Although surprising, such an observation is not unusual. Circannual rhythms have been found to be endogenous cycles that persist even under constant environmental conditions in several taxa [e.g. Gwinner, 1986; Woodfill et al., 1994]. Androgen levels significantly dropped throughout the course of the study, from May to September. If these data are an accurate reflection of natural changes, they suggest that testosterone levels would be high when males emerge from hibernation and then progressively decline throughout the course of the breeding period, becoming quite low by the time the breeding period has ended. A seasonal decline in hormone levels has been reported in other anurans in a number of studies [Licht et al., 1983; Gobbetti et al., 1991; Delgado et al., 1992; Herman, 1992], which also find that androgen levels during the nonbreeding period are predictably low [Girgenrath and Marsh, 2003]. These reported values are similar to those found in the present study.
Because we obtained our frogs from a commercial source, it is possible that androgen levels merely declined upon capture and subsequent entry into the lab. A previous study found that animals housed for longer periods in the lab had significantly diminished testosterone levels [Burmeister and Wilczynski, 2000]. We cannot rule this out, nor do we know how long or under what conditions the animals we obtained for this study remained with suppliers prior to our receiving them. However, examination of our records suggests that housing time alone does not result in a decline in androgens. We obtained animals throughout the breeding season and assigned them randomly to groups. Animals from the two years averaged different amounts of time in the lab prior to experimental use (2003 = 38 days, 2004 = 13 days). Despite being in the lab considerably longer, 2003 animals had higher average androgen levels compared to 2004 animals (F1 = 7.57, p = 0.014). If laboratory housing resulted in progressively lower androgen levels, then the animals from 2003 should have had lower levels compared to the animals from 2004.
Androgen levels were also modulated by social cues in the present study, which replicates previous findings from our lab in males of two different species [H. cinerea: Burmeister and Wilczynski, 2000; Rana sphenocephala: Chu and Wilczynski, 2001]. Similarly, call stimulation increases estrogen in female túngara frogs, Physalaemus pustulosus [Lynch and Wilczynski, 2006]. Inspection of the data shown in figure 1 and the results of the post-hoc tests reveal interesting seasonal trends. Early in the season in May, when breeding behavior is often at its peak, androgen levels are very high, and a ceiling effect may prevent any large increase by social cues. As the season is ending in September, the seasonal inactivation of the HPG axis may have reached a point where it has become somewhat refractory to stimulatory cues, and hence less able to elevate androgens. The greatest effects are in the middle of the breeding season. The overall pattern of ANOVAs argues that social stimulation does contribute to some percentage of the variance in androgen levels across much of the breeding season, with less effect at the beginning when baseline levels are highest.
As for androgens, seasonal changes were found in AVT-ir cell number and size. The AVT changes were apparent only in the NAcc, where more and larger immunoreactive cells are present late in the season when both androgen levels and behavior (spontaneous calling) are declining. This result is reminiscent of an earlier study in cricket frogs [Acris crepitans; Marler et al., 1999], which found that noncalling satellite males had more AVT staining and larger cells than vigorously calling males in the NAcc population. It is also similar to the results of Maruska et al. [2007] who found that AVT cell number and size was higher in female gobies during the nonspawning season compared to the spawning season. It is interesting that in prairie voles, Microtus ochragaster, where male aggression and paternal care are increased by experimentally elevating AVP levels, a decrease in AVP-ir staining (in the lateral septum and the lateral habenular nucleus) is associated with the expression of these behaviors, whereas staining is greater in control animals that are not behaving [Bamshad et al., 1993, 1994; Winslow et al., 1993]. All these cases suggest that the seasonal increase in AVT-ir number and size we found reflect a decline in its release. Unfortunately, we cannot test this hypothesis directly. A further caution is that our measures do not directly relate to AVT production, which may or may not be changing seasonally. A more direct measure of AVT production is necessary to obtain a full picture of seasonal AVT changes and to better interpret the meaning of the AVT-ir changes we found.
Unlike androgens, AVT-ir cell number and size are not sensitive to social stimulation. Therefore any seasonal decline in AVT activity would not be mitigated simply by the effects of listening to a chorus. In many vertebrates investigated, increased androgens increase AVT production or other measures of brain AVT levels. In our data, the relationship between AVT soma size and androgens is a complicated one because of the interacting effect of our seasonal variable and a relationship that seems to vary depending on the point in the season at which the animals are tested. When data are analyzed within each time point, there appears to be a positive relationship between AVT cell size and androgens. There may be a change in AVT production, induced by short-term socially stimulated androgen increases, which interacts with a general seasonal decline in overall androgen levels and AVT release, but we cannot disentangle such effects with the data we have.
Seasonal and Social Effects on Calling Behavior
A nearly universal feature of anuran male social behavior is that males call in response to conspecific calls. As expected, social stimulation via conspecific calls significantly increased evoked calling rate in our study compared to control animals stimulated acoustically with random tones. This is consistent with previous laboratory work using similar stimuli [Burmeister and Wilczynski, 2000]. Evoked calling did not appear to decline towards the end of the breeding season, suggesting that the machinery required for responding to social cues is stable throughout the year, or at least until the end of the breeding season. It is not known whether or not males of temperate-zone species similar to H. cinerea would also respond in the winter. However, Burmeister and Wilczynski [2001] found that gonadectomy abolished evoked calling in male H. cinerea, suggesting that when testosterone falls to negligible levels evoked calling would in fact stop. It seems likely that a threshold level is required for androgens to induce this behavior, and the September androgen levels remained above this level.
Spontaneous calling rate, or the amount of calling that occurs in the absence of a stimulus, did not vary with acoustic stimulation treatment. It did, however, decline from May to September, mirroring the seasonal decline in androgen levels. In fact across groups spontaneous calling and plasma androgen levels are correlated, which is similar to the relationship found by Burmeister and Wilczynski [2001] in intact and testosterone-implanted males. Spontaneous calling behavior likely reflects the overall excitability or motivation of a male to engage in reproductive social behavior. Spontaneous calling is important because males’ calling advertises their presence to females, which find and choose mates based on calls. The more males call, the greater their chance of attracting a female. In addition, high spontaneous calling in general predicts more evoked calling, as would be expected if it indicated the level of social motivation. This correlation between calling measures occurred even though androgens only correlate with spontaneous calling. Note, however, that Solis and Penna [1997] documented a significant correlation between plasma androgens and evoked calling in Batrachyla taeniata. The loss of significance in the androgen-evoked calling correlation may be a statistical issue as this chain of relationships – i.e. androgens predict spontaneous calling, then spontaneous calling predicts evoked calling, plus the added influence of locking evoked calls to the stimulus – ultimately loses the power to reveal a relationship between such separated variables.
As in other parts of this study, the statistical relationships between calling and AVT measures are complicated. Overall, the correlation between AVT-ir cell size and calling is positive and independent of androgen levels. However, mean levels of AVT-ir cell number and size decrease over the season just as spontaneous calling declines, and when the time points are analyzed separately the significant correlations vanish except for the September data when many males express little calling. A more complete analysis of relationships between calling and AVT measures, as well as a better understanding of how different AVT measures reflect production, storage, and release, is necessary to interpret these relationships.
Overall, it therefore seems likely that, as the breeding season progresses, males, regardless of their exposure to social cues, become less active in terms of acoustic behavior, and that this decline likely is due to declining androgen levels and perhaps changes in the neuromodulatory AVT population in the NAcc. We interpret the AVT-ir changes to indicate a seasonal decline in AVT release, based on other studies showing that AVT-ir measures are higher in behaviorally inactive individuals. The seasonal AVT changes are not directly influenced by social interactions, and therefore may represent one factor that is most closely tied to an endogenous circannual rhythm limiting the breeding season. On the other hand, both time within the breeding season and social stimulation by advertisement calls set the state of circulating androgens in the male green treefrogs we examined in this study. These results indicate that being exposed to social signals, as is generally the case when males of this species participate in a mating chorus, helps to maintain gonadal steroids at an elevated state relative to where they would be in the absence of social stimulation. Such an elevation would maintain an enhanced reproductive state in males throughout the long breeding season and possibly lengthen the effective breeding season in the most stimulated males by maintaining reproductive levels of androgens past the point where they would have declined to nonreproductive levels [Wilczynski et al., 2005]. Our results do show that the social stimulation could not extend breeding indefinitely, as androgen levels decline despite social stimulation and AVT systems change regardless of social situation. Nevertheless, socially stimulated androgen elevation, if linked to enhanced calling (as the correlations with spontaneous calling suggest to be the case) or elevated gamete production (which still remains to be demonstrated), could increase the potential reproductive success of males toward the later parts of the season. In this way, joining a chorus with other calling males may have physiologically based benefits in addition to any other selective advantage such social aggregations may impart.
Acknowledgements
The authors wish to thank Jennifer Gench and Glennis Julian for their assistance in this study and Deborah Lutterschmidt for comments on the manuscript. Supported by NIH F32 MH067390 (E.L.O.) and NIH R01 MH057066 (W.W.).
References
- Adkins E, Schlesinger L. Androgens and the social behavior of male and female lizards (Anolis carolinensis) Horm Behav. 1979;13:139–152. doi: 10.1016/0018-506x(79)90053-9. [DOI] [PubMed] [Google Scholar]
- Allison JD, Wilczynski W. Thalamic and midbrain auditory projections to the preoptic area and ventral hypothalamus in the green treefrog (Hyla cinerea) Brain Behav Evol. 1991;38:322–331. doi: 10.1159/000114398. [DOI] [PubMed] [Google Scholar]
- Allison JD, Wilczynski W. Efferents from the suprachiasmatic nucleus to basal forebrain nuclei in the green treefrog (Hyla cinerea) Brain Behav Evol. 1994;43:129–139. doi: 10.1159/000113630. [DOI] [PubMed] [Google Scholar]
- Arnold AP. The effects of castration and androgen replacement on song, courtship, and aggression in zebra finches (Poephila guttata) J Exp Zool. 1975;191:309–326. doi: 10.1002/jez.1401910302. [DOI] [PubMed] [Google Scholar]
- Borges RA, Oliveira RF, Almada VC, Canario AVM. Short-term social modulation of 11-ketotestosterone levels in males of the cichlid fish Oreochromis mossambicus during male-female interactions. Acta Ethologica. 1998;1:43–48. [Google Scholar]
- Boyd SK. Arginine vasotocin facilitation of advertisement calling and call phonotaxis in bullfrogs. Horm Behav. 1994;28:232–240. doi: 10.1006/hbeh.1994.1020. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Neighbor call amplitude influences aggressive behavior and intermale spacing in choruses of the pacific treefrog (Hyla regilla) Ethology. 1989;83:69–79. [Google Scholar]
- Burmeister S, Somes C, Wilczynski W. Behavioral and hormonal effects of exogenous vasotocin and corticosterone in the green treefrog. Gen Comp Endocrinol. 2001;122:189–197. doi: 10.1006/gcen.2001.7625. [DOI] [PubMed] [Google Scholar]
- Burmeister S, Wilczynski W. Social signals influence hormones independently of calling behavior in the treefrog (Hyla cinerea) Horm Behav. 2000;38:201–209. doi: 10.1006/hbeh.2000.1605. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Wilczynski W. Social context influences androgenic effects on calling in the green treefrog (Hyla cinerea) Horm Behav. 2001;40:550–558. doi: 10.1006/hbeh.2001.1723. [DOI] [PubMed] [Google Scholar]
- Chu J, Marler CA, Wilczynski W. The effects of arginine vasotocin on the calling behavior of male cricket frogs in changing social contexts. Horm Behav. 1998;34:248–261. doi: 10.1006/hbeh.1998.1479. [DOI] [PubMed] [Google Scholar]
- Chu J, Wilczynski W. Social influences on androgen levels in the southern leopard frog, Rana sphenocephala. Gen Comp Endocrinol. 2001;121:66–73. doi: 10.1006/gcen.2000.7563. [DOI] [PubMed] [Google Scholar]
- Crews D. Interrelationships among ecological, behavioral, and neuroendocrine processes in the reproductive cycle of Anolis carolinensis and other reptiles. In: Rosenblatt JS, Hinde RA, Beer CG, Busnel MC, editors. Advances in the Study of Behavior. vol 11. New York: Academic Press; 1980. pp. 1–74. [Google Scholar]
- Crews D, Traina V, Wetzel FT, Muller C. Hormonal control of male reproductive behavior in the lizard, Anolis carolinensis: role of testosterone, dihydrotestosterone, and estradiol. Endocrinology. 1978;103:1814–1821. doi: 10.1210/endo-103-5-1814. [DOI] [PubMed] [Google Scholar]
- Delgado MJ, Alonso-Gomez AL, Alonso-Bedate M. Role of environmental temperature and photoperiod in regulation of seasonal testicular activity in the frog, Rana perezi. Can J Physiol Pharmacol. 1992;70:1348–1352. doi: 10.1139/y92-189. [DOI] [PubMed] [Google Scholar]
- Deviche P, Gulledge CC. Vocal control region sizes of an adult female songbird change seasonally in the absence of detectable circulating testosterone concentrations. J Neurobiol. 2000;42:202–211. doi: 10.1002/(sici)1097-4695(20000205)42:2<202::aid-neu4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- DeVries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt HC. The significance of some spectral features in mating call recognition in the green treefrog (Hyla cinerea) J Exp Biol. 1974;61:229–241. doi: 10.1242/jeb.61.1.229. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC, Bee M. Recognition and localization of acoustic signals. In: Narins PN, Feng AS, Fay RR, Popper AN, editors. Hearing and Sound Communication in Amphibians: Springer Handbook of Auditory Research. New York: Springer-Verlag; 2007. pp. 113–146. [Google Scholar]
- Girgenrath M, Marsh RL. Season and testosterone affect contractile properties of fast calling muscles in the gray tree frog Hyla chrysoscelis. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1513–R1520. doi: 10.1152/ajpregu.00243.2002. [DOI] [PubMed] [Google Scholar]
- Gobbetti A, Zerani M, Bolelli GF, Botte V. Seasonal changes in plasma prostaglandin F2 alpha and sex hormones in the male water frog, Rana esculenta. Gen Comp Endocrinol. 1991;82:331–336. doi: 10.1016/0016-6480(91)90307-r. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:2–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Gozdowska M, Kleszczyńska A, Sokołowska E, Kulczykowska E. Arginine vasotocin (AVT) and isotocin (IT) in fish brain: diurnal and seasonal variations. Comp Biochem Physiol B. 2006;143:330–334. doi: 10.1016/j.cbpb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Gwinner E. Circannual Rhythms: Endogenous Annual Clocks in the Organization of Seasonal Processes. New York: Springer-Verlag; 1986. [Google Scholar]
- Hattori T, Wilczynski W. Comparison of arginine vasotocin immunoreactivity differences in dominant and subordinate green anole lizards. Physiol Behav. 2009;96:104–107. doi: 10.1016/j.physbeh.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Herman CA. Endocrinology. In: Feder ME, Burggren WW, editors. Environmental Physiology of the Amphibians. Chicago: University of Chicago Press; 1992. pp. 40–54. [Google Scholar]
- Holmes MM, Wade J. Seasonal plasticity in the copulatory neuromuscular system of green anole lizards: a role for testosterone in muscle but not motoneuron morphology. J Neurobiol. 2004;60:1–11. doi: 10.1002/neu.10334. [DOI] [PubMed] [Google Scholar]
- Kabelik D, Morrison JA, Goodson JL. Cryptic regulation of vasotocin neuronal activity but not anatomy by sex steroids and social stimuli in opportunistic desert finches. Brain Behav Evol. 2010;75:71–84. doi: 10.1159/000297522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelik D, Weiss SL, Moore MC. Arginine vasotocin (AVT) immunoreactivity relates to testosterone but not territorial aggression in the tree lizard, Urosaurus ornatus. Brain Behav Evol. 2008;72:283–294. doi: 10.1159/000174248. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Lieberburg I, McEwen BS, Pfaff DW. Autoradiographic and biochemical studies of steroid hormone-concentrating cells in the brain of Rana pipiens. Brain Res. 1978;140:287–305. doi: 10.1016/0006-8993(78)90461-4. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Morrell JI, Pfaff DW. Autoradiographic localization of hormone-concentrating cells in the brain of an amphibian, Xenopus laevis. I. Testosterone. J Comp Neurol. 1975;164:47–59. doi: 10.1002/cne.901640105. [DOI] [PubMed] [Google Scholar]
- Kime NM, Whitney TK, Davis ES, Marler CA. Arginine vasotocin promotes calling behavior and call changes in male Túngara frogs. Brain Behav Evol. 2007;69:254–265. doi: 10.1159/000099613. [DOI] [PubMed] [Google Scholar]
- Klomberg KF, Marler CA. The neuropeptide arginine vasotocin alters male call characteristics involved in social interactions in the grey treefrog, Hyla versicolor. Anim Behav. 2000;59:807–812. doi: 10.1006/anbe.1999.1367. [DOI] [PubMed] [Google Scholar]
- Licht P. Environmental control of annual testicular cycles in the lizard Anolis carolinensis. I. Interaction of light and temperature in the initiation of testicular recrudescence. J Exp Zool. 1967;165:505–516. doi: 10.1002/jez.1401650317. [DOI] [PubMed] [Google Scholar]
- Licht P. Regulation of the annual testis cycle by photoperiod and temperature in the lizard Anolis carolinensis. Ecology. 1971;52:240–252. [Google Scholar]
- Licht P, McCreery BR, Barnes R, Pang R. Seasonal and stress related changes in plasma gonadotropins, sex steroids and corticosterone in the bullfrog, Rana catesbeiana. Gen Comp Endocrinol. 1983;50:124–145. doi: 10.1016/0016-6480(83)90249-6. [DOI] [PubMed] [Google Scholar]
- Lindzey J, Crews D. Hormonal control of courtship and copulatory behavior in male Cnemidophorus inornatus, a direct sexual ancestor of a unisexual, parthenogenetic lizard. Gen Comp Endocrinol. 1986;64:411–418. doi: 10.1016/0016-6480(86)90077-8. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Gonadal steroids vary with reproductive stage in a tropically breeding female anuran. Gen Comp Endocrinol. 2005;143:51–56. doi: 10.1016/j.ygcen.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Social regulation of plasma estrogen concentration in a female anuran. Horm Behav. 2006;50:101–106. doi: 10.1016/j.yhbeh.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler CA, Boyd SK, Wilczynski W. Forebrain neuropeptide correlates of alternative male mating strategies. Horm Behav. 1999;36:53–61. doi: 10.1006/hbeh.1999.1524. [DOI] [PubMed] [Google Scholar]
- Marler CA, Chu JC, Wilczynski W. Arginine vastocin increases probability of calling but decreases aggressive nature of calls produced in the cricket frog, Acris crepitans. Horm Behav. 1995;29:554–570. doi: 10.1006/hbeh.1995.1286. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Mizobe MH, Tricas TC. Sex and seasonal co-variation of arginine vasotocin (AVT) and gonadotropin-releasing hormone (GnRH) neurons in the brain of the halfspotted goby. Comp Biochem Physiol A. 2007;147:129–144. doi: 10.1016/j.cbpa.2006.12.019. [DOI] [PubMed] [Google Scholar]
- O'Bryant EL, Wade J. Sexual dimorphisms in a neuromuscular system regulating courtship in the green anole lizard: effects of season and androgen treatment. J Neurobiol. 1999;40:202–213. doi: 10.1002/(sici)1097-4695(199908)40:2<202::aid-neu6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- O'Bryant EL, Wade J. Seasonal and sexual dimorphisms in the green anole forebrain. Horm Behav. 2002;41:384–395. doi: 10.1006/hbeh.2002.1778. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Almada VC, Canario AV. Social modulation of sex steroid concentrations in the urine of male cichlid fish Oreochromis mossambicus. Horm Behav. 1996;30:2–12. doi: 10.1006/hbeh.1996.0002. [DOI] [PubMed] [Google Scholar]
- Orchinik M, Licht P, Crews D. Plasma steroid concentrations change in response to sexual behavior in Bufo marinus. Horm Behav. 1988;22:338–350. doi: 10.1016/0018-506x(88)90006-2. [DOI] [PubMed] [Google Scholar]
- Riters LV, Absil P, Balthazart J. Effects of brain testosterone implants on appetitive and consummatory components of male sexual behavior in Japanese quail. Brain Res Bull. 1998;47:69–79. doi: 10.1016/s0361-9230(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Rudd CD, Short RV, Shaw G, Renfree MB. Testosterone control of male-type sexual behavior in the tammar wallaby (Macropus eugenii) Horm Behav. 1996;30:446–454. doi: 10.1006/hbeh.1996.0049. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Preoptic activation of frog mating behaviour. Behaviour. 1968;30:239–257. [Google Scholar]
- Schmidt RS. Neural correlates of frog calling: masculinization by androgens. Horm Behav. 1983;17:94–102. doi: 10.1016/0018-506x(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Semsar K, Godwin J. Social influences on the arginine vasotocin system are independent of gonads in a sex-changing fish. J Neurosci. 2003;23:4386–4393. doi: 10.1523/JNEUROSCI.23-10-04386.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J Neurosci. 1997;17:6001–6010. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis R, Penna M. Testosterone levels and evoked vocal responses in a natural population of the frog Batrachyla taeniata. Horm Behav. 1997;31:101–109. doi: 10.1006/hbeh.1997.1366. [DOI] [PubMed] [Google Scholar]
- Soma KK, Francis RC, Wingfield JC, Fernald RD. Androgen regulation of hypothalamic neurons containing gonadotropin-releasing hormone in a cichlid fish: integration with social cues. Horm Behav. 1996;30:216–226. doi: 10.1006/hbeh.1996.0026. [DOI] [PubMed] [Google Scholar]
- Stacey N, Kobayashi M. Androgen induction of male sexual behaviors in female goldfish. Horm Behav. 1996;30:434–445. doi: 10.1006/hbeh.1996.0048. [DOI] [PubMed] [Google Scholar]
- Tokarz RR, McMann S, Seitz L, John-Alder H. Plasma corticosterone and testosterone levels during the annual reproductive cycle of male brown anoles (Anolis sagrei) Physiol Zool. 1998;71:139–146. doi: 10.1086/515907. [DOI] [PubMed] [Google Scholar]
- Tokarz RR, McMann S, Smith LC, John-Alder H. Effects of testosterone treatment and season on the frequency of dewlap extensions during male-male interactions in the lizard Anolis sagrei. Horm Behav. 2002;41:70–79. doi: 10.1006/hbeh.2001.1739. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Rouse KL, Marler CA. Arginine vasotocin interacts with the social environment to regulate advertisement calling in the gray treefrog (Hyla versicolor) Brain Behav Evol. 2003;61:165–171. doi: 10.1159/000070700. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA. Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J Neurosci. 1999;19:476–483. doi: 10.1523/JNEUROSCI.19-01-00476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Gorbman A. Mate calling induced by electrical stimulation in freely moving leopard frogs, Rana pipiens. Horm Behav. 1977a;9:141–149. doi: 10.1016/0018-506x(77)90081-2. [DOI] [PubMed] [Google Scholar]
- Wada M, Gorbman A. Relation of mode of administration of testosterone to evocation of male sex behavior in frogs. Horm Behav. 1977b;8:310–319. doi: 10.1016/0018-506x(77)90005-8. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Allison JD. Acoustic modulation of neural activity in the hypothalamus of the leopard frog. Brain Behav Evol. 1989;33:317–324. doi: 10.1159/000115939. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Allison JD, Marler CA. Sensory pathways linking social and environmental cues to endocrine control regions of amphibian forebrains. Brain Behav Evol. 1993;42:252–264. doi: 10.1159/000114159. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Brenowitz EA. Acoustic cues mediate inter-male spacing in a neotropical frog. Anim Behav. 1988;36:1054–1063. [Google Scholar]
- Wilczynski W, Lynch KS, O'Bryant EL. Current research in amphibians: studies integrating endocrinology, behavior, and neurobiology. Horm Behav. 2005;48:440–450. doi: 10.1016/j.yhbeh.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfill CJ, Wayne NL, Moenter SM, Karsch FJ. Photoperiodic synchronization of a circannual reproductive rhythm in sheep: identification of season-specific time cues. Reproductive Biol. 1994;50:965–976. doi: 10.1095/biolreprod50.4.965. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Sakata JT, Crews D. Evolutionary insights into the regulation of courtship behavior in male amphibians and reptiles. Physiol Behav. 2004;83:347–360. doi: 10.1016/j.physbeh.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Moore FL. Seasonal changes in luteinizing hormone-releasing hormone concentrations in microdissected brain regions of male rough-skinned newts (Taricha granulosa) Gen Comp Endocrinol. 1985;58:222–230. doi: 10.1016/0016-6480(85)90338-7. [DOI] [PubMed] [Google Scholar]