Abstract
Background
The most appropriate timing of chemotherapy and hormone therapy administration is a critical issue in early breast cancer patients. The purpose of our study was to compare the efficacy of concurrent vs sequential administration of adjuvant chemotherapy and tamoxifen.
Methods
Women with node-positive primary breast cancer were randomly assigned to receive tamoxifen (20 mg/d for 5 years) during (concurrent arm) or after (sequential arm) adjuvant chemotherapy. Chemotherapy consisted of alternating regimens of cyclophosphamide, epidoxorubicin, and 5-fluorouracil and cyclophosphamide, methotrexate, and 5-fluorouracil every 21 days for a total of 12 cycles. The primary endpoint was overall survival (OS), and secondary endpoints were toxic effects and disease-free survival (DFS). No provision for interim analyses was made in the original study protocol. Survival curves were estimated by the Kaplan–Meier method. Multivariable Cox regression models, adjusted for age, menopausal status, tumor stage, and lymph node and hormone receptor status, were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical tests were two-sided.
Results
From 1985 to 1992, 431 patients were randomly assigned and studied according to the intention-to-treat principle. After a maximum of 15.4 years of follow-up (median 12.3 years), the estimated actuarial 10-year OS was equivalent for the two study arms (concurrent arm: 111 patients, 66%, 95% CI = 59% to 72%; sequential arm: 114 patients, 65%, 95% CI = 59% to 72%, P = .86). No differences in DFS and toxic effects were evident. Four interim analyses were performed, but no alpha error adjustment was necessary because of the largely negative results of this final analysis (sequential vs concurrent arm: HR of death = 1.06, 95% CI = 0.78 to 1.44, P = .76; HR of relapse = 1.16, 95% CI = 0.88 to 1.52, P = .36).
Conclusions
No statistically significant differences in OS, DFS, and toxic effects between concurrent and sequential adjuvant chemo- and hormone therapies were observed. Our study does not support the superiority of one schedule of chemo- and hormone-therapy administration over the other. However, because of the limited statistical power of the study, these results must be considered with caution.
CONTEXTS AND CAVEATS
Prior knowledge
The combination of tamoxifen and adjuvant chemotherapy has been shown to be an effective treatment for early breast cancer. However, it is not known whether concurrent or sequential administration of these treatments is more beneficial.
Study design
In a randomized phase III trial (1985–1992), 431 women with node-positive primary breast cancer were randomly assigned to receive tamoxifen concurrently with or following chemotherapy.
Contribution
After a median 12.3-year follow-up, there was no difference in overall survival, disease-free survival, or toxic effects between the two study arms.
Implication
Combining tamoxifen with chemotherapy works equally well, whether administered concurrently or sequentially.
Limitations
The already low statistical power of the study was exacerbated by 23 deaths unrelated to breast cancer. Women with negative and unknown hormone receptor status were included in the study. Therefore, the results could differ if the study arms included only women with hormone-responsive tumors.
From the Editors
Meta-analyses (1) by the Early Breast Cancer Trialists’ Collaborative Group have shown that both polychemotherapy and tamoxifen in the adjuvant setting are very effective as single modality treatments in prolonging patient survival. The combination of both modalities results in a better outcome in terms of overall survival (OS) and disease-free survival (DFS), with a statistically significant reduction in the risk of relapse and death (1).
The rationale for combining the two modalities was based on the hypothesis that the side effects and mechanisms of action are different (2), but several in vitro investigations on the interaction between tamoxifen and chemotherapeutic agents yielded discordant results (3–8). In the 1980s, it was reported that an alteration in tumor cell kinetics, such as the G1-S blockade induced by tamoxifen, antagonizes the antitumor effect of chemotherapy (3–5). In contrast, some researchers observed a synergism between tamoxifen and 5-fluorouracil (8) or anthracyclines (6) in hormone-responsive breast cancer cells.
The two competing hypotheses of antagonism and synergism led to the debate on the best timing for chemo- and hormone therapy administration, whether sequential or concurrent (9). It was thought that the concurrent schedule would avoid the delay in delivering endocrine therapy and would exploit the synergistic pharmacological interactions. Alternatively, the sequential schedule would avoid the kinetic and dynamic antagonism between tamoxifen and chemotherapy.
The trial presented here, which began in 1985, compared concurrent with sequential administration of chemotherapy and hormone therapy in patients with early breast cancer. To our knowledge, this was the first randomized phase III trial that addressed the timing of adjuvant chemotherapy and hormone therapy in breast cancer patients.
Methods
Patients
Women younger than 65 years with histologically confirmed breast cancer who had undergone radical mastectomy or breast-conserving surgery, in addition to full ipsilateral axillary lymph node dissection, were eligible for enrollment if they had at least one involved node. Both pre- and postmenopausal patients without clinical or radiological evidence of distant metastases were eligible. A performance status of 1.0 or less (Eastern Cooperative Oncology Group Scale) and adequate hepatic, renal, bone marrow, and cardiac functions were also required inclusion criteria. Hormone receptor status, whether positive or negative, was not an exclusion criterion. Estrogen receptor and progesterone receptor status were defined as positive when 10% or more positive cells were revealed by immunohistochemistry, or when 10 fmol/mg or more of cytosol proteins were detected by dextran-coated charcoal assay.
Study Endpoints
The primary study endpoint was OS as estimated from the date of random assignment to the date of last contact or death from any cause; DFS and toxic effects, scored by World Health Organization criteria (10), were secondary endpoints. The DFS events included local relapse, distant relapse, contralateral breast cancer, or death from any cause, whichever came first.
Study Design, Treatments, and Allocation Procedures
This open-label randomized phase III trial was conducted at six Italian centers in accordance with the Declaration of Helsinki (1964) and with the Italian regulatory requirements, which at the time mandated only oral informed consent (11). The study was approved by the Ethics Committee of the National Cancer Research Institute of Genoa (Italy). Eligible and consenting patients were randomly assigned via telephone to treatment by the Trial Center of the National Cancer Research Institute of Genoa. Patients were assigned to their treatment arm according to stratified random lists that were balanced in blocks of various sizes in random sequence. Patients were randomly assigned to receive chemotherapy and tamoxifen either concurrently or sequentially.
All patients received the same adjuvant chemotherapy consisting of alternating regimens of cyclophosphamide 600 mg/m2, epirubicin 60 mg/m2, and 5-fluorouracil 600 mg/m2 (CEF) and cyclophosphamide 600 mg/m2, methotrexate 40 mg/m2, 5-fluorouracil 600 mg/m2 (CMF) every 21 days for a total of 12 cycles. The first chemotherapy cycle was started within 30 days after surgery (12). Patients who had been enrolled in our perioperative chemotherapy trial were also included in the study, as planned by the original protocol (Supplementary Methods, available online). Therefore, they had been randomly assigned to receive their first postoperative chemotherapy cycle within 3 days after surgery (12). The hormone therapy consisted of tamoxifen at 20 mg/d orally for 5 years, administered concurrently with chemotherapy in the concurrent arm and 30 days after the last chemotherapy cycle in the sequential arm.
Although the original protocol scheduled reduction of dose according to toxicity grades, no drug modifications were performed, but a treatment delay of up to 2 weeks was permitted before each cycle to allow for recovery when patients had fewer than 3000 leucocytes per microliter and/or 100 000 platelets per microliter, according to clinical practice in our institutions at that time. Radiation therapy limited to the breast began 1 month after the last chemotherapy cycle and was planned only for patients who underwent conservative surgery.
Evaluations
Initial staging consisted of a medical history, physical examinations, complete blood counts, and blood chemistry analysis. A bone scan, chest x-ray, liver ultrasonography, electrocardiography, and mammography were also required before random assignment.
During the first 5 years of follow-up, physical examination, complete blood counts, and blood chemistry analysis were repeated every 3 months. A bone scan, chest x-ray, liver ultrasound, and mammography were repeated every 12 months during the first 5 years of follow-up. Thereafter, patients were annually examined with mammography, complete blood counts, and any procedure as circumstances required. Patients were asked to report any change in their health. The vital status of any patient failing to present for examination was investigated by telephoning or consulting the municipal office.
Statistical Methods
All randomly assigned subjects were included in the analyses according to the intention-to-treat principle. OS was computed from the date of random assignment to the date of death or censored at the date of last information on survival status. DFS was estimated from the date of random assignment to the date of event occurrence. OS and DFS curves were obtained from the Kaplan–Meyer product-limit estimator (13). The primary comparison between the two study arms was performed with the log-rank test (14). To assess the presence of a statistically significant effect on treatment efficacy of several factors that have been indicated as prognostic and/or effect modifiers of adjuvant treatment, two multivariable Cox proportional hazards models were fitted to the data; one for OS and one for DFS (15). The graphical representation of log {log[S(t)]} against log t, where S(t) is the cumulative survival in each stratum at time t and t is the follow-up time, was used to confirm the assumption of proportionality. The subgroup analysis for tumor size, lymph node status, age, menopausal status, and hormone receptor status was planned in the original protocol. The following covariates were included in each model: treatment arm (concurrent vs sequential); tumor size (pT1 vs ≥pT2); number of involved nodes (≤3 vs 4–9, vs ≥10); menopausal status (pre- vs postmenopausal); age (≤50 vs >50); positive hormone receptor status (estrogen receptor positive and/or progesterone receptor positive) vs negative hormone receptor status (combined estrogen receptor negative and progesterone receptor negative) vs unknown status. To evaluate the presence of heterogeneity in the efficacy of sequential vs concurrent therapy across strata of the prognostic factors, the appropriate treatment by covariate interaction terms were included in the model one at a time, which is considered appropriate for subgroup analyses (16). The statistical significance of each interaction term (test for interaction) was obtained by means of a step-down procedure based on the likelihood ratio test, starting from the full model, with the five main effects, and the interaction term. No correction for the multiple significance test was included, and a relaxed (P = .10) significance level was used to retain variables in the multivariable model (15). As a consequence, these analyses must be considered exploratory, and in their interpretation, the correction for the number of tests (n = 10) must be accounted for. Using a classical Bonferroni correction, the critical P value required to declare statistical significance with α = .05 is P = .005. All statistical tests were two-sided. The results of subgroup analyses were graphically summarized using Forest plots (16). SPSS software (Version 15.0 for Windows, Chicago, IL) was used for all statistical analysis.
This study was conducted as part of a multi-trial study that included the main trial on perioperative chemotherapy (12). No formal sample size projection was included in the original study protocol (Supplementary Methods, available online) for this trial. The accrual of the study was stopped in 1992 after the conclusion of the perioperative trial, which provided more than half of the patients enrolled in this study (12), concomitantly with the beginning of our dose-dense randomized trial (17). Posterior statistical power estimates, based on the observed number of events (170 deaths and 209 DFS events), indicate that the present analysis has an 80% statistical power (for α = .05, two-sided) to detect a hazard ratio (HR) for OS of 0.64, and a hazard ratio for DFS of 0.68.
Furthermore, no provision for interim analyses was made in the original study protocol. However, four interim analyses were conducted in 1990 (18), 1991 (19), and 1992 (20) while patient accrual was still ongoing, and the last in 2000 (21), when accrual had been completed. None of the interim analyses was intended to result in stopping the study, and none was considered to be the final analysis. The largely negative results of the final analysis make it unnecessary to adjust the alpha error for multiple measures. The cutoff date for the analysis presented here was June 1, 2001. Update of the follow-up after that time was not possible because of financial constraints.
Results
Patients
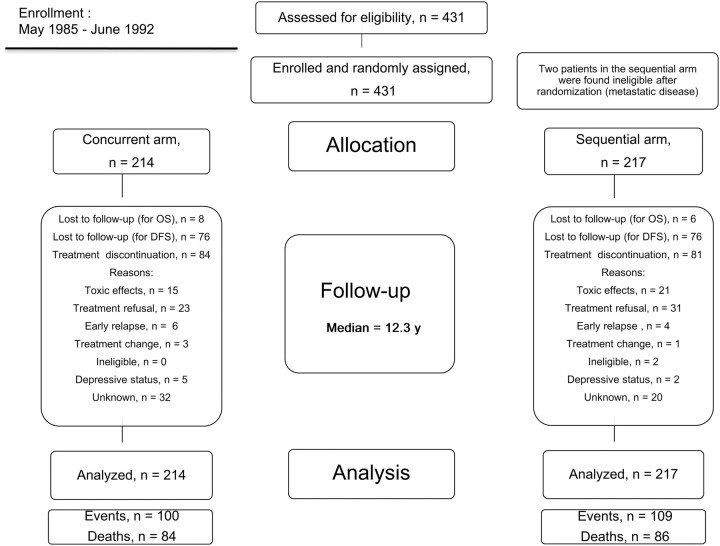
From May 1985 to June 1992, 431 patients were randomly assigned. Two patients were found ineligible (because of metastatic disease) after random assignment but were included in the analysis in accordance with the intention-to-treat principle. Of 431 patients, 214 were randomly assigned to receive concurrent treatment and 217 to receive sequential treatment (Figure 1). From our perioperative randomized trial, 230 of 431 patients (115 of 214 in the concurrent arm and 115 of 217 in the sequential arm) were included (12).
Figure 1.
CONSORT diagram. Patient disposition during the study. Concurrent arm = tamoxifen concurrent with chemotherapy; sequential arm = tamoxifen after completion of chemotherapy. DFS = disease-free survival; OS = overall survival.
The two treatment arms were comparable in terms of patients and characteristics (Table 1). Of 431 randomly assigned women, 220 (51%) were premenopausal. Hormone receptor status was unknown in 105 (24%) of 431 patients. Of the remaining patients, 227 (53%) were hormone receptor positive and 99 (23%) were hormone receptor negative.
Table 1.
Patient characteristics*
| Characteristic | Concurrent arm (n = 214) | Sequential arm (n = 217) |
| No. (%) | No. (%) | |
| Age, y | ||
| Median | 49 | 51 |
| Range | 30–65 | 28–65 |
| Age group, y | ||
| ≤50 | 111 (51.8) | 107 (49.3) |
| >50 | 103 (48.1) | 110 (50.7) |
| Menopausal status | ||
| Premenopausal | 113 (52.8) | 107 (49.3) |
| Postmenopausal | 101 (47.2) | 110 (50.7) |
| Surgical treatment | ||
| Conservative | 66 (30.8) | 65 (30.0) |
| Mastectomy | 146 (68.2) | 149 (68.6) |
| Unknown | 2 (0.9) | — |
| Pathological tumor stage† | ||
| pT1 | 85 (39.7) | 84 (38.7) |
| ≥pT2 | 127 (59.6) | 131 (60.3) |
| pTis | — | 2 (0.9) |
| Unknown | 2 (0.9) | — |
| No. of metastatic axillary lymph nodes | ||
| 1–3 | 128 (59.8) | 120 (55.2) |
| 4–9 | 53 (24.7) | 58 (26.7) |
| ≥10 | 31 (14.4) | 38 (17.5) |
| Unknown‡ | 2 (0.9) | 1 (0.5) |
| Hormone receptor status | ||
| ER- | 68 (31.7) | 51 (23.5) |
| ER+ | 100 (46.7) | 105 (48.3) |
| ER status unknown | 46 (21.4) | 61 (28.1) |
| PgR− | 76 (35.5) | 63 (29.0) |
| PgR+ | 66 (30) | 71 (32.7) |
| PgR status unknown | 72 (33.6) | 83 (38.2) |
| Hormone negative, ER− and PgR− | 59 (27.5) | 40 (18.4) |
| Hormone positive, ER+ and/or PgR+ | 111 (51.8) | 116 (53.4) |
| Hormone status unknown | 44 (20.5) | 61 (28.1) |
Concurrent arm, tamoxifen concurrent with chemotherapy; sequential arm, tamoxifen after completion of chemotherapy. ER− = estrogen receptor negative; ER+ = estrogen receptor positive; PgR− = progesterone receptor negative; PgR+ = progesterone receptor positive.
pT1, 2 = primary tumor stage 1 and 2; pTis = carcinoma in situ, according to the American Joint Committee on Cancer Staging System.
At least one lymph node.
Treatment Administration and Toxic Effects
Completion of treatment did not differ between the two groups: 130 (61%) of 214 patients in the concurrent arm and 136 (63%) of 217 patients in the sequential arm completed the 12 cycles of chemotherapy. Seventy-four percent of patients received more than 80% (as percentage of planned doses) of CEF and CMF doses. The high incidence of G3–G4 nausea and vomiting (approximately 60%) was the dose-limiting factor, inducing most people to drop off the last two cycles. However, no differences in the incidence of side effects between the two arms were detected (Table 2). Virtually all patients experienced alopecia (G3–G4 in approximately 75% of patients). Leukopenia occurred in 44% of patients, but only a very small minority of patients experienced G3–G4 toxic effects (1.6% and 3.6% in the concurrent and sequential arm, respectively). Thrombocytopenia was rare (<5%). No thromboembolic events were reported in this trial, and no death attributable to toxic effects occurred in either arm of the study.
Table 2.
Toxic effects*
| Toxic effect† | Concurrent arm (n = 214), % |
Sequential arm (n = 217), % |
||
| G1–2 | G3–4 | G1–2 | G3–4 | |
| Nausea and vomiting | 37.8 | 58.0 | 35.0 | 60.9 |
| Alopecia | 22.3 | 74.0 | 23.3 | 76.1 |
| Cardiac | 4.1 | 0.5 | 5.1 | 1.5 |
| Hepatic | 2.1 | 1.0 | 7.6 | 0.5 |
| Renal | 0.5 | — | — | |
| Leukopenia | 46.1 | 1.6 | 41.1 | 3.6 |
| Thrombocytopenia | 4.1 | — | 4.6 | 0.5 |
| Stomatitis | 25.3 | 2.6 | 20.3 | 4.1 |
| Infections | 3.6 | — | 3.5 | — |
| Cystitis | 16.5 | — | 10.6 | — |
| Spotting | 5.2 | — | 3.0 | — |
| Hot flashes | 35.7 | 1.0 | 27.9 | 0.5 |
Concurrent arm, tamoxifen concurrent with chemotherapy; sequential arm, tamoxifen after completion of chemotherapy. G1–4 = toxic effect grades according to World Health Organization criteria.
There were no statistically significant differences in any of the toxic effects between the concurrent and sequential arm.
Efficacy
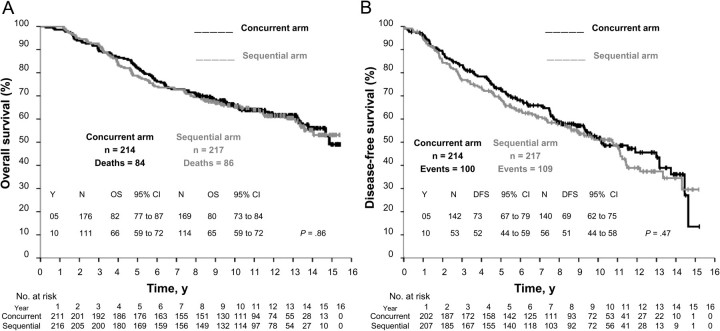
After a maximum of 15.4 years of follow-up (median 12.3 years), 170 deaths were observed, 84 in the concurrent arm and 86 in the sequential arm. The two treatments were equivalent in terms of OS and DFS. The estimated actuarial 10-year OS was 66% (111 patients, 95% CI = 59% to 72%) in the concurrent arm and 65% (114 patients, 95% CI = 59% to 72%) in the sequential arm (Figure 2, A) (P = .86). There were 209 DFS events recorded, 100 in the concurrent arm and 109 in the sequential arm (Table 3). The estimated actuarial 10-year DFS was 52% (95% CI = 44% to 59%) in concurrent arm and 51% (95% CI = 44% to 58%) in the sequential arm (P = .47) (Figure 2, B). In the multivariable analysis, lymph node status was the only variable influencing OS and DFS (Table 4). No difference in risk of death (HR of death = 1.06, 95% CI = 0.78 to 1.44, P = .76, Figure 3) or relapse (HR of relapse = 1.16, 95% CI = 0.88 to 1.52, P = .36, Figure 4) was evident between the sequential and concurrent arm after adjustment for prognostic factors.
Figure 2.
Kaplan–Meier survival curves for all randomly assigned patients. A) Overall survival and B) Disease-free survival. P values from two-sided log-rank test. Concurrent arm = tamoxifen concurrent with chemotherapy; sequential arm = tamoxifen after completion of chemotherapy; CI = confidence interval; DFS = disease-free survival; N = number of patients at risk; OS = overall survival; Y = year.
Table 3.
Events by treatment arm*
| Events | Concurrent arm, No. of patients† | Sequential arm, No. of patients‡ |
| Contralateral breast cancer | 7 | 6 |
| Local relapse only | 5 | 9 |
| Distant metastasis only | 62 | 62 |
| Local relapse§ and distant metastasis (concurrently) | 4 | 6 |
| Unspecified site of relapse | 11 | 14 |
| Death without report of progression | 11 | 12 |
Disease-free survival events were local relapse, distant relapse, contralateral breast cancer, or death from any cause, whichever came first. Concurrent arm, tamoxifen concurrent with chemotherapy; sequential arm, tamoxifen after completion of chemotherapy.
In the concurrent arm, 100 (46.7%) of 214 patients experienced an event.
In the sequential arm, 109 (50.2%) of 217 patients experienced an event.
Local relapses were cytologically and/or histologically confirmed and included tumor relapses in the ipsilateral breast, thoracic wall, and axillary and supraclavicular nodes.
Table 4.
Multivariable analysis of prognostic factors, overall survival, and disease-free survival*
| Characteristic | Overall survival |
Disease-free survival |
||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age group, y | .39 | .86 | ||
| ≤50 | 1.0 (referent) | 1.0 (referent) | ||
| >50 | 1.22 (0.78 to 1.90) | 0.89 (0.60 to 1.32) | ||
| Menopausal status | .66 | .81 | ||
| Premenopausal | 1.0 (referent) | 1.0 (referent) | ||
| Postmenopausal | 0.93 (0.60 to 1.45) | 1.11 (0.75 to 1.65) | ||
| Pathological tumor stage† | .24 | .07 | ||
| pT1 | 1.0 (referent) | 1.0 (referent) | ||
| ≥pT2 | 1.20 (0.86 to 1.69) | 1.32 (0.98 to 1.79) | ||
| No. of metastatic axillary lymph nodes | <.001 | <.001 | ||
| 1–3 | 1.0 (referent) | 1.0 (referent) | ||
| 4–9 | 1.99 (1.38 to 2.88) | 1.90 (1.37 to 2.62) | ||
| ≥10 | 4.50 (3.15 to 6.54) | 3.26 (2.30 to 4.63) | ||
| Hormone receptor status | .56 | .36 | ||
| Hormone negative, ER−, PgR− | 1.0 (referent) | 1.0 (referent) | ||
| Hormone positive, ER+ and/or PgR+ | 0.83 (0.57 to 1.21) | 0.86 (0.61 to 1.19) | ||
| Hormone receptor status unknown | 0.85 (0.54 to 1.33) | 0.73 (0.48 to 1.09) | ||
CI = confidence interval; ER+ = estrogen receptor positive; ER− = estrogen receptor negative; HR = hazard ratio; PgR+ = progesterone receptor positive; PgR− = progesterone receptor negative. Concurrent arm, tamoxifen concurrent with chemotherapy; sequential arm, tamoxifen after completion of chemotherapy. HR and CI were obtained from a Cox model in which all variables were initially included as covariates. Covariates not statistically significantly (P > .10) associated with the outcome were excluded from the model by means of a step-down procedure that was based on the likelihood ratio test. All variables were categorical and implied established factors associated with prognosis and/or response to chemotherapy and hormone therapy. All statistical tests are two-sided.
pT1,2 = primary tumor stage 1,2 according to the American Joint Committee on Cancer Staging System.
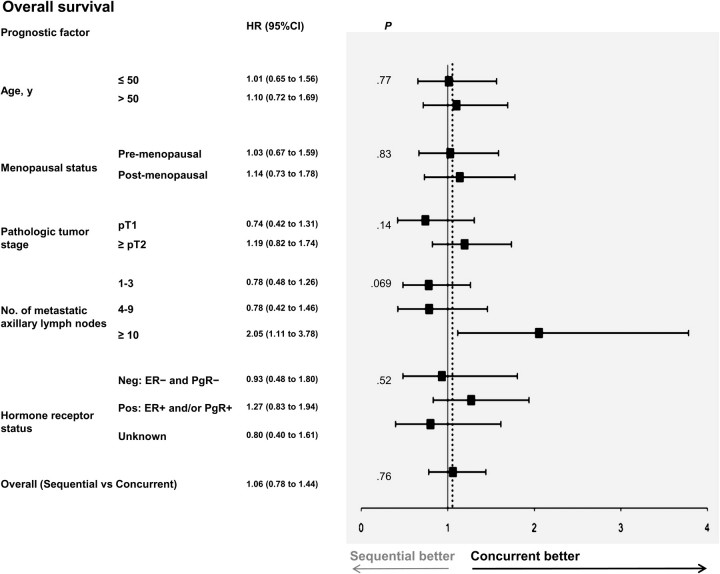
Figure 3.
Forest plot for overall survival of subgroups. Comparison of the concurrent vs the sequential arm within strata formed by each prognostic factor. The solid line shows the no effect point, and the dotted line shows overall treatment effect for the whole dataset. HR and CI were obtained from a Cox model in which all variables were included as covariates. All variables were categorical and implied established factors associated with prognosis and/or response to chemotherapy and hormone therapy. Interaction terms assessing the homogeneity of the effect of experimental treatment across strata of each covariate were introduced in the model one at a time. P values are from two-sided likelihood ratio tests. All P values shown are from tests for interaction, except for the last at the bottom, which is the overall comparison of sequential arm vs concurrent arm adjusted for all prognostic factors. The solid line corresponds to no effect and the dotted line shows overall treatment effect for the whole dataset. Concurrent = tamoxifen concurrent with chemotherapy; sequential = tamoxifen after completion of chemotherapy. CI = confidence interval; ER = estrogen receptor; HR = hazard ratio; Neg = negative; PgR = progesterone receptor; Pos = positive; pT1,2 = primary tumor stage 1,2, according to the American Joint Committee on Cancer staging system staging system.
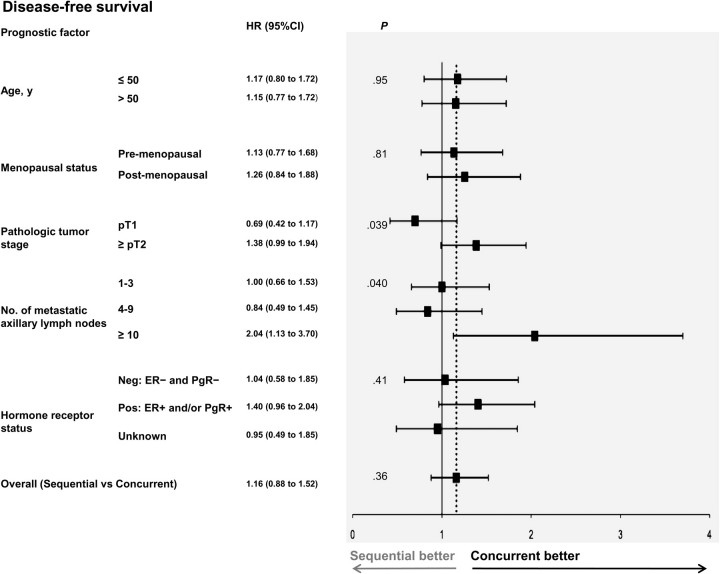
Figure 4.
Forest plot for disease-free survival of subgroups. Comparison of the concurrent vs the sequential arm within strata formed by each prognostic factor. The solid line shows the no effect point, and the dotted line shows overall treatment effect for the whole dataset. HR and CI were obtained from a Cox model in which all variables were included as covariates. All variables were categorical and implied established factors associated with prognosis and/or response to chemotherapy and hormone therapy. Interaction terms assessing the homogeneity of the effect of experimental treatment across strata of each covariate were introduced in the model one at a time. P values are from two-sided likelihood ratio tests. All P values shown are from tests for interaction, except for the last at the bottom, which is the overall comparison of sequential arm vs concurrent arm adjusted for all prognostic factors. Concurrent = tamoxifen concurrent with chemotherapy; sequential = tamoxifen after completion of chemotherapy. CI = confidence interval; ER = estrogen-receptor; HR = hazard ratio; Neg = negative; PgR = progesterone receptor; Pos = positive; pT1,2 = primary tumor stage 1,2, according to the American Joint Committee on Cancer Staging System.
In the exploratory subgroup analyses, no heterogeneity in the effect of sequential as compared with concurrent therapy was seen across the strata of age, menopausal status, and hormone receptor status. However, in DFS analyses, an interaction between random assignment and both nodal status and tumor size was noted (P = .040 and P = .039 for nodal and tumor size status comparisons, respectively; not statistically significant with Bonferroni correction). This interaction suggests that a sequential therapy may be associated with an increased risk of relapse in high-risk subgroups, namely patients with 10 or more metastatic axillary lymph nodes (HR of relapse = 2.04, 95% CI = 1.13 to 3.70) and a pathological tumor stage or at least pT2 (HR of relapse = 1.38, 95% CI = 0.99 to 1.94). The same two associations were also seen in OS analyses (without statistical significance).
We also analyzed the hormone receptor–positive patients. In these patients, a 40% increase in the probability of relapse was observed in the sequential arm (HR = 1.40, 95% CI = 0.96 to 2.04) with a similar difference in OS (HR of death = 1.27, 95% CI = 0.83 to 1.94), but the test for interaction was not statistically significant (P = .41 and P = .52 for DFS and OS, respectively).
Discussion
In this study, no differences in OS and DFS between concurrent and sequential adjuvant chemo- and hormone therapies in node-positive breast cancer patients were observed. This trial was conceived on the basis of two contrasting hypotheses underlying the concurrent or sequential administration of hormone therapy and chemotherapy in the adjuvant treatment of early breast cancer. The concurrent modality could avoid any delay in prompting synergistic pharmacological interactions; the sequential modality could circumvent the expected kinetic antagonism of chemotherapy and hormone therapy. The results of this study fail to support either hypothesis.
Some limitations of the study must be considered because they are, at least in part, a reflection of the assumptions and attitudes of the time when the study was designed. First, the size of the study is inadequate by today’s standards. In fact, the study had the statistical power (ie, >80%) to detect a 36% reduction in death rate and a 32% reduction in recurrence rate, and we now know that an effect of this magnitude is a rare event (1,22). As a consequence, any negative finding in this study must be interpreted in the light of its low statistical power.
The problem of low statistical power in this study is enhanced by the occurrence of 23 deaths that were not preceded by a report of progression. Deaths unrelated to breast cancer might have diluted not only OS but also DFS comparisons. Even though the distribution of these 23 deaths was similar in the two groups (11 and 12 in the concurrent and sequential group, respectively), it is possible to speculate that the convergence of the DFS and OS curves with increasing follow-up may be due, at least in part, to the diluting effect of these deaths. According to standard statistical practices, it is not possible to ignore these deaths by censoring these patients at the time of death because of the risk of bias and because some of these case patients may indeed have suffered an unreported relapse. The latter hypothesis is supported by the fact that most of these patients were in their early 60s when they died (median age at death = 63 years), an age when deaths from competing risks are much less frequent than deaths from breast cancer in a high-risk population such as the one in this study (10-year DFS approximately 50%).
Second, patients were eligible for this study regardless of their hormone receptor status, an understandable omission, considering that the study was conceived before the first Early Breast Cancer Trialists’ Collaborative Group overview (published in 1988), in which tamoxifen was reported effective in women 50 aged years or older (23). It was only in 1998 that the Oxford Overview (24) definitely attributed true predictive value to estrogen receptor status. Therefore, a major weakness of our study is represented by the inclusion of one-fourth of patients with negative hormone receptor status and one-fourth of patients with unknown hormone receptor status.
As a consequence, the hazard ratio of 0.64 and 0.68 (for OS and DFS, respectively), which could be detected with a statistical power of 80% in the entire study population, should be entirely driven by the approximately two-thirds of patients with tumors sensitive to hormonal treatments: The reductions in the hazard of relapse and of death in this subgroup that were necessary to obtain these overall effects were 47% and 53%, respectively, much larger than the difference one might reasonably expect when comparing sequential and concurrent tamoxifen.
The subgroup analyses were planned in advance and included in the original study protocol. However, given the number of tests performed (n = 10) and the small size of the study, none of them achieved the critical P value required for statistical significance after correction for multiplicity (P = .005). When the analysis was restricted to the hormone receptor–positive patients, a 40% increase in the risk of relapse was seen in the sequential arm, with a similar difference in OS, but the test for interaction was not statistically significant (P = .41 and P = .52 for DFS and OS, respectively). Although this finding cannot provide evidence to support the superiority of one treatment over the other, it suggests that concurrent tamoxifen might not be detrimental (sequential vs concurrent arm: HR of relapse = 1.40, 95% CI = 0.96 to 2.04 and HR of death = 1.27, 95% CI = 0.83 to 1.94). Two other planned subgroup analyses, by tumor size and by nodal status, corroborate the same interpretation. Indeed, in both analyses, a statistically significant treatment–covariate interaction was found for DFS (P = .040 and P = .039 for nodal and tumor size status comparisons, respectively; not statistically significant with Bonferroni correction), suggesting that the sequential regimen may be less effective than the concurrent one in at least two high-risk subgroups (HR of relapse = 1.38, 95% CI = 0.99 to 1.94 in the pT ≥2 group and HR of relapse = 2.04, 95% CI = 1.13 to 3.70 in patients with ≥10 metastatic axillary lymph nodes). These results are mirrored in OS analyses.
Two other randomized studies similarly assessed the administration of chemo- and hormone therapy (25–27). In the Spanish Breast Cancer Research Group (GEICAM) study (25), a randomized trial involving node-positive postmenopausal women not selected by hormone receptor status or with unknown status, tamoxifen was administered concurrently or sequentially with epirubicin–cyclophosphamide adjuvant chemotherapy. After a follow-up of 4.6 years, the GEICAM study (25), which started in 1995, did not detect any statistically significant difference in outcome between concurrent and sequential administration. However, considering the number of events detected (52 OS and 96 DFS events), the statistical power of the GEICAM study is very low, and this weakness strongly reduces the relevance of the observation.
Conversely, the preliminary analysis of the Southwest Oncology Group (SWOG) 8814 trial, presented in 2002, showed a statistically significant improvement in DFS in the sequential arm over the concurrent arm (27). That large trial, started in 1989, compared three arms: chemotherapy (cyclophosphamide, doxorubicin and 5-fluorouracil [CAF]) given concurrently or sequentially with tamoxifen vs tamoxifen alone. A study of such magnitude (ie, 89% statistical power to detect a 33% increase in the HR for concurrent vs sequential administration, for α = .05, one-sided) and one that is focused on a specific set of node-positive patients (ie, hormone receptor positive and postmenopausal) represents the ideal setting for assessing the relative role of sequential vs concurrent combination of chemotherapy and hormone therapy. After the presentation of the SWOG trial results (27), clinical practice did change, and the general consensus among clinical oncologists was to administer the two treatments sequentially (28). Furthermore, on the basis of the SWOG preliminary results, the 2005 St Gallen consensus recommended that patients receiving chemotherapy should not start tamoxifen until completion of treatment (29). However, the final results of the SWOG trial, with a median follow-up of 8.9 years, did not fully support the findings of the preliminary SWOG analysis (26). We caution that a longer follow-up could eventually reduce the advantage, as happened for this study. In fact, the findings of our interim analyses were reversed after longer follow-up. Early data suggested that the concurrent arm was better (18–20), whereas this advantage was greatly reduced later (21). Even in the present report, the DFS curves progressively diverge until the eighth year of observation and then converge at the end of follow-up; a similar trend was observed for OS.
Although in the SWOG study the authors championed the use of anthracycline-based chemotherapy followed by tamoxifen in clinical practice, they failed to prove a clear superiority of the sequential treatment over the concurrent one, because the OS and DFS analyses did not reach the statistical significance (HR = 0.90, 95% CI = 0.73 to 1.10, P = .30 and HR = 0.84, 95% CI = 0.70 to 1.01, P = .06, for OS and DFS, respectively) (26). Nevertheless, the combined therapy of CAF and tamoxifen (given either sequentially or concurrently) was superior to tamoxifen alone in terms of DFS (HR = 0.76, 95% CI = 0.64 to 0.91, P = .002) (26). Therefore, the SWOG study upheld the legacy of previous trials with respect to the superiority of chemo- and hormone therapy over hormone therapy alone, when an anthracycline-containing regimen was used, but did not favor any specific modality for administering the drugs (30,31).
To date, neither this study nor the GEICAM (25) or SWOG (26) studies have been able to clearly indicate the best timing for administering the chemotherapy and hormone therapy combination. When these three studies were pooled together in the Oxford Overview (unpublished data; presented at the 2008 San Antonio Breast Cancer Meeting), no difference was seen between the use of tamoxifen given concurrently or sequentially after chemotherapy (9).
Recently, the role of concurrent or sequential treatment was retrospectively investigated in pre- and post-menopausal hormone receptor–positive patients, who were accrued to two more recent randomized adjuvant trials at our Institutes (32). Although there was no statistically significant difference in either OS or event-free survival between patients receiving tamoxifen concurrently with or sequentially to chemotherapy, a statistically significant decreasing trend in the hazard of death (P = .015) for sequential therapy was associated with increasing age. This finding suggests that concurrent therapy might be more effective than sequential therapy in younger patients and/or in premenopausal patients. The authors speculated that starting tamoxifen as soon as possible together with chemotherapy could counterbalance the poor prognosis reported in young premenopausal patients with estrogen receptor–positive tumors, who are treated with chemotherapy alone (32–34).
To our knowledge, only this study prospectively investigated the timing of the two modalities in premenopausal patients, but because of its design, this study does not provide sufficient evidence to support or reject either aforementioned hypothesis. Therefore, we believe that the definition of the best timing of chemo- and hormone therapy in premenopausal patients is a pending subject, given the concern of many clinicians to delay the endocrine treatment.
Both our study and the SWOG study (26) used an anthracycline-based chemotherapy regimen of longer duration compared with the modern chemotherapy standards. In addition, the chemotherapy regimen chosen for our trial is not comparable to the current standard in terms of schedule, dosage, and drugs. These variables probably affected the survival of the study population, even if the overall poor outcomes observed in trials started in the 1980s were also affected by the less than adequate staging available at the time.
In our report, nausea and vomiting (G3–G4 in approximately 60% of subjects) represented the dose-limiting factors. This is not surprising considering the high emetogenic activity of anthracycline-based regimens in the absence of premedication with modern antiemetic drugs (5-HT3 antagonists), which were unavailable at the time of this trial. It is noteworthy that although an increased risk of thromboembolic complications with concurrent tamoxifen and chemotherapy was reported by others (35,36), no differences in toxic effects were seen in this trial or in the GEICAM (25) and SWOG (26) trials. Even if we were aware of the cardiovascular risks (36,37) no relevant toxic effect was associated with the concurrent treatment either in this study or in our dose-density phase III trials, in which patients were similarly treated with concomitant or sequential tamoxifen (17).
No data are available so far about the best timing of administration of chemotherapy and aromatase inhibitors in early breast cancer. These drugs represent the other major form of adjuvant endocrine therapy. In view of their different pharmacodynamics and pharmacokinetics compared with tamoxifen, the results of studies evaluating the timing of chemotherapy and tamoxifen cannot be applied to aromatase inhibitors (26). Because aromatase inhibitors are particularly active in switching off the proliferation and taxanes are particularly active in highly proliferating cancers, concerns about their concurrent administration exist. However, in vivo synergism between these drugs could not be excluded. Intriguingly, Watanabe et al. (38) recently observed that the rate of pathological complete response obtained by neoadjuvant concurrent administration of an aromatase inhibitor (anastrozole) and chemotherapy (CEF followed by paclitaxel) was not lower than those of previous chemotherapy only trials. The antagonism of these clashing hypotheses (ie, antagonism or synergism) recapitulates the same rationale behind this study.
In conclusion, the debate about the best timing for adjuvant chemo- and hormone therapy is far from resolved, even considering that new chemotherapeutic, biological, and endocrine agents are presently available.
Funding
This study was supported by a grant from the Italian National Research Council, Rome [Special Project Oncology (8800601.44)].
Supplementary Material
Footnotes
The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the article. The random allocation sequence was generated at the National Cancer Research Institute of Genoa under the supervision of PB. The authors have no affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the article. The following centers and investigators also contributed to patient accrual: Dr Pier Franco Conte (Department of Medical Oncology, S. Chiara Hospital, Pisa), Dr Elio Paganini (Medical Oncology Unit, Villa Scassi Hospital, Genoa, Italy); Dr Fulvio Brema (Department of Oncology, S. Paolo Hospital, Savona, Italy), Dr Pietro Gallotti (Clinical Oncology, Beato Matteo Clinical Institute, Vigevano, Italy). DB thanks Dr Francesco Barone-Adesi (Cancer Epidemiology Unit, CeRMS and CPO-Piemonte, University of Turin, Turin, Italy) and Dr Pietro Blandini (U.C. Sampdoria, Genoa, Italy) for their useful statistical advice. The authors thank Ms. Shari Lama (National Institutes of Health Library, Bethesda, MD, USA) for help in editing for the English format. Davide Bedognetti is a participant in the NIH Graduate Partnership Program and a graduate student at University of Genoa. Davide Bedognetti's scholarship is partially supported by the Conquer Cancer Foundation of the American Society of Clinical Oncology (2011 Young Investigator Award).
References
- 1.Abe O, Abe R, Enomoto K, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Sertoli MR, Scarsi PG, Rosso R. Rationale for combining chemotherapy and hormonal therapy in breast cancer. J Steroid Biochem. 1985;23(6B):1097–1103. doi: 10.1016/0022-4731(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 3.Osborne CK, Kitten L, Arteaga CL. Antagonism of chemotherapy-induced cytotoxicity for human breast cancer cells by antiestrogens. J Clin Oncol. 1989;7(6):710–717. doi: 10.1200/JCO.1989.7.6.710. [DOI] [PubMed] [Google Scholar]
- 4.Hug V, Hortobagyi GB, Drewinko B, Finders M. Tamoxifen-citrate counteracts the antitumor effects of cytotoxic drugs in vitro. J Clin Oncol. 1985;3(12):1672–1677. doi: 10.1200/JCO.1985.3.12.1672. [DOI] [PubMed] [Google Scholar]
- 5.Osborne CK. Combined chemo-hormonal therapy in breast cancer: a hypothesis. Breast Cancer Res Treat. 1981;1(2):121–123. doi: 10.1007/BF01805864. [DOI] [PubMed] [Google Scholar]
- 6.Leonessa F, Jacobson M, Boyle B, Lippman J, McGarvey M, Clarke R. Effect of tamoxifen on the multidrug-resistant phenotype in human breast cancer cells: isobologram, drug accumulation, and M(R)-170,000 glycoprotein (Gp170) binding studies. Cancer Res. 1994;54(2):441–447. [PubMed] [Google Scholar]
- 7.Kurebayashi J, Nukatsuka M, Nagase H, et al. Additive antitumor effect of concurrent treatment of 4-hydroxy tamoxifen with 5-fluorouracil but not with doxorubicin in estrogen receptor-positive breast cancer cells. Cancer Chemother Pharmacol. 2007;59(4):515–525. doi: 10.1007/s00280-006-0293-7. [DOI] [PubMed] [Google Scholar]
- 8.Benz C, Cadman E, Gwin J, et al. Tamoxifen and 5-fluorouracil in breast cancer: cytotoxic synergism in vitro. Cancer Res. 1983;43(11):5298–5303. [PubMed] [Google Scholar]
- 9.Pritchard KI. Combining endocrine agents with chemotherapy: which patients and what sequence? Cancer. 2008;112(3):718–722. doi: 10.1002/cncr.23189. [DOI] [PubMed] [Google Scholar]
- 10.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Wager E, Tooley PJ, Emanuel MB, Wood SF. How to do it. Get patients’ consent to enter clinical trials. BMJ. 1995;311(7007):734–737. doi: 10.1136/bmj.311.7007.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sertoli MR, Bruzzi P, Pronzato P, et al. Randomized cooperative study of perioperative chemotherapy in breast cancer. J Clin Oncol. 1995;13(11):2712–2721. doi: 10.1200/JCO.1995.13.11.2712. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 14.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 15.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187–220. [Google Scholar]
- 16.Cuzick J. Forest plots and the interpretation of subgroups. Lancet. 2005;365(9467):1308. doi: 10.1016/S0140-6736(05)61026-4. [DOI] [PubMed] [Google Scholar]
- 17.Venturini M, Del Mastro L, Aitini E, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. J Natl Cancer Inst. 2005;97(23):1724–1733. doi: 10.1093/jnci/dji398. [DOI] [PubMed] [Google Scholar]
- 18.Pronzato P, Sertoli MR, Amoroso D, et al. A randomized study of concurrent versus sequential chemotherapy and tamoxifen in stage II breast cancer. 1990;1:298–304. Proceeding of the Sixth International Conference on the Adjuvant Therapy of CancerTucson, Arizona. [Google Scholar]
- 19.Sertoli MR, Pronzato P, Amoroso D, et al. A randomized study of concurrent versus sequential chemotherapy and tamoxifen in stage II breast cancer. Proc Am Soc Clin Oncol. 1991;10:48. Abstract 65. [Google Scholar]
- 20.Sertoli M, Pronzato P, Queirolo P, et al. A randomized study of concurrent vs sequential chemo-ormonotherapy in stage II breast cancer. 1992 Proceeding of the Fourth International Conference on the Adjuvant Therapy of Primary Breast CancerSt. Gallen, Switzerland. Abstract 71. [Google Scholar]
- 21.Sertoli MR, Pronzato P, Venturini M, et al. A randomized study of concurrent versus sequential adjuvant chemotherapy and tamoxifen in stage II breast cancer. Proc Am Soc Oncol. 2002;21(182):46. Abstract 182. [Google Scholar]
- 22.Martin M, Segui MA, Anton A, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363(23):2200–2210. doi: 10.1056/NEJMoa0910320. [DOI] [PubMed] [Google Scholar]
- 23.Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. Early Breast Cancer Trialists’ Collaborative Group. N Engl J Med. 1988;319(26):1681–1692. doi: 10.1056/NEJM198812293192601. [DOI] [PubMed] [Google Scholar]
- 24.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351(9114):1451–1467. [PubMed] [Google Scholar]
- 25.Pico C, Martin M, Jara C, et al. Epirubicin-cyclophosphamide adjuvant chemotherapy plus tamoxifen administered concurrently versus sequentially: randomized phase III trial in postmenopausal node-positive breast cancer patients. A GEICAM 9401 study. Ann Oncol. 2004;15(1):79–87. doi: 10.1093/annonc/mdh016. [DOI] [PubMed] [Google Scholar]
- 26.Albain KS, Barlow WE, Ravdin PM, et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9707):2055–2063. doi: 10.1016/S0140-6736(09)61523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albain KS, Green SJ, Ravdin PM, et al. Adjuvant chemohormonal therapy for primary breast cancer should be sequential instead of concurrent: initial results from intergroup trial 0100 (SWOG-8814) Proc Am Soc Clin Oncol. 2002;21(182) Abstract 143. [Google Scholar]
- 28.Swain SM, Jeong JH, Geyer CE, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362(22):2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16(10):1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- 30.Fisher B, Redmond C, Legaultpoisson S, et al. Postoperative chemotherapy and tamoxifen compared with tamoxifen alone in the treatment of positive-node breast cancer patients aged 50 years and older with tumors responsive to tamoxifen : results from the National Surgical Adjuvant Breast and Bowel Project-B-16. J Clin Oncol. 1990;8(6):1005–1018. doi: 10.1200/JCO.1990.8.6.1005. [DOI] [PubMed] [Google Scholar]
- 31.Namer M, Fargeot P, Roche H, et al. Improved disease-free survival with epirubicin-based chemoendocrine adjuvant therapy compared with tamoxifen alone in one to three node-positive, estrogen-receptor-positive, postmenopausal breast cancer patients: results of French Adjuvant Study Group 02 and 07 trials. Ann Oncol. 2006;17(1):65–73. doi: 10.1093/annonc/mdj022. [DOI] [PubMed] [Google Scholar]
- 32.Del Mastro L, Dozin B, Aitini E, et al. Timing of adjuvant chemotherapy and tamoxifen in women with breast cancer: findings from two consecutive trials of Gruppo Oncologico Nord-Ovest-Mammella Intergruppo (GONO-MIG) Group. Ann Oncol. 2008;19(2):299–307. doi: 10.1093/annonc/mdm475. [DOI] [PubMed] [Google Scholar]
- 33.Aebi S, Castiglione M. The enigma of young age. Ann Oncol. 2006;17(10):1475–1477. doi: 10.1093/annonc/mdl330. [DOI] [PubMed] [Google Scholar]
- 34.Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. 2002;13(2):273–279. doi: 10.1093/annonc/mdf039. [DOI] [PubMed] [Google Scholar]
- 35.Saphner T, Tormey DC, Gray R. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. J Clin Oncol. 1991;9(2):286–294. doi: 10.1200/JCO.1991.9.2.286. [DOI] [PubMed] [Google Scholar]
- 36.Pritchard KI, Paterson AHG, Paul NA, Zee B, Fine S, Pater J. Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. J Clin Oncol. 1996;14(10):2731–2737. doi: 10.1200/JCO.1996.14.10.2731. [DOI] [PubMed] [Google Scholar]
- 37.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102(1):14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe N, Ootawa Y, Kodama K. Concurrent administration of chemo-endocrine therapy for postmenopausal breast cancer patients. Breast Cancer. 2010;17(4):247–253. doi: 10.1007/s12282-009-0144-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.