Abstract
Background
Therapeutic endpoints based on reduced clinical worsening represent clinically relevant and realistic goals for patients suffering from progressive neurodegenerative disorders such as Alzheimer's disease (AD).
Methods
Data from 906 patients (388 receiving placebo; 518 receiving donepezil) with mild-to-moderate AD [Mini-Mental State Examination (MMSE) score 10–27] were pooled from 3 randomized, double-blind placebo-controlled studies. Clinical worsening was defined as decline in (1) cognition (MMSE), (2) cognition and global ratings (Clinician's Interview-Based Impression of Change plus Caregiver Input/Gottfries-Bråne-Steen scale) or (3) cognition, global ratings and function (various functional measures).
Results
At week 24, lower percentages of donepezil-treated patients than placebo patients met the criteria for clinical worsening, regardless of the definition. The odds of declining were significantly reduced for donepezil-treated versus placebo patients (p < 0.0001; all definitions). Among patients meeting criteria for clinical worsening, mean declines in MMSE scores were greater for placebo than donepezil-treated patients.
Conclusion
In this population, donepezil treatment was associated with reduced odds of clinical worsening of AD symptoms. Moreover, patients worsening on donepezil were likely to experience less cognitive decline than expected if left untreated. This suggests that AD patients showing clinical worsening on donepezil may still derive benefits compared with placebo/untreated patients.
Key Words: Donepezil, Alzheimer's disease, Cognition, Global function, Clinical worsening
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by a gradual decline in cognition, decreased ability to perform activities of daily living (ADL) and, often, neuropsychiatric/behavioral problems [1]. Despite the progressive nature of AD, it is frequently assumed that treatment benefits in patients can be demonstrated only by an improvement or stabilization of cognitive, functional and/or behavioral symptoms. In support of this notion, authorities tend to license AD therapy and provide guidance on the use of this therapy based on demonstrable symptomatic improvements [2]. However, while improvement and, to a lesser extent, stabilization are ideal outcomes of treatment, therapeutic end points based on reduced worsening or less than expected decline are also clinically relevant and, at present, potentially more realistic goals for patients suffering from progressive dementia [3,4,5].
The cholinesterase inhibitor donepezil is licensed for the treatment of mild to moderately severe AD in many countries worldwide and has been approved for the treatment of severe AD in several countries, including the USA. In randomized, double-blind, placebo-controlled clinical trials, donepezil has been shown to provide benefits in cognition, function and behavior in patients with mild-to-moderate, moderate-to-severe and severe AD [6,7,8,9,10,11,12]. As with all dementia therapy, some patients treated with donepezil do not show an improvement or stabilization of symptoms. These patients may initially be characterized as unresponsive to treatment, but some may still show less of a decline than would be expected if left untreated. The failure to identify this effect may result not only from perceptions regarding what constitutes a positive outcome in AD, but also from the difficulty in measuring a less than expected decline in an individual patient.
Useful information regarding the relevance of response definitions in patients with AD may be provided by ‘responder’ analyses. These analyses allow identification of patient subpopulations meeting specific ‘response’ criteria and, thus, facilitate evaluation of various outcomes within these subpopulations. Previous responder analyses have tended to focus on positive outcomes of treatment in patients receiving AD therapy [13] and have therefore focused on improvement in cognitive and/or functional and global measures. However, this does not take into account the continued progression of the disease in the majority of patients. In addition, an important treatment benefit may be overlooked if the possibility of reduced clinical worsening is not considered as a potential treatment goal in the setting of a progressive degenerative disease.
In an attempt to assess the utility of treatment in preventing/delaying the worsening of clinical symptoms in AD patients, we performed an analysis in which ‘response’ was considered not as improvement, but as reduced worsening. In this type of analysis, patients receiving placebo would be expected to respond less, that is worsen more, than patients under active therapy. We investigated whether donepezil-treated patients with mild-to-moderate AD are less likely to experience clinical worsening compared with placebo patients. An additional objective was to determine, in those patients meeting similar criteria for clinical worsening, whether donepezil-treated patients show a reduced degree of cognitive decline compared with those on placebo. For the current analyses, several criteria were used to define clinical worsening based on concurrent decline on commonly used scales assessing cognition, global outcomes and function. The overall purpose was to provide greater insight into the range of responses to donepezil, particularly in patients without symptomatic stabilization or improvement.
Methods
Study Selection
Studies were selected for inclusion in the present analyses if they met the following prespecified criteria: (1) a randomized, double-blind, placebo-controlled parallel-group study of the use of donepezil in the treatment of AD; (2) conducted within the donepezil clinical development program and with available individual patient level data; (3) at least 24 weeks in duration; (4) available postbaseline cognition data as measured by the Mini-Mental State Examination (MMSE) [14]; (5) available postbaseline global data as measured by the Clinician's Interview-Based Impression of Change plus Caregiver Input (CIBIC+) [15] or the Gottfries-Bråne-Steen (GBS) scale [16,17], and (6) available postbaseline function data measured using any functional scale. In total, 3 studies met the prespecified inclusion criteria and were included in the subsequent analyses (table 1). One study was conducted at multiple sites in the USA (302 study), 1 at sites throughout northern Europe (Nordic study) and 1 at sites in Canada, Australia and France [Moderate to Severe AD (MSAD) study]. Detailed descriptions of study designs for the individual trials have been published previously [7,10,11]. The 302 and Nordic studies enrolled patients with baseline MMSE scores of 10–26, whereas the MSAD study enrolled patients with MMSE scores of 5–17. To limit the current analyses to a population with mild-to-moderate AD, individual patient data were pooled only from the subset of patients with baseline MMSE scores of at least 10.
Table 1.
Randomized, double-blind placebo-controlled trials included in analyses
| Study | MMSE | Study duration weeks | Patients randomized to treatment | |||
|---|---|---|---|---|---|---|
| inclusion range | pretreatment range of means | placebo | donepezil | total | ||
| 302 (Rogers et al. [10]) | 10–26 | 18.9–19.2 | 301 | 162 | 3112 | 473 |
| Nordic (Winblad et al. [11]) | 10–26 | 19.3–19.4 | 52 | 144 | 142 | 286 |
| MSAD (Feldman et al. [7]) | 5–17 | 11.7–12.0 | 24 | 146 | 144 | 290 |
Study included a donepezil treatment period of 24 weeks, followed by a 6-week washout phase.
Study included 5 mg/day (n = 154) and 10 mg/day (n = 157) donepezil dosage arms.
Patients
All patients enrolled in the original trials had a diagnosis of possible or probable AD based on criteria of the National Institute of Neurological and Communicative Disorders and Stroke – Alz-heimer's Disease and Related Disorders Association [18]. Patients enrolled in the 302 and Nordic studies were also required to meet criteria for dementia of Alzheimer's type from the third or fourth edition of the Diagnostic and Statistical Manual of Mental Disorders [19]. In general, inclusion/exclusion criteria were similar across the 3 studies [7,10,11]. In the 302 study, patients were randomized to either donepezil 5 mg/day, donepezil 10 mg/day or placebo. A blinded, forced-dose escalation method was used for the higher-dose group, in which subjects received 5 mg/day for 1 week and then 10 mg/day for the remainder of the study. In the Nordic and MSAD studies, donepezil was given at 10 mg/day following 4 weeks of treatment with the dose 5 mg/day. In the Nordic and MSAD studies, a dose adjustment back to 5 mg/day was allowed at any time to improve tolerability, if required. All studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Outcome Measures
All 3 studies used the MMSE to measure cognition. The MMSE is a brief psychometric test that evaluates the cognitive status of the patient and includes aspects of memory, orientation, language and praxis. Scores range from 0 to 30, with lower scores indicating greater cognitive impairment. In the 302 study, cognition was also assessed using the Alzheimer's Disease Assessment Scale cognitive subscale (ADAS-cog) [20].
In the 302 and MSAD studies, global outcomes were assessed using the CIBIC+. The 302 study also assessed global outcomes using the Clinical Dementia Rating Sum of the Boxes (CDR-SB) [21]. In the Nordic study, global outcomes were assessed using the GBS scale, as well as the Global Deterioration Scale [22,23].
Function was assessed in the Nordic study using the Progressive Deterioration Scale (PDS) [23] and in the MSAD study using the Disability Assessment for Dementia [24], the modified Instrumental Activities of Daily Living scale and the Physical Self-Maintenance Scale [26]. Function was not formally assessed in the 302 study but could be evaluated via 3 domains of the CDR-SB that are comparable to domains measured in most ADL scales (i.e. community affairs, home and hobbies, personal care).
Behavior was assessed in the Nordic and MSAD studies using the Neuropsychiatric Inventory [27]. Behavior was not assessed in the 302 study.
In all studies, efficacy measures were utilized at weeks 12 and 24.
Definitions of Clinical Worsening
Three sets of criteria were used to define clinical worsening at week 24:
• cognition (COG): decline in cognitive function; for all patients, the criterion for this definition was any decline in MMSE;
• cognition + global (COG + G): decline in cognition and global ratings; for patients in the 302 and MSAD studies, criteria for this definition were any decline in MMSE and any decline on the CIBIC+; for patients in the Nordic study, criteria were any decline in MMSE and any decline on the GBS;
• cognition + global + function (COG + G + F): decline in cognition, global ratings and function; for patients in the 302 study, criteria for this definition were any decline in MMSE and any decline on the CIBIC+ and any decline in the sum of the 3 function-related domains of the CDR-SB (community affairs, home and hobbies and personal care); for patients in the Nordic study, criteria were any decline in MMSE and any decline on the GBS and Global Deterioration Scale and PDS; for patients in the MSAD study, criteria were any decline in MMSE and any decline on the CIBIC+ and any decline on the Instrumental Activities of Daily Living scale and Disability Assessment for Dementia and Physical Self-Maintenance Scale.
A behavioral outcome was not included within the selected definitions of clinical worsening because the 302 study did not include a measure of behavior, and therefore sample sizes available for analysis would have been limited. In addition, subjects for these studies were not selected for the presence of behavioral problems.
Statistical/Data Analyses
Initial analyses focused on determining the proportion of the overall population who met the specified criteria for each definition of clinical worsening at week 24. Analyses were performed using both the last observation carried forward (LOCF) and observed cases (OC) methods. For the OC analyses, patients showing any change (improvement or worsening) in any of the 3 domains (cognition, global or function) were included in the analysis population, even if data were missing for the other domains. χ2 tests were used to compare the proportion of patients meeting criteria for clinical worsening between the donepezil and placebo groups. Subsequent analyses assessed the mean change in MMSE scores from baseline to weeks 12 and 24 among patients meeting the criteria for clinical worsening. These analyses are reported using the OC method only. All analyses were based on the intent-to-treat population, defined as patients who took at least 1 dose of study medication and provided at least 1 postbaseline efficacy assessment. The random effects model, with study as random effect and baseline value and treatment as fixed effects, was used in the analyses.
Results
Patient Population
In total, 1,049 patients were randomized to donepezil or placebo in the original trials and were assessed for eligibility for inclusion in the current analyses. From this population, 906 patients (388 receiving placebo; 518 receiving donepezil) had baseline MMSE scores ≥10 and available postbaseline data and were included in the current analyses.
Population Demographics
Patient characteristics for the analysis population are shown in table 2. No clinically relevant differences were noted between the donepezil-treated and placebo populations.
Table 2.
Population characteristics of patients in the pooled data set
| Characteristic | Placebo | Donepezil |
|---|---|---|
| Age | ||
| Number | 388 | 517 |
| Mean ± SD, years | 73.1±7.7 | 73.0±8.1 |
| Range, years | 48–90 | 49–92 |
| Sex | ||
| Male | 155 (40.0%) | 182 (35.1%) |
| Female | 233 (60.0%) | 336 (64.9%) |
| Baseline MMSE | ||
| Number | 388 | 518 |
| Mean ± SD | 18.0±4.7 | 18.4±4.7 |
| Range | 10–26 | 10–27 |
| Comorbidities | ||
| Number | 388 | 517 |
| Mean ± SD | 5.1±2.3 | 5.1±2.4 |
| Range | 1–15 | 1–15 |
| Concomitant medications used | ||
| Number | 388 | 518 |
| Mean ± SD | 4.1±3.5 | 4.1±3.7 |
| Range | 0–20 | 0–27 |
Analysis Outcomes
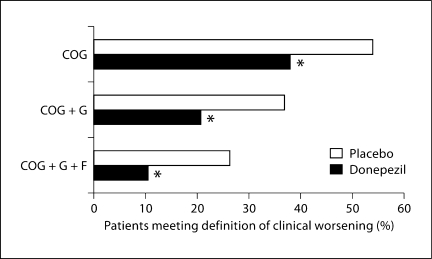
A significantly greater percentage of placebo patients than donepezil-treated patients met the specified criteria for all 3 definitions of clinical worsening (fig. 1; table 3). In both the OC and LOCF analyses, the odds ratios for clinical worsening were significantly reduced for donepezil-treated patients compared with placebo patients (p < 0.0001 for all definitions using OC and LOCF methods; table 3). As expected, as the definition criteria became more stringent, the percentage of patients with clinical worsening became smaller, irrespective of treatment allocation.
Fig. 1.
Percentage of patients meeting criteria for clinical worsening in the overall mild-to-moderate AD population. Data are shown for the COG (placebo, 188/348; donepezil, 172/453), COG + G (placebo, 129/350; donepezil, 93/451) and COG + G + F (placebo, 92/350; donepezil, 47/453) definitions. Intent-to-treat population, OC analysis; ∗ p < 0.0001.
Table 3.
Distribution of patients meeting criteria for clinical worsening at week 24
| Assessment used to define clinical worsening | Proportion of patients showing clinical worsening | Odds ratio | 95% CI | p value | ||
|---|---|---|---|---|---|---|
| placebo, n/N | donepezil, n/N | |||||
| COG | OC | 188/348 (54.0) | 172/453 (38.0) | 0.52 | 0.39–0.69 | <0.0001 |
| LOCF | 204/381 (53.5) | 190/504 (37.7) | 0.53 | 0.40–0.69 | <0.0001 | |
| COG + G | OC | 129/350 (36.9) | 93/451 (20.6) | 0.45 | 0.32–0.61 | <0.0001 |
| LOCF | 137/385 (35.6) | 102/507 (20.1) | 0.46 | 0.34–0.62 | <0.0001 | |
| COG + G + F | OC | 92/350 (26.3) | 47/453 (10.4) | 0.32 | 0.22–0.48 | <0.0001 |
| LOCF | 97/384 (25.3) | 53/510 (10.4) | 0.34 | 0.24–0.49 | <0.0001 | |
Odds ratios are based on odds of clinical worsening for donepezil-treated patients compared with placebo patients.
Figures in parentheses are percentages. CI = Confidence interval; n/N = actual number out of total number.
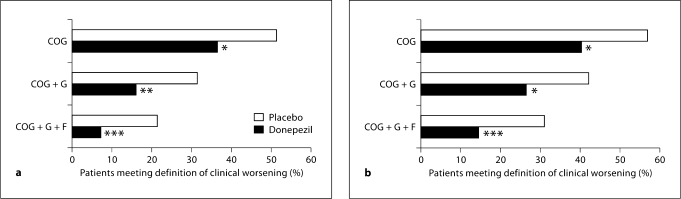
In subgroups of patients with milder (MMSE, 18–26) and more moderate AD (MMSE, 10–17), the pattern of treatment benefit mirrored that seen for the overall population. In both subgroups, the percentage of placebo patients showing clinical worsening was significantly greater than the percentage of donepezil-treated patients meeting the same criteria (fig. 2). For the most stringent definition of clinical worsening (COG + G + F), more than twice as many placebo patients as donepezil-treated patients met the specified criteria (milder subgroup, 21.3 vs. 7.2%; more moderate subgroup, 30.9 vs. 14.4%). Regardless of the definition of clinical worsening employed or the treatment allocation, the percentage of patients showing clinical worsening was greater in the more moderate subgroup than the milder subgroup.
Fig. 2.
a Percentage of patients with milder AD (MMSE, 1826) meeting criteria for clinical worsening. Data are shown for the COG (placebo, 86/168; donepezil, 91/251), COG + G (placebo, 53/169; donepezil, 40/250) and COG + G + F (placebo, 36/169; donepezil, 18/251) definitions. b Percentage of patients with more moderate AD (MMSE, 1017) meeting criteria for clinical worsening. Data are shown for the COG (placebo, 102/180; donepezil, 81/202), COG + G (placebo, 76/181; donepezil, 53/201) and COG + G + F (placebo, 56/181; donepezil, 29/202) definitions. Intent-to-treat population, OC analysis; ∗ p < 0.01, ∗∗ p < 0.001, ∗∗∗ p < 0.0001.
The COG criterion for clinical worsening was defined as any decline in MMSE score. However, analysis of the degree of cognitive decline in patients who met criteria for clinical worsening showed that placebo patients tended to decline by a greater extent over the 6-month study period compared with their donepezil-treated counterparts (table 4). In the week 12 and week 24 OC analyses, MMSE scores worsened by a significantly greater degree in placebo patients meeting criteria for the COG and COG + G definitions. Among patients meeting criteria for the most stringent definition (COG + G + F), mean declines in MMSE score from baseline to week 12 and week 24 were numerically greater for placebo patients, but the between-treatment differences did not reach statistical significance (table 4).
Table 4.
Least squares (LS) mean changes in MMSE scores among patients meeting criteria for clinical worsening (intention to treat, OC analysis)
| Assessment used to define clinical worsening | Number | Change from baseline at week 12 | Number | Change from baseline at week 24 | |
|---|---|---|---|---|---|
| COG | Placebo | 181 | −1.81 | 188 | −3.41 |
| Donepezil | 162 | −0.82 | 172 | −2.95 | |
| Difference | 0.99 | 0.46 | |||
| p value | 0.0008 | 0.0388 | |||
| COG + G | Placebo | 123 | −1.90 | 129 | −3.89 |
| Donepezil | 86 | −0.99 | 93 | −3.25 | |
| Difference | 0.92 | 0.65 | |||
| p value | 0.0158 | 0.0306 | |||
| COG + G + F | Placebo | 87 | −2.12 | 92 | −4.12 |
| Donepezil | 40 | −1.29 | 47 | −3.68 | |
| Difference | 0.84 | 0.44 | |||
| p value | NS | NS | |||
p values are based on an analysis of random effects model with study as random effect and baseline value and treatment as fixed effects using a 2- tailed t test, and least significant difference procedure for pairwise comparisons. NS = Non significant (p > 0.05).
Discussion
While the 3 original trials showed significant benefits for many patients treated with donepezil by traditional measures of improvement [7,10,11], the current pooled data approach demonstrated the benefits of donepezil in reducing the worsening of cognition, global outcomes and function in patients with mild-to-moderate AD. In this pooled population, there was a significant difference in the course of the measured symptoms of the disease between donepezil-treated patients and those treated with placebo, with a significantly greater proportion of placebo patients showing clinical worsening over the 6-month study period. This outcome was also apparent when milder (MMSE, 18–26) and more moderate (MMSE, 10–17) subgroups were analyzed separately. In both the overall population and the 2 separate subgroups, the percentage of donepezil-treated patients who showed combined worsening on combined measures of cognition, global outcomes and function was less than half that of the patients receiving placebo.
The outcomes presented here extend findings of previous studies showing positive effects of antidementia therapy on clinical worsening of symptoms in patients with AD [4,28,29]. In a study by Lopez et al. [29], 60% of patients treated with cholinesterase inhibitors were classified as ‘slow progressors’, defined as patients showing a decline of ≤2 points on the MMSE over 1 year. In contrast, only 30% of untreated patients were classified as ‘slow progressors’. Furthermore, in a previous study of the effects of the cholinesterase inhibitor rivastigmine on preventing decline in each individual symptom domain (cognition, global and ADL), the authors reported that only 22% of patients in the high-dose rivastigmine range (6–12 mg) worsened by 4 points on the ADAS-cog over 6 months compared with 36% receiving placebo [28]. Unlike the current analyses, the study reported by Raskind et al. [28] did not incorporate a composite, multidomain definition, but separately showed reduced levels of decline in the global assessment (CIBIC+) and in a measure of functional impairment (PDS). The study concluded that the benefits of AD therapy should be seen in the context of the progressive nature of this condition and that benefit may be obtained not only from improvement, but also from stabilization and reduced worsening of symptoms.
Analyzing single domains may be confounded by fluctuations in test performance. A more robust assessment has previously been undertaken in a pooled analysis of 6 randomized controlled trials of memantine in mild-to-severe AD [4]. As in the current analyses, the memantine study used a more exacting definition of worsening across multiple domains. In the memantine study, patients were deemed to have shown marked clinical worsening if they demonstrated a worsening in functional and global assessments and had deteriorated by ≥4 points on the ADAS-cog (mild-to-moderate studies) or ≥5 points on the Severe Impairment Battery (moderate-to-severe studies). Results showed that about twice as many placebo patients (21%) showed this marked and clinically significant deterioration compared with those taking memantine (11%). The strength of analyzing the combination of assessments lies in the use of all available information in the key domains of AD to determine whether the patient is worsening or not.
The current analyses also indicated that donepezil-treated patients who met criteria for clinical worsening tended to show a lesser degree of cognitive decline compared with placebo patients meeting the same criteria of clinical worsening. For the COG and COG + G definitions, the difference in the degree of decline was statistically significant in favor of donepezil at both week 12 and week 24; for the most stringent composite definition, including a combined decline in COG + G + F, the trend continued toward a less than expected cognitive decline with donepezil treatment but was not statistically significant.
Interestingly, there was almost no difference in the magnitude or significance of the difference between the treatment groups in the OC or LOCF analyses. In this study, which is examining worsening rather than improvement, imputed values from an earlier time point are likely to have worsened less than observed values at the end of the study. Therefore, imputing those values did not have the commonly observed effect of decreasing the effect size in the LOCF analysis. Similarly, the magnitude of the difference between the groups of the least squares mean change in MMSE score among those who met criteria for clinical worsening was notably greater at 12 weeks than at 24 weeks, indicating a greater effect of donepezil on MMSE score at 12 weeks compared to 24 weeks (table 4). This extends the finding of a peak effect of donepezil on MMSE scores at 12–18 weeks that has previously been observed in donepezil AD clinical trials [7,10,11].
A potential weakness of the current analysis is that although the included studies used a standardized measure of cognition, i.e. the MMSE, they used different scales to measure global and functional responses. As a result, the criteria used for the COG + G and COG + G + F definitions of clinical worsening varied among the 3 studies and the relative sensitivity to change of these different scales criteria is unclear. However, since the criteria were equally applied to the donepezil-treated and placebo patient groups, statistical comparisons between these patient populations remain valid.
In conclusion, the current analyses demonstrated that during the mild-to-moderate stages of AD significantly fewer patients treated with donepezil experienced symptomatic worsening compared with those receiving placebo. Moreover, patients who did worsen while receiving donepezil were likely to experience less cognitive decline than would be expected if left untreated. These results suggest that many donepezil-treated patients initially characterized as ‘nonresponders’ according to traditional markers of treatment success (i.e. those based on improvement) may still derive benefits over placebo/untreated patients. Moreover, results from the current analyses, as well as previous related studies [4,28,29] suggest that assessment of treatment benefit, both in clinical trials and in the clinic, should include end points based on reduced worsening and less than expected decline to effectively assess the range of benefits of antidementia therapy.
Conflicts of Interest
The analyses described within this research article resulted from discussions undertaken by an expert working group initiated and funded by Eisai Inc. and Pfizer Inc. Drs. Schindler, Schwam and Xu are employees of Pfizer Inc. All other authors received honoraria from Pfizer Inc. for participation in the expert working group. Dr. Cummings also reports having provided consultation services to Eisai Inc. and Pfizer Pharmaceuticals. Dr. Feldman also reports having received grant/research support from, having acted as a consultant for and having participated in CME programs sponsored by Eisai and Pfizer.
Acknowledgements
In the preparation of the manuscript, editorial support was provided by Richard Daniel, PhD, of Parexel; it was funded by Eisai Inc. and Pfizer Inc.
References
- 1.Cummings JL. Alzheimer's disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health and Clinical Excellence: TA111 Alzheimer's disease – donepezil, galantamine, rivastigmine (review) and memantine. NICE Web site. http://www.nice.org.uk/Guidance/TA111 (accessed January 5, 2009).
- 3.Geldmacher DS, Frolich L, Doody RS, Erkinjuntti T, Vellas B, Jones RW, Banerjee S, Lin P, Sano M. Realistic expectations for treatment success in Alzheimer's disease. J Nutr Health Aging. 2006;10:417–429. [PubMed] [Google Scholar]
- 4.Wilkinson D, Andersen HF. Analysis of the effect of memantine in reducing the worsening of clinical symptoms in patients with moderate to severe Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:138–145. doi: 10.1159/000105162. [DOI] [PubMed] [Google Scholar]
- 5.Winblad B, Brodaty H, Gauthier S, Morris JC, Orgogozo JM, Rockwood K, Schneider L, Takeda M, Tariot P, Wilkinson D. Pharmacotherapy of Alzheimer's disease: is there a need to redefine treatment success? Int J Geriatr Psychiatry. 2001;16:653–666. doi: 10.1002/gps.496. [DOI] [PubMed] [Google Scholar]
- 6.Black SE, Doody R, Li H, McRae T, Jambor KM, Xu Y, Sun Y, Perdomo CA, Richardson S. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology. 2007;69:459–469. doi: 10.1212/01.wnl.0000266627.96040.5a. [DOI] [PubMed] [Google Scholar]
- 7.Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer's disease. Neurology. 2001;5:613–620. doi: 10.1212/wnl.57.4.613. [DOI] [PubMed] [Google Scholar]
- 8.Holmes C, Wilkinson D, Dean C, Vethanayagam S, Olivieri S, Langley A, Pandita-Gunawardena ND, Hogg F, Clare C, Damms J. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology. 2004;63:214–219. doi: 10.1212/01.wnl.0000129990.32253.7b. [DOI] [PubMed] [Google Scholar]
- 9.Homma A, Imai Y, Tago H, Asada T, Shigeta M, Iwamoto T, Takita M, Arimoto I, Koma H, Ohbayashi T. Donepezil treatment of patients with severe Alzheimer's disease in a Japanese population: results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement Geriatr Cogn Disord. 2008;25:399–407. doi: 10.1159/000122961. [DOI] [PubMed] [Google Scholar]
- 10.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT, Donepezil Study Group A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 11.Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm AL, Zhang R, Haglund A, Subbiah P, Donepezil Nordic Study Group a 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489–495. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]
- 12.Winblad B, Kilander L, Eriksson S, Minthon L, Batsman S, Wetterholm AL, Jansson-Blixt C, Haglund A. Donepezil in patients with severe Alzheimer's disease: double-blind, parallel-group, placebo-controlled study. Lancet. 2006;367:1057–1065. doi: 10.1016/S0140-6736(06)68350-5. [DOI] [PubMed] [Google Scholar]
- 13.Burns A, Yeates A, Akintade L, Del Valle M, Zhang RY, Schwam EM, Perdomo CA. Defining treatment response to donepezil in Alzheimer's disease: responder analysis of patient-level data from randomized, placebo-controlled studies. Drugs Aging. 2008;25:707–714. doi: 10.2165/00002512-200825080-00007. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state': a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, Schmitt FA, Grundman M, Thomas RG, Ferris SH. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 16.Brane G, Gottfries CG, Winblad B. The Gottfries-Brane-Steen scale: validity, reliability and application in anti-dementia drug trials. Dement Geriatr Cogn Disord. 2001;12:1–14. doi: 10.1159/000051230. [DOI] [PubMed] [Google Scholar]
- 17.Gottfries CG, Brane G, Gullberg B, Steen G. A new rating scale for dementia syndromes. Arch Gerontol Geriatr. 1982;1:311–330. doi: 10.1016/0167-4943(82)90031-0. [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. ed 4, rev. Washington: American Psychiatric Association; 2000. [Google Scholar]
- 20.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 21.Berg L, Miller J, Baty J, Rubin E, Morris J, Figiel G. Mild senile dementia of the Alz-heimer type. 4. Evaluations of intervention. Ann Neurol. 1992;31:242–249. doi: 10.1002/ana.410310303. [DOI] [PubMed] [Google Scholar]
- 22.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 23.Eisdorfer C, Cohen D, Paveza GJ, Ashford JW, Luchins DJ, Gorelick PB, Hirschman RS, Freels SA, Levy PS, Semla TP. An empirical evaluation of the Global Deterioration Scale for staging Alzheimer's disease. Am J Psychiatry. 1992;149:190–194. doi: 10.1176/ajp.149.2.190. [DOI] [PubMed] [Google Scholar]
- 24.DeJong R, Osterlund OW, Roy GW. Measurement of quality-of-life changes in patients with Alzheimer's disease. Clin Ther. 1989;11:545–554. [PubMed] [Google Scholar]
- 25.Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. Am J Occup Ther. 1999;53:471–481. doi: 10.5014/ajot.53.5.471. [DOI] [PubMed] [Google Scholar]
- 26.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 27.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 28.Raskind M, Kumar V, Malaty L, Messina J, Hartman R, Anand R. Rivastigmine for Alzheimer's disease: improvement versus reduced worsening. Prim Care Companion J Clin Psychiatry. 2000;2:134–138. doi: 10.4088/pcc.v02n0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez OL, Becker JT, Saxton J, Sweet RA, Klunk W, De Kosky ST. Alteration of a clinically meaningful outcome in the natural history of Alzheimer's disease by cholinesterase inhibition. J Am Geriatr Soc. 2005;53:83–87. doi: 10.1111/j.1532-5415.2005.53015.x. [DOI] [PubMed] [Google Scholar]