Abstract
Aim
To examine if the body mass index (BMI) in midlife is related to cognitive function 30 years later in a dementia-free sample.
Methods
BMI was reported in 1963 at age 50–60 years, and cognitive abilities were examined 30 years later in a longitudinal design with 5 measurement occasions at 2-year intervals (n = 417). The cognitive abilities examined included tests of long-term memory, short-term memory, speed, verbal and spatial ability.
Results
Multilevel modeling adjusting for demographic and lifestyle factors, and relevant diseases showed that a higher BMI in midlife predicted lower test performance 30 years later. Significant associations between BMI and level of performance were found in all cognitive abilities; however, a higher midlife BMI was not associated with steeper cognitive decline.
Conclusion
Our results indicate that midlife overweight is related to lower overall cognitive function in old age. The fact that BMI-related effects were noted in mean-level cognitive performance, whereas only one ability showed differences in slopes, suggests that the negative effect of overweight has an onset before the entry into very old age.
Key Words: Adiposity, Obesity, Cognition, Mental ability
Introduction
The evidence that overweight in middle age is related to increased dementia risk in old age is growing [1,2,3,4]. However, few studies have addressed the question if overweight affects cognitive abilities among those who do not develop dementia. Our hypothesis is that overweight is likely to assert a negative effect on cognition even in individuals who remain nondemented.
Cross-sectional studies on the association between body mass index (BMI) and cognitive function in midlife have produced mixed and nonconclusive findings. Significant associations between high BMI and low cognitive test performance have been reported in two studies using samples covering the whole adult age span [5,6]. However, in the study by Dore et al. [6], the association between BMI and cognition was to a large extent attenuated by adjusting for physical activity. In another study, a significant association between BMI and cognition was found only in executive function [7]. In one further study, based on an older sample (54–81 years), significant associations were found in only 3 out of 11 cognitive tests [8]. Cross-sectional studies on older age cohorts show even more contradictory results, where some indicate that higher BMI is associated with better cognitive function [9,10,11], whereas others have found that higher BMI is associated with lower cognitive function [12,13]. Taken together, findings based on cross-sectional studies do not convincingly demonstrate a significant association between BMI and cognitive function at younger ages. In older ages, the association is often blurred by the inclusion of individuals diagnosed with dementia and those in a preclinical phase of dementia, knowing that people often lose weight prior to diagnosis [14] and early in the dementia phase [15].
There are only a few prospective studies including young elderly and elderly people, all with a follow-up time of only 6 years or less. In a report from the Framingham study [16], using 4–6 years of follow-up (mean age 66 years), high BMI predicted lower cognitive function in only 2 out of 8 tests among men, and no associations were found among women. On the contrary, slight overweight (BMI 23–27) predicted lower risk of cognitive decline across a 5-year follow-up in the French PAQUID study [9]. Findings from the Chicago Health and Aging Project underline the importance of taking into account those who are already diagnosed with dementia or in a preclinical phase, showing that when this group is included, higher BMI may appear to be a protective factor against cognitive decline [11]. However, when those with an MMSE score of 24 or less were excluded, there was no association between BMI and cognitive function.
Previous findings regarding the effect of overweight on cognitive function are typically based on either cross-sectional studies or on prospective studies in young old age with a limited follow-up time. An exception is a recent study from the UK [17], in which BMI assessed on 3 occasions across midlife (first assessment at 35–55 years) was used to predict cognitive function (MMSE, memory, and executive function) at the age of 52–72 years. Using overweight and obesity as long-term predictors of cognitive function, overweight at the first occasion predicted lower memory performance, whereas obesity at the second occasion was related to lower performance in executive function.
The aim of our study is to examine the long-term effects of midlife overweight on cognitive abilities in old age. Specifically, we will examine whether BMI measured in midlife is related to cognition 30 years later among nondemented individuals. Further, given that no study has examined the association between overweight in midlife and cognitive function with such an extensive follow-up period, all available cognitive domains in our study will be explored. This will be done by using cognitive tests that cover a range of cognitive abilities, including long-term memory, short-term memory, speed, verbal and spatial ability. Further, we will examine if BMI is related to steeper cognitive decline measured at 2-year intervals across a period of 8 years (age interval 80+). Analyses will include relevant covariates, including midlife physical activity. Given recent findings that overweight in midlife increases risk of dementia, we hypothesize that overweight will be associated with lower performance across cognitive domains in a sample of nondemented elderly people. Finally, given some indications that BMI may have differential effects on cognitive function in men and women, data will be analyzed separately for both sexes.
Methods
Participants
The participants in this study were drawn from the Swedish Twin Registry which was established in the late 1950s to study smoking and alcohol consumption in relation to potential risk of cancer and cardiovascular diseases [18,19]. In 1963, the participants answered questionnaires including questions about health and diseases, smoking habits, alcohol consumption, physical activity, and information on weight and height. Between the years 1991 and 1993, the first wave of the longitudinal study ‘Origins of Variance in the Old-Old: Octogenarian Twins’ (OCTO-Twin) was started [20], including those who had participated in the survey in 1963. For the purpose of the present study, information from the survey in the sixties and information from the OCTO-Twin study have been linked.
The OCTO-Twin study is based on the oldest cohort of the Swedish Twin Registry and includes 702 people aged 80 years and older at the time of the first examination. It has a longitudinal design with repeated assessments at a 2-year time schedule. The participants were evaluated continuously across the study period with respect to dementia and vascular risk factors. All individuals were assessed with a cognitive test battery during an in-person visit. Individuals suspected of having dementia were given a diagnostic workup, including an interview with an informant about memory and cognitive problems, review of in-person cognitive test protocols and medical records. Clinical diagnoses of dementia followed the DSM-III-R criteria.
Given the purpose of the present study, that is to examine the effect of overweight in midlife on later cognitive function among those who do not develop dementia, the following individuals were excluded: (1) those who did not have valid data on BMI from the midlife assessments in 1963 (n = 30), (2) those who were older than 60 years in 1963 (n = 39), (3) those who received a dementia diagnosis (n = 203), and (4) those who did not have any valid cognitive data (n = 13). The total sample at the first wave of the longitudinal study includes 417 individuals (170 men and 277 women). Attrition across the 5 waves is described in table 1; as can be seen, the main cause is due to death.
Table 1.
Sample size and attrition across the 5 waves
| Wave 1 (1991– 1993) | Wave 2 (1993– 1995) | Wave 3 (1995– 1997) | Wave 4 (1997– 1999) | Wave 5 (1999– 2001) | |
|---|---|---|---|---|---|
| Participation | 417 | 339 | 274 | 211 | 167 |
| Reason for attrition | |||||
| Death | 51 | 53 | 53 | 41 | |
| Refusal | 24 | 12 | 9 | 3 | |
| Other reason | 3 | 0 | 1 | 0 |
All participants were informed about the study in accordance with the ethics committee of the Karolinska Institute, the Swedish Data Inspection Board, and the institutional board at the Pennsylvania State University.
Measures
Body Mass Index. Weight and height were based on self-reported information from the survey in 1963. BMI was calculated by dividing the weight in kilos by the height in square meters (kg/m2). In the data analyses, BMI was used as a continuous variable. Further, for descriptive purposes only, BMI was also classified according to World Health Organization standards [21], where overweight is defined as a BMI between 25 and 30, whereas obesity is defined as a BMI greater than 30. As heights and weights were self-reported, they are potentially subject to bias. However, analyses completed in a related sample from the Twin Registry comparing self-reported and measured information on heights and weights (within a 1-year period) resulted in a correlation of 0.97 for height and 0.95 for weight [22]. The mean differences ± SD between self-reported and measured values were 1.2 ± 2.4 cm for height and 0.8 ± 4.0 kg for weight.
Smoking and Alcohol. The information on smoking and alcohol habits comes from the survey in the sixties as well as from the OCTO-Twin study. The smoking variable was coded as 0 for nonsmoker and 1 for current or previous smoker. The alcohol variable was coded as 0 for alcohol consumers and 1 for those who never use alcohol.
Physical Activity. The information on physical activity in midlife comes from the survey in the sixties. Respondents were asked if they exercised, and the level of exercise was measured on a 4-point scale (1 = no exercise, 2 = light exercise, 3 = regular exercise, 4 = heavy exercise).
During the OCTO-Twin Study, the participants were asked for permission to review their medical records. Medical records were ordered from hospitals, outpatient clinics, district physicians, and primary health care centers, and multiple requests were made to secure the quality of information and to make sure that the records covered the entire time period and also contained a summary of diseases earlier in life. A physician made a concurrent review of (1) medical records, including reported medical history, (2) medicine use, and (3) self-reported information about diseases. An independent second opinion on the classifications, performed by another physician, in a 20% subsample produced only marginal amendment. Diagnoses were classified according to the ICD-10 [23]. Diagnoses of special interest for the present study were hypertension, diabetes, and stroke.
Hypertension. Blood pressure was assessed on every test occasion in the OCTO-Twin Study (maximum of 5 times). Hypertension was diagnosed in cases (1) with a diastolic blood pressure value above 95 mm Hg and/or a systolic pressure value higher than 160 mm Hg in line with the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure, and/or (2) where the medical records contained information on specific hypertension treatment, and/or (3) with self-reported hypertension in the survey in 1963.
Diabetes. Information on type 2 diabetes mellitus was obtained from the medical records and includes all types of the disease from a time point when the diagnostic level of fasting blood sugar was 6.7 mmol/l. Diagnoses were classified according to the American Diabetes Association recommendations [24].
Stroke. A diagnosis of stroke was mainly based on medical exam information in the medical records. Self-reports of stroke were accepted as valid in 10% of the cases despite a lack of record confirmation.
Cognitive Testing
The participants were investigated in their home. A complete testing session, including rest periods, took about 3.5–4.0 h. The longitudinal design for survivors encompassed at maximum 5 measurement occasions at 2-year intervals beginning in 1991–1993. The cognitive test battery was designed to represent the domains of fluid and crystallized intelligence and specific abilities such as memory, spatial ability and speed. A detailed description of the test battery can be seen elsewhere [25].
Principal component analyses were used to construct latent factors from the individual tests within each cognitive ability reflecting long-term memory, short-term memory, speed, and verbal ability. Spatial ability is only represented by the Block Design Test. For the ease of presentation, cognitive scores were transformed into a t score metric with a mean of 50 and standard deviation of 10.
Long-Term Memory. Three tests reflecting long-term episodic memory functioning were used: the Prose Recall Test (a Swedish version of the Logical Memory in the Wechsler Memory Scale), the Thurstone's Picture Memory Test [26] and the Memory-in-Reality Test [27,28].
Short-Term Memory. Two measures reflecting short-term memory were used: the Digit Span Forward and the Digit Span Backward [29].
Speed. Two tests measured speed: the Symbol Digit Task, a modified version of the Digit Symbol Substitution Test from the revised Wechsler Adult Intelligence Scale [29], that requires an oral (rather than written) response, and the Perceptual Speed Test from a Swedish test battery [30].
Verbal Ability. Two tests were used to assess verbal ability: the Swedish version of the Information Task [31], and the Verbal Meaning Test [30].
Spatial Ability. The Block Design Test [30] was used to measure spatial ability.
Data Analyses
Weight group comparisons for means (BMI, age, and years of formal education) were conducted with one-way analyses of variance. Weight group comparisons in gender distribution, smoking, alcohol consumption, midlife physical activity, hypertension, diabetes, and stroke were analyzed with χ2 tests.
To analyze the potential association between midlife BMI and later cognitive function and rate of change, random coefficient modeling using SAS Proc Mixed [32] was used to estimate individual-level change and predictors of change while properly accounting for the dependency associated with twin pair status. The multilevel model is characterized by a fixed part which contains average effects for the intercept (initial status) and slope (rate of change) and a random part which contains individual differences (variance) in the intercept, slope, and the within-person residual. The models were tested using the missing at random assumption [33] for missing cognitive outcomes. A 3-level linear growth model is composed of a level 1 component of individual outcomes over time, a level 2 component which models individual fixed and random effects of initial status and change over time (person-level covariates can be added at this level), and a level 3 component which models variance associated with twin pair status. This is done to handle the fact that a twin sample is used to draw conclusions about non-twins in which higher intraclass correlations are expected for monozygotic (MZ) than for dizygotic (DZ) twins. Thus, a 3-level structure is characterized by longitudinal measurements nested within individuals who are nested within groups (twin dyad).
The baseline model specifies a growth model with no covariates and is used to evaluate the fit of the growth model parameters. In this and subsequent models, zygosity is not modeled as a fixed effect because the expectation is for no or random differences among MZ and DZ twins in terms of average slope and rate of change. Random effects are, however, estimated separately by zygosity because of an expectation of higher intraclass correlations for MZ than for DZ twins. Finally, we permit different estimates of the residual (level 1) variance for the two zygosity groups. The intercept was centered in all models at age 83, education of 7 years, and midlife BMI of 24.
All models were estimated for each cognitive outcome stratified by sex. However, as the analyses revealed a similar pattern for men and women, the models were run on the total sample and presented accordingly. First, a baseline model was estimated to determine the initial status (level) and rate of change (linear and quadratic slopes) for all cognitive abilities. The second model included midlife BMI as a continuous variable and was adjusted for demographic data only, that is, age (continuous variable), sex (0 = man, 1 = woman), and education (continuous variable). Further, to measure the potential interaction between BMI and rate of change in cognitive function, two interaction terms between BMI and slope were added, one for the linear slope and one for the quadratic slope. The third model was a fully adjusted model additionally including smoking (0 = never smoked, 1 = ever smoked), alcohol consumption (0 = never, 1 = ever), physical activity in midlife (1 = no exercise, 2 = light exercise, 3 = regular exercise, 4 = heavy exercise), years with hypertension (continuous variable), years with diabetes (continuous variable), stroke (0 = no stroke, 1 = stroke), BMI and BMI and slope interaction terms (linear and quadratic).
Results
The participant characteristics across weight groups are presented in table 2. The analyses of variance revealed no significant difference between groups in age (p > 0.05), whereas a significant difference was found for education reflecting higher education in the normal weight group in comparison with the overweight and obese groups [F(2, 414) = 7.25; p = 0.001]. The average educational level in this study is low, although it is representative of this old cohort in Sweden. It shows that the majority of the participants only had the basic 6 years of elementary school. Further, the groups did differ in gender distribution reflecting a predominance of women in the obese group (p < 0.05). The obese group reported a lower frequency of smoking (p < 0.05) compared to the normal weight group, whereas more people in the normal weight group reported using alcohol as compared to the overweight group (p < 0.05). The normal weight group also had a significantly lower prevalence of diabetes as compared to the overweight group (p < 0.05).
Table 2.
Participant characteristics by weight group (n = 417)
| Characteristics | Normal weight (n = 264) | Overweight (n = 135) | Obese (n = 18) |
|---|---|---|---|
| Age in wave 1 (1991–1993), years | 82.9 ±2.5 (80–90) | 83.0 ±2.4 (80–90) | 82.7 ±2.1 (80–90) |
| BMI in 1963 | 22.7± 1.7 (16.3–24.9) | 26.7 ± 1.3 (25.0–29.9) | 31.2 ±1.4a (30.0–35.4) |
| BMI in wave 1 | 23.4 + 3.2 (15.6–32.9) | 26.1 + 3.6 (14.3–35.6) | 31.4+ 4.6a (22.9–38.6) |
| Education, years | 7.±±2.8b (2–23) | 6.9 ± 1.±(3–16) | 6.1 ±1.2 (4–10) |
| Women | 179 (68) | 81 (60) | 17 (94)c |
| No physical activity in midlife | 24 (9) | 11 (8) | 2 (11) |
| Ever smoked | 115 (44)d | 40 (37) | 2 (11) |
| Ever used alcohol | 201 (76)e | 86 (64) | 11 (61) |
| Hypertension | 117 (44) | 62 (46) | 13 (72) |
| Diabetes | 30 (11)e | 30 (22) | 4 (22) |
| Stroke | 52 (20) | 27 (20) | 4 (22) |
Figures are means ± SD with ranges in parentheses or numbers with percentages in parentheses.
p < 0.05 for all pairwise comparisons;
p < 0.05 for normal weight versus overweight and obese;
p < 0.05 for obese versus normal weight and overweight;
p < 0.05 for normal weight versus obese;
p < 0.05 for normal weight versus overweight. All information on vascular risk factors indicates lifetime exposure.
Associations between Midlife BMI and Cognitive Abilities in Old Age
For the purpose of description only, means and standard deviations in the cognitive abilities at the first measurement occasion are reported across weight groups in table 3. The table shows that higher BMI in midlife is related to poorer cognitive performance in old age.
Table 3.
Cognitive performance across abilities at the first wave (1991–1993) by weight group
| Cognitive abilities | Normal weight | Overweight | Obese |
|---|---|---|---|
| Long-term memory | 51.3±8.9 (10.1–66.5) | 49.4±8.4 (26.9–66.1) | 39.6±14.0 (10.1–53.3) |
| Short-term memory | 52.7±11.2 (7.8–84.3) | 50.1±10.0 (23.7–81.0) | 45.3± 13.2 (7.8–61.9) |
| Speed | 52.5±9.3 (20.4–83.2) | 48.5±10.0 (26.8–79.1) | 41.2± 12.7 (20.4–57.9) |
| Verbal ability | 51.1±9.4 (24.3–68.0) | 49.8±9.6 (26.4–67.2) | 38.2±9.4 (21.2–56.2) |
| Spatial ability | 51.2±10.4 (32.1–79.8) | 48.4±9.5 (32.1–68.3) | 42.3±10.4 (32.1–63.9) |
All cognitive scores were transformed into a t score metric with a mean of 50 and standard deviation of 10. Figures indicate means ± SD with ranges in parentheses.
In the multilevel modeling, midlife BMI was entered as a continuous variable. All models were run separately for men and women. However, as the analyses revealed a similar pattern for men and women, the models were run on the total sample and presented accordingly. The baseline model indicated that the estimates of the average intercept and rate of change across measurement occasions were significant for all cognitive abilities. Thus, there was a general significant decline over time in all cognitive abilities.
The second model allows us to explore whether variation in intercepts and slopes is related to midlife BMI, after accounting for age, sex, and differences in education. The results showed that midlife BMI was a significant predictor of mean-level performance for all cognitive abilities such that higher BMI predicts poorer performance. Although higher BMI was related to lower overall performance in the cognitive abilities, it was not associated with steeper decline (slope) with the exception of verbal ability.
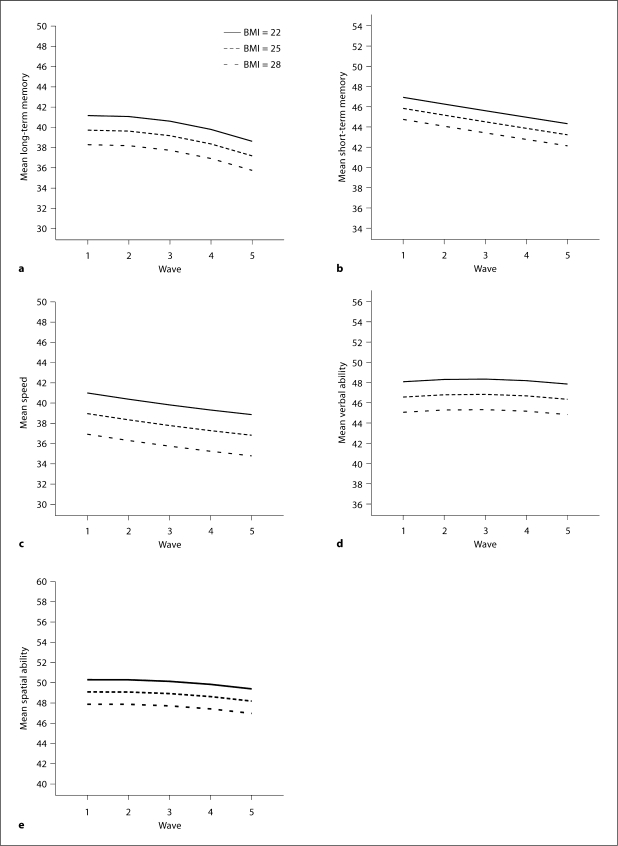
The fully adjusted model was adjusted for lifestyle factors such as smoking and alcohol use, midlife physical activity, as well as hypertension, diabetes, and stroke. These results are illustrated in table 4 and figure 1. The inclusion of these factors did not alter the association between midlife BMI and cognitive function.
Table 4.
Parameter estimates of the fixed effects for each cognitive abilities in fully adjusted modelsa
| Cognitive abilities | F ixed effects |
||
|---|---|---|---|
| estimate | linear slope | quadratic slope | |
| Long-term memory | |||
| Intercept | 51.70∗∗ (3.30) | 0.03 (0.22) | −0.09∗∗ (0.03) |
| BMI | −0.48∗ (0.19) | 0.05 (0.08) | −0.01 (0.01) |
| Short-term memory | |||
| Intercept | 54.95∗∗ (2.55) | −0.74∗∗ (0.22) | 0.01 (0.03) |
| BMI | −0.36∗ (0.17) | 0.07 (0.08) | 0.00 (0.01) |
| Speed | |||
| Intercept | 55.96∗∗ (3.20) | −0.75∗∗ (0.29) | 0.04 (0.05) |
| BMI | −0.68∗∗ (0.20) | 0.10 (0.11) | −0.01 (0.02) |
| Verbal ability | |||
| Intercept | 59.13∗∗ (2.93) | 0.25∗ (0.12) | −0.08∗∗ (0.02) |
| BMI | −0.50∗∗ (0.17) | 0.08 (0.04) | −0.01∗∗ (0.01) |
| Spatial ability | |||
| Intercept | 59.17∗∗ (2.92) | 0.12 (0.17) | −0.08∗∗ (0.02) |
| BMI | −0.40∗ (0.17) | −0.05 (0.06) | 0.01 (0.01) |
Fully adjusted models including age, sex, education, smoking, alcohol use, physical activity in midlife, lifetime hypertension, lifetime diabetes, and lifetime stroke as covari-ates. Figures in parentheses indicate SE.
p < 0.05;
p < 0.01.
Fig. 1.
Level and change in cognitive abilities across the 5 waves in relation to BMI in midlife.
The estimates in the fully adjusted models in table 4 are interpreted as the additional lowering in cognitive test performance related to one-unit increase in BMI score. For example, the average person, defined as being 83 years of age and having a BMI of 24 and 7 years of education, scored 51.70 points (intercept) in long-term memory and showed a significant curvilinear decline of 0.09 points per 2-year interval (quadratic slope). One-unit increase in BMI was related to 0.48 points lower average performance in long-term memory; however, BMI was not related to additional change across time as can be seen in the nonsignificant estimate of both the linear (0.05) and the quadratic (−0.01) slopes related to BMI.
Discussion
The aim of the study was to examine the association between overweight in midlife and cognitive function in old age. Our findings demonstrate that being overweight in midlife is related to lower cognitive function 30 years later independently from dementia. Moreover, this association was evident even when age, education, physical activity, and factors related to vascular risk were controlled for. The association was demonstrated across several cognitive abilities, including long-term memory, short-term memory, speed, verbal ability, and spatial ability.
The finding is novel because, to our knowledge, no previous study has examined the long-term effects of high BMI in midlife on later cognitive function among individuals who remain nondemented, using a prospective design with an extensive longitudinal follow-up time. Our results support and extend some findings based on cross-sectional [12,13] and prospective studies [17] in showing a negative association between BMI and cognitive function in old age. On the other hand, the results contradict cross-sectional findings in old samples that show a positive association between BMI and cognitive function [9,10]. This may be explained by how prevalent dementia cases and preclinical dementia are accounted for in the analyses. For example, in the study by Sturman et al. [11], the analyses were performed with and without people with an MMSE score lower than 24. When all cases were included in the analyses, a higher BMI was associated with less cognitive decline, but when people with a low MMSE score were excluded, the association was attenuated and no longer significant. Findings like this suggest that the association is likely to be influenced by preclinical and mild dementia. Thus, overweight is unlikely to be a protective factor in relation to late-life cognitive function.
There is some evidence for a sex difference in the association between adiposity and cognitive function. For example, findings from the Framingham study [16] showed that obese men performed significantly lower on some cognitive tasks whereas no such effects were found in women. In the present study, data were first analyzed separately for men and women. Our analyses revealed the same pattern among men and women and therefore do not support previous findings of sex differences.
Concerning the question whether overweight is related to performance across cognitive abilities, our findings support the notion of an overall negative influence of high BMI in midlife. Thus, it can be concluded that the negative association between BMI and cognition prevails across a range of cognitive abilities.
The fact that high BMI in midlife predicted poorer overall cognitive performance but not steeper decline across the longitudinal follow-up period in old age, with one exception, raises the question of when the difference in the level of cognitive function related to high BMI becomes manifest. As we do not have any measure of midlife cognitive function in our study, we do not know for sure whether the observed BMI-related differences in the level of cognitive function in old age were already manifest in midlife. It could be hypothesized that the difference in cognitive function related to BMI is a lifelong phenomenon. This is partially supported by evidence from studies showing that childhood cognition predicts adiposity in adult life [34,35]. On the other hand, when educational attainment was considered in these studies, cognitive ability did no longer predict adult overweight [34,35]. Further, in an attempt to control for potential differences in cognitive function in midlife we made complementary analyses in which we used verbal ability as a covariate. The rationale is that verbal ability is a good proxy for premorbid cognitive ability and that it is a stable ability across adult life. However, using verbal ability as a covariate did not alter the association between midlife BMI and later cognitive function. Thus, our findings, based on analyses in which the educational level was included as a covariate, and the complementary analyses using verbal ability as a proxy for premorbid cognitive function, support the notion that overweight in midlife predicts lower cognitive function in old age. Cross-sectional studies on the association between BMI and cognitive function in midlife do not provide conclusive information as to whether there really is an association [6,7,8]. A more feasible explanation then to why we do not find differences in the cognitive slopes related to BMI may be that the negative effect of overweight has an onset before the late entry into the study. This is supported by recent findings from our own research group, based on a younger sample (50 years and older), where high BMI was related to lower mean-level cognitive performance 18 years later, as well as a steeper decline in cognitive function [36].
The accumulating evidence, supporting the notion of a negative effect of adiposity on cognitive health, highlights the need for explanatory mechanisms. At this time, several hypotheses are being proposed, although no single one is at the front. Potential mechanisms by which long-term overweight might affect cognitive function in later life include vascular disease, diabetes, genetics, and inflammatory processes. Overweight increases the risk of hypertension, diabetes mellitus, and stroke, all three known risk factors for cognitive impairment. Our study, along with other studies, show that even after controlling for these comorbid risk factors, overweight remains as a significant independent risk factor. Concerning the genetic factor, APOE ∊4 carrier status is a known risk factor for vascular disease and dementia [37,38]. In a recent study from our research group [4], we found that overweight did not interact with APOE ∊4 status on dementia risk, supporting earlier findings from the Finnish CAIDE study [39]. Finally, inflammation has been shown to be associated with vascular disease [40], obesity [41,42], cognitive decline [43], and dementia [44]. More research is needed to understand the complex mechanism explaining the association between adiposity and cognitive health. Detailed reviews on the topic have been conducted by Gustafson [45] and Beydoun et al. [46].
The strengths of this study lie in its prospective design, long follow-up time, an extensive cognitive test battery with repeated measurements over a critical period for cognitive change in old age, and the range of included covariates. When interpreting our results it should be kept in mind that the inclusion age into the OCTO-Twin study was 80 years or older, which implies that the generalizability is restricted to those who survive to that age. The implication of this fact may be that our results are an underestimation of the negative effect of BMI given that there is a greater attrition among those with high BMI. A potential limitation is the midlife BMI measure which is based on self-reports on weight and height. However, correlation analyses conducted on a subsample from the Swedish Twin Registry [22], comparing self-reported and measured data, provide support for the validity of self-reports. In contrast, new findings from our research group, based on a different sample, show that in old age, there is a small but significant increase in the mean difference between self-reported and measured BMI, which is likely due to unawareness of changes in height over time [47].
In conclusion, the present study provides further support for a negative effect of midlife overweight. The 30-year follow-up indicates that there are long-term effects of adiposity on cognitive health. Further, the observed effects could not be ascribed to vascular risk factors, diabetes, physical activity, or educational attainment. Given the epidemic features of overweight and obesity in Western societies, further research and prevention is essential to minimize the burden for individuals as well as for societies.
Acknowledgements
This study was supported by a grant from The Bank of Sweden Tercentenary Foundation. Data for the analyses were drawn from the OCTO-Twin supported by National Institute of Aging Grants (AG04563, AG08724, AG08861, AG10175). None of the funding organizations played a role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
References
- 1.Gorospe EC, Dave JK. The risk of dementia with increased body mass index. Age Ageing. 2007;36:23–29. doi: 10.1093/ageing/afl123. [DOI] [PubMed] [Google Scholar]
- 2.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–326. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 4.Hassing LB, Dahl AK, Thorvaldsson V, Berg S, Gatz M, Pedersen NL, Johansson B. Overweight in midlife and risk of dementia: a 40-year follow-up study. Int J Obes (Lond) 2009;33:893–898. doi: 10.1038/ijo.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cournot M, Marquie JC, Ansiau D, Martinaud C, Fonds H, Ferrieres J, Ruidavets JB. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 6.Dore GA, Elias MF, Robbins MA, Budge MM, Elias PK. Relation between central adiposity and cognitive function in the Maine-Syracuse Study: attenuation by physical activity. Ann Behav Med. 2008;35:341–350. doi: 10.1007/s12160-008-9038-7. [DOI] [PubMed] [Google Scholar]
- 7.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Waldstein SR, Katzel LI. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int J Obes (Lond) 2006;30:201–207. doi: 10.1038/sj.ijo.0803114. [DOI] [PubMed] [Google Scholar]
- 9.Deschamps V, Astier X, Ferry M, Rainfray M, Emeriau JP, Barberger-Gateau P. Nutritional status of healthy elderly persons living in Dordogne, France, and relation with mortality and cognitive or functional decline. Eur J Clin Nutr. 2002;56:305–312. doi: 10.1038/sj.ejcn.1601311. [DOI] [PubMed] [Google Scholar]
- 10.Kuo HK, Jones RN, Milberg WP, Tennstedt S, Talbot L, Morris JN, Lipsitz LA. Cognitive function in normal-weight, overweight, and obese older adults: an analysis of the Advanced Cognitive Training for Independent and Vital Elderly cohort. J Am Geriatr Soc. 2006;54:97–103. doi: 10.1111/j.1532-5415.2005.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sturman MT, de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology. 2008;70:360–367. doi: 10.1212/01.wnl.0000285081.04409.bb. [DOI] [PubMed] [Google Scholar]
- 12.Jeong SK, Nam HS, Son MH, Son EJ, Cho KH. Interactive effect of obesity indexes on cognition. Dement Geriatr Cogn Disord. 2005;19:91–96. doi: 10.1159/000082659. [DOI] [PubMed] [Google Scholar]
- 13.Kilander L, Nyman H, Boberg M, Lithell H. Cognitive function, vascular risk factors and education. A cross-sectional study based on a cohort of 70-year-old men. J Intern Med. 1997;242:313–321. doi: 10.1046/j.1365-2796.1997.00196.x. [DOI] [PubMed] [Google Scholar]
- 14.Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR, Launer LJ. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 15.White H, Pieper C, Schmader K. The association of weight change in Alzheimer's disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc. 1998;46:1223–1227. doi: 10.1111/j.1532-5415.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- 16.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Obesity, diabetes and cognitive deficit: the Framingham Heart Study. Neurobiol Aging. 2005;26(suppl 1):11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. Am J Clin Nutr. 2009;89:601–607. doi: 10.3945/ajcn.2008.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlstrom E, Bjork C, Svartengren M, Wolk A, Klareskog L, de Faire U, Schalling M, Palmgren J, Pedersen NL. The Swedish Twin Registry in the third millennium: an update. Twin Res Hum Genet. 2006;9:875–882. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- 20.McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . Physical Status: The Use and Interpretation of Anthropometry. Geneva: World Health Organization; 1995. [PubMed] [Google Scholar]
- 22.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med. 1990;322:1483–1487. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson SE, Johansson B, Berg S, Karlsson D, McClearn GE. A comparison of diagnosis capture from medical records, self-reports, and drug registrations: a study in individuals 80 years and older. Aging Clin Exp Res. 2002;14:178–184. doi: 10.1007/BF03324433. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association Clinical practice recommendations. Diabetes Care. 1998;21:1–30. [Google Scholar]
- 25.Hassing LB, Grant MD, Hofer SM, Pedersen NL, Nilsson SE, Berg S, McClearn G, Johansson B. Type 2 diabetes mellitus contributes to cognitive decline in old age: a longitudinal population-based study. J Int Neuropsychol Soc. 2004;10:599–607. doi: 10.1017/S1355617704104165. [DOI] [PubMed] [Google Scholar]
- 26.Thurstone LL, Thurstone TG. Manual to SRA Primary Mental Abilities. Chicago: Science Research Associates; 1949. [Google Scholar]
- 27.Fiske A, Gatz M. The Apartment Test: validity of a memory measure. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:441–461. doi: 10.1080/13825580600611294. [DOI] [PubMed] [Google Scholar]
- 28.Johansson B. The MIR – Memory in Reality Test. Stockholm: Psykologiförlaget AB; 1988/89. [Google Scholar]
- 29.Wechsler D. Manual for the Wechsler Adult Intelligence-Scale Revised. New York: Pychological Corporation; 1991. [Google Scholar]
- 30.Dureman I, Sälde H. Psykometriska och experimental-psykologiska metoder för klinisk tillämpning. Uppsala: Almqvist & Wiksell; 1959. [Google Scholar]
- 31.Jonsson CO, Molander L. Manual till CVB-skalan. Stockholm: Psykologi Förlaget; 1964. [Google Scholar]
- 32.SAS Version 9.1. Cary, SAS Institute Inc, 2002–2003.
- 33.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 34.Chandola T, Deary IJ, Blane D, Batty GD. Childhood IQ in relation to obesity and weight gain in adult life: the National Child Development (1958) Study. Int J Obes (Lond) 2006;30:1422–1432. doi: 10.1038/sj.ijo.0803279. [DOI] [PubMed] [Google Scholar]
- 35.Lawlor DA, Clark H, Davey Smith G, Leon DA. Childhood intelligence, educational attainment and adult body mass index: findings from a prospective cohort and within sibling-pairs analysis. Int J Obes (Lond) 2006;30:1758–1765. doi: 10.1038/sj.ijo.0803330. [DOI] [PubMed] [Google Scholar]
- 36.Dahl A, Hassing LB, Fransson E, Berg S, Gatz M, Reynolds C, Pedersen NL. Being overweight in midlife is associated with lower cognitive ability and steeper cognitive decline in late life. J Gerontol A Biol Sci Med Sci. 2010;65:57–62. doi: 10.1093/gerona/glp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 38.Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 40.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 41.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 42.Bruun JM, Pedersen SB, Kristensen K, Richelsen B. Effects of pro-inflammatory cytokines and chemokines on leptin production in human adipose tissue in vitro. Mol Cell Endocrinol. 2002;190:91–99. doi: 10.1016/s0303-7207(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 43.Yaffe K. Metabolic syndrome and cognitive decline. Curr Alzheimer Res. 2007;4:123–126. doi: 10.2174/156720507780362191. [DOI] [PubMed] [Google Scholar]
- 44.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gustafson D. Adiposity indices and dementia. Lancet Neurol. 2006;5:713–720. doi: 10.1016/S1474-4422(06)70526-9. [DOI] [PubMed] [Google Scholar]
- 46.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dahl A, Hassing LB, Fransson E, Pedersen NL. Agreement between self-reported and measured height, weight, and body mass index in late life – a longitudinal study with 20 years of follow-up. Age Ageing. 2010;39:445–451. doi: 10.1093/ageing/afq038. [DOI] [PMC free article] [PubMed] [Google Scholar]