Abstract
Objective
Scavenger receptor BI (SR-BI) is an HDL receptor. Recent studies revealed that SR-BI protects against sepsis via modulating innate immunity. However, its role in adaptive immunity is unclear.
Methods and Results
SR-BI null mice exhibited impaired lymphocyte homeostasis as shown by splenomegaly and imbalanced expansion of T and B lymphocytes in the spleens. Importantly, the activated T and B lymphocytes were increased 3–4-fold, indicating a heightened active status of T and B lymphocytes. More importantly, in line with the accumulation of the activated T and B lymphocytes, SR-BI null mice developed systemic autoimmune disorders characterized by the presence of autoantibodies in circulation, the deposition of immune complexes in glomeruli, and the leukocyte infiltration in kidney. Further analyses revealed that SR-BI deficiency enhances lymphocyte proliferation, causes imbalanced IFN-g and IL-4 production in lymphocytes and elevated inflammatory cytokine production in macrophages. Furthermore, HDL from SR-BI null mice exhibited less capability of suppressing lymphocyte proliferation.
Conclusions
SR-BI regulates lymphocyte homeostasis likely through its roles in modulating the proliferation of lymphocytes, the cytokine production by lymphocytes and macrophages, and the function of HDL. Its deficiency leads to impaired lymphocyte homeostasis and autoimmune disorders. Our findings reveal a previously unrecognized role of SR-BI in adaptive immunity.
Keywords: lymphocytes, adaptive immunity, scavenger receptor BI, Scarb1, HDL
Lymphocytes are essential regulators and effectors of the adaptive immune system. Different from the innate immune cells, activated T and B lymphocytes form long-lived immunologic memory which provides protective immune responses to a diversity of pathogens.1 However, improper activation of T and B lymphocytes breakdowns the lymphocyte homeostasis that leads to a variety of diseases such as autoimmune disorders.
Scavenger receptor BI (SR-BI or Scarb1) is a well-established HDL receptor.2–3 It mediates intracellular uptake of cholesterol ester from HDL, which plays a key role in regulating plasma HDL cholesterol levels. 4–7 Mice lacking SR-BI exhibit a 2-fold increase in plasma cholesterol levels and are susceptible to atherosclerosis. 4–12 Recent reports showed that mutations in human SR-BI cause elevated plasma HDL concentration associated with infertility and changes in cholesterol metabolism in macrophages and platelets, indicating a role of SR-BI in human.13–15 In addition to regulate HDL metabolism, recent studies indicated that SR-BI is a multi-functional protein. It activates eNOS in endothelial cells in the presence of HDL,16–19 induces apoptosis in the absence of HDL/eNOS 20, and negatively regulates cytokine and IgM generation in B lymphocytes.21 Recent studies revealed that SR-BI protects against endotoxic and septic animal death via multiple mechanisms including protection against nitric oxide (NO)-induced oxidative damage and suppression of inflammatory cytokine generation in macrophages. 22–24 In spite of this established role in innate immunity, the function of SR-BI in adaptive immunity remains largely unknown. In this study, we report that mice deficient in SR-BI exhibit impaired lymphocyte homeostasis and develop autoimmunity. Further studies reveal that SR-BI deficiency enhances lymphocyte proliferation, causes imbalanced interferon-gamma (IFN-g) and interleukin-4 (IL-4) production in lymphocytes and elevated inflammatory cytokine production in macrophages, and reduces inhibitory effect of HDL on lymphocyte proliferation. The current findings extend our understanding about SR-BI and its role in autoimmunity.
Materials and Methods
SR-BI null mice
SR-BI+/− (B6;129S2-Scarb1tm1Kri/J) mice were from the Jackson Laboratory. SR-BI−/− mice were generated by breeding SR-BI+/− mice, and SR-BI+/+ littermates were used as controls. The animals were fed a standard laboratory diet (0.015% wt/wt cholesterol, 5.7% wt/wt fat, Harlan Tekland 2018). Animal care and experiments were approved by the Institutional Animal Care and Use Committee of the University of Kentucky. Both male and female mice were used. For a detailed description of Materials and Methods see the supplemental materials.
Results
Splenomegaly, imbalanced T and B lymphocyte expansion, and heightened lymphocyte activation in mice lacking SR-BI
To understand the significance of SR-BI in adaptive immunity, we examined the lymphatic organs from SR-BI null and wild type littermates. SR-BI null mice exhibited splenomegaly (0.173 ± 0.051g vs. 0.083 ± 0.031g wild type) associated with a 2.1-fold increase in total splenocyte number (Fig. 1a and b). Histological examination of the enlarged spleens revealed disrupted spleen structure as shown by increased numbers of small lymphoid nodules that lack a defined marginal zone (Fig. 1c). Immunofluorescent staining showed that these small lymphoid nodules contain densely packed T and B lymphocytes (Supplement Fig. 2a). Enlarged lymph nodes were more frequently observed in SR-BI null mice than wild type controls (Supplement Fig. 2b). There was no significant difference in thymus weight between SR-BI null and wild type littermates, but the thymus cellularity in SR-BI null mice was slightly decreased (Supplement Fig. 2c).
Figure 1. Splenomegaly, disrupted splenic architecture and imbalanced T and B cell expansion in mice lacking SR-BI.

(a) Splenomegaly and increased spleen/body weight in SR-BI−/− mice.
(b) Increased splenic cellularity in SR-BI−/− mice.
(c) Disrupted splenic architecture in SR-BI−/− mice. HE staining shows increased numbers of small lymphoid nodules (arrows) that lack a defined marginal zone in SR-BI−/− mice.
(d to g) Imbalanced T and B cell expansion in SR-BI−/− mice. FACS analysis indicates a 2-fold increase B cell populations and a 1.4 increase in T and populations (d and e), a 30% decrease in the percentages CD3+, CD4+, CD8+ T cell (d and f), and a 30% decrease in T/B cell ratio (g) in SR-BI−/− spleen. Fluorescence was analyzed on gated cells with forward and side light scatter properties of lymphocytes.
Spleens were harvested from 20-26-week-old SR-BI−/− and SR-BI+/+ littermates. n = 30 each group. ***p < 0.001 vs. SR-BI+/+ mice.
FACS analysis revealed that, compared to wild type littermates, SR-BI null mice had a 2-fold increase in the number of B lymphocytes in the spleen (Fig. 1e). Interestingly, compared to the marked increase in the number of B lymphocytes, the T lymphocytes were less expanded (1.4-fold) (Fig. 1e). Actually, the percentages of CD3+, CD4+ and CD8+ T lymphocytes were moderately decreased (Fig. 1d and f), and there was a 30% reduction in T to B cell ratio compared to wild type controls (Fig. 1g), indicating an imbalanced T and B cell expansion in mice lacking SR-BI. A 1.8-fold increase in B lymphocytes and a 30% reduction in T to B cell ratio were also observed in circulating blood (Supplement Fig. 2d).
We next investigated the effect of SR-BI deficiency on lymphocyte activation. Both T and B cells exhibited an overall hyperreactive feature, as shown by significant increases in the percentages of activated CD3CD69high T and CD19 CD69high B cells (Fig. 2a), and 3–4-fold increases in the number of activated CD3CD69high T and CD19 CD69high B cells (Fig. 2b),
Figure 2. Heightened activation of T and B lymphocytes in mice lacking SR-BI.
(a and b) Increased accumulation of activated T and B cells in SR-BI−/− mice. CD69 expression was analyzed on gated T (CD3+) or B (CD19+) cells, respectively. The percentages and cell numbers of activated CD69high cells in gated T or B cells were shown respectively.
(c and d) Increased accumulation of memory CD4+ cells in SR-BI−/− mice. Naïve (CD4+CD44lowCD62Lhigh), central memory (CD4+CD44highCD62Lhigh) and effector memory (CD4+CD44highCD62Llow) T cells were analyzed on gated CD4+ cells by FACS. The percentage and the cell numbers in CD4+ cells were shown.
(e) Elevated splenic plasmablasts (CD138+) in SR-BI−/− mice.
Spleens were harvested from 20-26-week-old SR-BI−/− and SR-BI+/+ littermates. n = 17 each group. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. SR-BI+/+ mice. The isotype control stainings for CD69 and CD138 were shown in Supplement Fig. 3.
We further looked at the effect of SR-BI deficiency on memory T cell using CD44 as marker. Mice lacking SR-BI had significant increases in both the percentage and the number of effector memory (CD4+CD44highCD62Llow) CD4+ T lymphocytes (Fig. 2c and d). In contrast, there was a significant decrease in the ratio of naïve (CD4+CD44lowCD62Lhigh) CD4+ T lymphocytes (Fig. 2c). The increase in memory T cell ratio and decrease in naïve T cell ratio indicate a heightened active status of T lymphocytes and accumulation of the memory T cells.
We also examined activated B lymphocytes using CD138 as marker.25 While there was no significant change in the percentage of plasmablasts (CD138+), the number of plasmablasts was increased 2.2-fold in SR-BI null mice compared to those of wild type mice (Fig. 2e).
SR-BI deficiency did not affect T or B lymphocyte development
To understand how SR-BI deficiency leads to disrupted lymphocyte homeostasis, we investigated the effect of SR-BI on lymphocyte development in thymus, bone marrow, and spleen of adult mice. We analyzed the CD4/CD8 profile in the thymus for T lymphocyte development. No difference was observed between SR-BI null and wild type littermates (Supplement Fig. 4a), suggesting that SR-BI deficiency does not affect T lymphocyte development in the thymus. We then examined the development of B lymphocytes. Immature B lymphocytes are first produced in bone marrow; after reaching the IgM+ immature stage, these B cells migrate to the spleen, where they continue to develop and differentiate into mature B lymphocytes. As shown in Supplement Fig. 4b and c, there was no difference in the percentages of B lymphocyte subsets in bone marrow or spleen between SR-BI null and wild type littermates. To determine whether SR-BI deficiency affects T or B cell development in early age, we analyzed the T and B cell development in 5–6-week old mice. No difference was observed between SR-BI null and wild type littermates (data not shown).
Taken together, these data suggest that the disordered lymphocyte homeostasis in SR-BI null mice may not be the consequence of lymphocyte development.
Increased in vivo lymphocyte proliferation in mice lacking SR-BI
We then asked whether an increase in lymphocyte proliferation is responsible for the accumulation of lymphocytes in the spleen. We administered BrdU in drinking water and quantified in vivo lymphocyte proliferation by FACS analysis of the BrdU incorporated cells. Compared to wild type littermates, SR-BI null mice displayed a 2-fold increase in proliferating B cell (B220+BrdU+) populations, and a slight increase in the percentage of proliferating B cells (Fig. 3a); there was a slight increase in the proliferating T cell (CD3+BrdU+) populations and a moderate decrease in the percentage of proliferating CD3+ cells (Fig. 3b). Interestingly, the number of proliferating CD4+CD44highBrdU+ T cells was significantly increased, but the percentage of proliferating CD4+CD44highBrdU+ T cells was slightly decreased in SR-BI null mice compared to wild type littermates (Fig. 3b and c). This may not be surprising considering a longer lifespan of the memory T cells which results in accumulation of the long-lived memory T cells.
Figure 3. Increased in vivo lymphocyte proliferation in mice lacking SR-BI.
SR-BI−/− and SR-BI+/+ littermates (26-week-old) were administered BrdU in the drinking water for 6 days. Splenocytes were isolated and stained with indicated cell surface markers and anti-BrdU antibody. n = 4 each group. *p < 0.05 and **p < 0.01 vs. SR-BI+/+ mice.
(a) Proliferation of B lymphocytes. BrdU fluorescence was analyzed on gated B220+ cells. SR-BI−/− mice display a 2-fold increase in the number of daughter B cells (B220+BrdU+).
(b) Proliferation of T lymphocytes. BrdU fluorescence was analyzed on gated CD3+ cells.
(c) Proliferation of memory T lymphocytes. BrdU fluorescence was analyzed on gated CD4+CD44high cells. SR-BI−/− mice display a significant increase in the number of daughter memory T cells (CD4+CD44highBrdU+).
SR-BI deficiency caused enhanced in vitro lymphocyte proliferation and HDL from SR-BI null mice lost inhibitory effect on lymphocyte proliferation in response to TCR/BCR stimuli
To understand the mechanisms of how SR-BI deficiency contributes to the impaired lymphocyte homeostasis, we assessed the effect of SR-BI deficiency on lymphocyte proliferation and cytokine production. We isolated T and B cells from spleen and detected SR-BI expression in T and B cells. Moderate SR-BI expression was detected in T and B cells (Fig. 4a and b), suggesting that SR-BI may have an intrinsic effect on lymphocyte. Indeed, as shown in Fig. 4c and d, T and B cells from SR-BI null mice had a 2-fold and 1.5-fold increase in proliferating rate compared to wild type controls, respectively, indicating enhanced proliferation in basal condition in the absence of SR-BI.
Figure 4. SR-BI deficiency caused enhanced in vitro lymphocyte proliferation and HDL from SR-BI null mice lost inhibitory effect on lymphocyte proliferation in response to TCR/BCR stimuli.
(a and b) SR-BI expression in T and B cells. CD3+ T and B220+ B cells were isolated by FACS from spleens and SR-BI expression was detected by quantitative RT-PCR. Liver was used as positive control. Splenocytes were from 20-week-old SR-BI−/− and SR-BI+/+ littermates. n = 6 per group.
(c and d) SR-BI deficiency caused enhanced T and B lymphocyte proliferation in basal status. Splenocytes were cultured in 10% FBS, and T and B cell proliferation was quantified by BrdU incorporation assay.
(e and f) HDL from SR-BI null mice had less capability of suppressing lymphocyte proliferation in response to TCR or BCR stimuli by anti-CD3 and LPS, respectively. Splenocytes were stimulated with anti-CD3 (10 µg/mL) or LPS (10 µg/mL) in the presence/absence of 40 µg/mL HDL isolated from SR-BI null or wild type littermates for 96 h and lymphocyte proliferation was determined by BrdU incorporation assay.
(g to i) SR-BI deficiency caused imbalanced IFN-g/IL-4 production in response to TCR stimuli.
Splenocytes were cultured in 10% FBS in the presence of Con A (1µg/mL), PMA (6.5 ng/mL) plus ionomycin (500 ng/mL), anti-CD3 (10 µg/mL) or anti-CD3 plus anti-CD28 (10 µg /mL) antibodies. After 48 h, the IFN-g and IL-4 levels in the culture supernatant were quantified.
For Figs. c to i, splenocytes were from 8-10-week-old SR-BI−/− and SR-BI+/+ littermates. Data represent 3 independent experiments. For each experiment, 3 mice per genotype were analyzed. *p < 0.05 and **p < 0.01 vs. SR-BI+/+ mice. Means without a common letter differ, p < 0.05.
We next looked at the effect of SR-BI deficiency on lymphocyte proliferation in stimulated conditions. Unexpectedly, T or B cells from SR-BI null mice did not display a difference in proliferation in response to TCR stimulus by anti-CD3 IgG or BCR stimulus by LPS in the presence of FBS (Fig. 4e and f, FBS). A possible explanation for the difference between the basal and stimulated conditions is that the strong stimuli by TCR or BCR ligands overshadowed the effect of SR-BI.
Early reports showed that HDL has inhibitory effect on T and B cell proliferation in stimulated status.26–28 SR-BI is an HDL receptor and mice deficient in SR-BI have abnormal HDL as shown by larger HDL particles with 2-fold increase in cholesterol contents, and changes in associated proteins. 4–7, 29 To test whether this HDL loses its inhibitory effect on lymphocyte proliferation, we stimulated splenocytes with TCR or BCR ligands in the presence/absence of HDL. In agreement with the early reports, HDL from wild type mice displayed 30% inhibition on CD4+ T cell and marked inhibition on B cell proliferation rate (Fig. 4e and f, SR-BI+/+ HDL) but HDL from SR-BI null mice displayed no inhibition on CD4+ T cell and much less inhibition on B cell proliferation rate (Fig. 4e and f, SR-BI−/− HDL). Similar results were obtained with respect to CD8+ T cell proliferation (data not shown).
We further looked at the effect of SR-BI on cytokine production in response to TCR stimuli. Interestingly, despite no difference in IFN-g production (Fig. 4g), T cells from SR-BI null mice produced markedly less IL-4 (Fig. 4h), and displayed a 2–3-fold increase in IFN-g/IL-4 ratio (Fig. 4i) in response to anti-CD3 stimulus. Similar data were obtained when cells were stimulated by both anti-CD3 and anti-CD28 which is a co-stimulator of CD3 to stimulate T cells. We also examined the effect of SR-BI on cytokine production stimulated by ConA or PMA/ionomycin which bypass the TCR to induce T cell activation and found no difference suggesting that the SR-BI likely functions via TCR signaling (Fig. 4g and h). It is worth to note that IFN-g belongs to proinflammatory cytokine (Th1 type) and IL-4 belongs to anti-inflammatory cytokine (Th2 type). Thus, SR-BI deficiency causes imbalanced Th1/Th2 cytokine production, which may lead to an inflammatory status and contribute to impaired lymphocyte homeostasis.
Increased monocyte/macrophage populations and elevated proinflammatory cytokine production in mice lacking SR-BI
Recent studies indicated that SR-BI suppresses inflammatory cytokine production by macrophages via regulating TLR4/NF-κB signaling pathway. 24 Macrophage cytokines such as IL-6 can act as growth factor for lymphocytes.30 So, it is possible that SR-BI deficiency may affect lymphocyte homeostasis partly through its role in modulating cytokine production in macrophages. To test such a possibility, we quantified CD11c−CD11b+F4/80+ cells, a major type of monocytes/macrophages in the spleen.31 As shown in Fig. 5a, there was a 1.9-fold increase in the number of CD11c−CD11b+F4/80+ cells in SR-BI null mice compared to wild type littermates. Importantly, in line with the significant increase in monocytes/macrophages, SR-BI null mice had 3–4-fold increases in TNF-α, IL-6 and iNOS expression and significant increases in TNF-α, IL-6 and nitrite/nitrate (NOx) levels in circulation (Fig. 5b to d).
Figure 5. Increased monocyte/macrophage populations and inflammatory cytokine expression and production in mice lacking SR-BI.
(a) Increased accumulation of monocytes/macrophages (CD11c-CD11b+F4/80+) in SR-BI−/− mouse spleen. FACS analysis of splenocytes from 20-26-week-old SR-BI−/− and SR-BI+/+ littermates. n = 12 each group. *p < 0.05 vs. SR-BI+/+ mice.
(b to d) Increased TNF-α, IL-6 and iNOS expression and production in SR-BI −/− mouse. Total splenic RNA was isolated from SR-BI−/− and SR-BI+/+ littermates and quantitative RT-PCR was conducted. The data were normalized to 18s rRNA expression. n = 6 each group. The serum TNF-α, IL-6 and NOx levels were analyzed with corresponding kit. n=16 each group *p < 0.05 vs. SR-BI+/+ mice.
Development of autoimmunity in aged SR-BI null mice
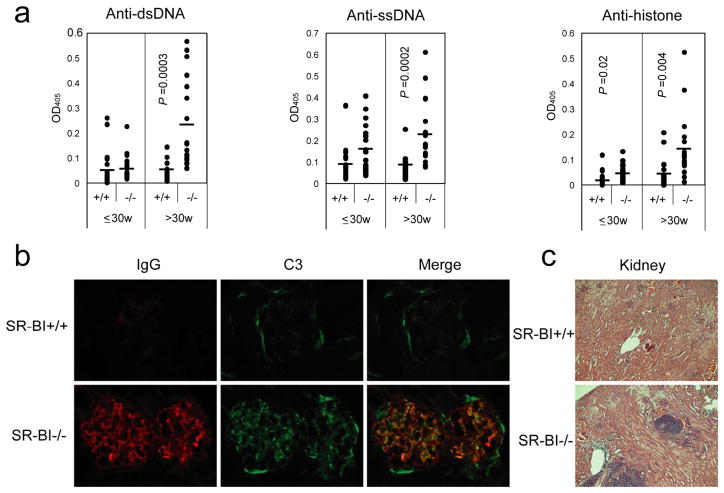
Impaired lymphocyte homeostasis with over activation of T and B lymphocytes, especially the accumulation of long-lived memory CD4+ T cells, is characteristics of systemic autoimmune diseases such as human systemic lupus erythematosus (SLE) and SLE-prone mouse.32–33 To assess whether SR-BI deficiency leads to autoimmunity, we quantified circulating autoantibodies in adult and aged mice. The autoantibody levels in adult SR-BI null mice ( 30 w) were slightly elevated compared to wild type littermates. For aged SR-BI null mice (> 30 w), there were marked increases in circulating autoantibodies against dsDNA, ssDNA and histone (Fig. 6a), indicating age-dependent development of autoimmunity in mice lacking SR-BI.
Figure 6. Development of autoimmunity in aged SR-BI null mice.
(a) Elevated autoantibodies in aged SR-BI−/− mice. Sera from SR-BI+/+ (n = 24 for mice ≤ 30w; n = 22 for mice > 30w) and SR-BI−/− (n = 22 for mice ≤ 30w; n = 19 for mice > 30w) mice were analyzed by ELISA for detection of anti-dsDNA, anti-ssDNA, and anti-histone antibodies.
(b) Deposition of IgG and C3 in the glomeruli of aged SR-BI−/− mice. Kidneys were isolated from 40-46-week-old SR-BI−/− and SR-BI+/+ littermates and frozen in OCT media. Sections were stained with Cy3-conjugated anti-IgG (red) or FITC conjugated anti-C3 (green) antibodies. n = 7 each group.
(c) Lymphocyte infiltration in the kidneys of aged SR-BI−/− mice. Kidneys were isolated from 40-46-week-old SR-BI−/− and SR-BI+/+ littermates. Sections were stained with H&E. n = 7 each group.
Circulating autoantibodies can deposit as immune complexes in kidney glomeruli through entrapment of circulating immune complexes as well as formation of immune complexes in situ following cross-reaction with components of glomerular basement membranes, which is a common feature of systemic autoimmune disorders. 32–33 As shown in Fig. 6b, using fluorochrome-labeled anti-mouse IgG and C3 antibodies, we detected profound positive staining of IgG and C3 in the glomeruli of SR-BI null mice (1 out of 7 in wild type vs. 7 out of 7 in SR-BI null mice were positive for IgG staining; 2 out of 7 in wild type vs. 7 out of 7 in SR-BI null mice were positive for C3 staining). Furthermore, most of the deposition of IgG was co-localized with C3 which suggests the formation of IgG/C3 immune complexes. We also evaluated leukocyte infiltration in the kidney, another common feature of systemic autoimmune disorders. 32–33 We found that there were large perivascular accumulation of leukocytes in SR-BI null mice that were seldom observed in control mice (Fig. 6c). All of these observations were evidence of age-dependent development of autoimmunity in SR-BI deficient mice.
Discussion
In this study, we report that SR-BI deficiency leads to impaired lymphocyte homeostasis characterized by splenomegaly and imbalanced expansion of T and B lymphocytes. Importantly, T and B lymphocytes in SR-BI null mice exhibited a heightened active status as shown by 3–4-fold increases in activated T and B lymphocytes. More importantly, in line with the accumulation of the activated T and B lymphocytes, SR-BI null mice developed systemic autoimmune disorders characterized by the presence of autoantibodies in circulation, the deposition of immune complexes in glomeruli, and the leukocyte infiltration in kidney.
To understand the mechanisms of how SR-BI deficiency contributes to the impaired lymphocyte homeostasis, we assessed the effect of SR-BI on lymphocyte proliferation and cytokine production. We found that SR-BI is moderately expressed in both T and B cells and its deficiency enhances lymphocyte proliferation in basal status and causes imbalanced IFN-g/IL-4 production in stimulated status. Furthermore, HDL from SR-BI null mice exhibited less capability of suppressing lymphocyte proliferation. An early report by Zhu et al demonstrated that SR-BI negatively regulates TLR9-dependent B cell activation induced by CpG.21 Combined with the current finding, these studies suggest that SR-BI has both intrinsic and extrinsic effects on lymphocyte activation and function.
As SR-BI has been shown to suppress inflammatory cytokine production by macrophages via regulating TLR4/NF-κB signaling pathway, 24 and the NF-κB signaling in macrophages plays an important role in lymphocyte activation, 30 we elucidated the effect of SR-BI on macrophage populations and proinflammatory cytokine production. We found a significant increase in the number of splenic monocytes/macrophages, marked increases in TNF-α, IL-6 and iNOS expression and significant increases in serum TNF-α, IL-6 and NOx levels in mice lacking SR-BI.
Taken together, these findings suggest that SR-BI regulates lymphocyte homeostasis likely through multiple ways by modulating the proliferation of lymphocytes, the cytokine production by lymphocytes and macrophages, and the function of HDL.
Glucocorticoid is an immunosuppressive hormone. As an HDL receptor, SR-BI plays an essential role in providing cholesterol for glucocorticoid synthesis in stressed conditions such as endotoxemia, sepsis and long-term fasting.23–24, 34 However, SR-BI deficiency does affect glucocorticoid production in physiological condition,23–24, 34 which suggests that the impaired lymphocyte homeostasis may not be caused by a change in glucocorticoid levels.
Recent studies revealed that excess accumulation of cellular cholesterol due to a defect in HDL-mediated cholesterol efflux disrupts lymphocyte homeostasis in mice lacking LXRβ, ABCA1/ABCG1 or ApoA-1/LDL receptor31, 35–38. SR-BI is an HDL receptor which mediates intracellular uptake of cholesterol from HDL. This raises a possibility that SR-BI may modulate lymphocyte homeostasis via regulating cellular cholesterol levels. To address this speculation, we isolated T and B cells from the spleen and analyzed their cholesterol contents. No difference in free or esterified cholesterol levels was found between SR-BI null and wild type littermates (Supplement Fig. 5a). We also assessed the plasma membrane cholesterol level of T and B cells by filipin staining and no difference was observed between SR-BI null and wild type control mice (Supplement Fig. 5b). Our finding is consistent with a recent report by Ji et al demonstrating that SR-BI expression enhances both cell cholesterol efflux and cholesterol influx from HDL, but does not lead to altered cellular cholesterol mass.39 These data suggest that the impaired lymphocyte homeostasis may not be caused by a change in cellular cholesterol contents.
Regulatory T cells (Tregs) and regulatory B cells (Bregs) play important role in lymphocyte proliferation and activation. To examine whether SR-BI deficiency affects Tregs or Bregs, we quantified Tregs and Bregs using CD4+CD25+Foxp3+ and CD19+CD1dhighCD5+ as markers, respectively. As shown in Supplement Fig. 6, SR-BI null mice displayed no change in the percentage of Trges and a moderate increase in the number of Tregs; the percentage of Bregs was significantly decreased but the number of Bregs remained unchanged. Further investigation is required to determine a role of SR-BI in regulatory T cells and regulatory B cells and their contribution to lymphocyte homeostasis.
Early studies showed that mice lacking SR-BI exhibit extramedullary erythropoiesis due to defects in erythrocyte maturation40–41. To assess the contribution of the erythrocytes to the observed splenic hypercellularity, we quantified erythrocytes (Ter119+) with FACS in the splenocyte suspension after ACK treatment and found that about 14% of the splenocytes in SR-BI null mice were Ter119+ cells while only 3% of the splenocytes in wild type littermates were Ter119+ cells (Supplement Fig. 7a). Further analysis showed that most of the Ter119+ cells were premature erythrocytes (Supplement Fig. 7b). Thus, the accumulation of erythrocytes accounted for about a 10% increase in splenocyte populations after ACK treatment. We also prepared single splenocyte suspension without ACK lysis to determine erythrocyte populations. As shown in supplemental Fig. 7c and d, SR-BI null mice had a 20% increase in Ter119+ cells compared with wild type littermates (48.2% ± 9.6% in SR-BI−/− vs. 28.5% ± 3.2% in SR-BI+/+), and a great portion of the erythrocytes were premature, as illustrated by a significant increase in the ratio of early (Ter119highCD71high, region II)-to-late (Ter119highCD71low, region IV) phase erythrocytes in SR-BI null mice. Thus, accumulation of erythrocytes accounted for about 20% increase in total splenic cellularity observed in SR-BI null mice. These data confirmed the presence of extramedullary erythropoiesis in the spleens of SR-BI null mice, but it only partly contributed to the observed splenic hypercellularity. It is of interest to determine whether and how extramedullary erythropoiesis contributes to the impaired lymphocyte homeostasis in SR-BI null mice. With the current available evidence, it is difficult to establish a causal relationship between impaired lymphocyte homeostasis and extramedullary erythropoiesis, which warrants further investigations.
In summary, SR-BI plays critical roles in modulating lymphocyte activation, proliferation and cytokine production and its deficiency leads to impaired lymphocyte homeostasis and autoimmune disorders. Our findings reveal a previously unrecognized role of SR-BI in adaptive immunity.
Supplementary Material
Acknowledgments
We thank Dr. Subbarao Bondada of the University of Kentucky for his invaluable advice.
Funding Sources
This work was supported by grants from American Heart Association (0530241N), NIH (R01GM085231) and the Children’s Miracle Network.
Nonstandard abbreviations
- BSA
bovine serum albumin
- BrdU
bromodeoxyuridine
- Con A
concanavalin A
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- IFN-g
interferon-gamma
- IL-4
interleukin-4
- IL-6
Interleukin-6
- LPS
lipopolysacharide
- PMA
phorbol-12 myristate 13-acetate
- SR-BI
scavenger receptor BI
- TLR4
toll like receptor 4
- TNF
tissue necrosis factor
Footnotes
Disclosures
None
References
- 1.Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 2.Krieger M. CHARTING THE FATE OF THE “GOOD CHOLESTEROL”: Identification and Characterization of the High-Density Lipoprotein Receptor SR-BI. Annu Rev Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- 3.Saddar S, Mineo C, Shaul PW. Signaling by the High-Affinity HDL Receptor Scavenger Receptor B Type I. Arterioscler Thromb Vasc Biol. 2010;30:144–150. doi: 10.1161/ATVBAHA.109.196170. [DOI] [PubMed] [Google Scholar]
- 4.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 5.Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414–417. doi: 10.1038/387414a0. [DOI] [PubMed] [Google Scholar]
- 6.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. PNAS. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraemer FB. Adrenal cholesterol utilization. Mol Cell Endocrinol. 2007;265–266:42–45. doi: 10.1016/j.mce.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Arai T, Wang N, Bezouevski M, Welch C, Tall AR. Decreased Atherosclerosis in Heterozygous Low Density Lipoprotein Receptor-deficient Mice Expressing the Scavenger Receptor BI Transgene. J Biol Chem. 1999;274:2366–2371. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- 9.Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- 10.Kozarsky KF, Donahee MH, Glick JM, Krieger M, Rader DJ. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol. 2000;20:721–727. doi: 10.1161/01.atv.20.3.721. [DOI] [PubMed] [Google Scholar]
- 11.Yu H, Zhang W, Yancey PG, Koury MJ, Zhang Y, Fazio S, Linton MF. Macrophage apolipoprotein E reduces atherosclerosis and prevents premature death in apolipoprotein E and scavenger receptor-class BI double-knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:150–156. doi: 10.1161/01.ATV.0000194096.89476.73. [DOI] [PubMed] [Google Scholar]
- 12.Van Eck M, Twisk J, Hoekstra M, Van Rij BT, Van Der Lans CA, Bos IS, Kruijt JK, Kuipers F, Van Berkel TJ. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and the liver. J Biol Chem. 2003;278:23699–23705. doi: 10.1074/jbc.M211233200. [DOI] [PubMed] [Google Scholar]
- 13.Vergeer M, Korporaal SJ, Franssen R, Meurs I, Out R, Hovingh GK, Hoekstra M, Sierts JA, Dallinga-Thie GM, Motazacker MM, Holleboom AG, Van Berkel TJ, Kastelein JJ, Van Eck M, Kuivenhoven JA. Genetic variant of the scavenger receptor BI in humans. N Engl J Med. 2011;364:136–145. doi: 10.1056/NEJMoa0907687. [DOI] [PubMed] [Google Scholar]
- 14.West M, Greason E, Kolmakova A, Jahangiri A, Asztalos B, Pollin TI, Rodriguez A. Scavenger Receptor Class B Type I Protein as an Independent Predictor of High-Density Lipoprotein Cholesterol Levels in Subjects with Hyperalphalipoproteinemia. J Clin Endocrinol Metab. 2009;94:1451–1457. doi: 10.1210/jc.2008-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yates M, Kolmakova A, Zhao Y, Rodriguez A. Clinical impact of scavenger receptor class B type I gene polymorphisms on human female fertility. Human Reproduction. 2011 doi: 10.1093/humrep/der124. [DOI] [PubMed] [Google Scholar]
- 16.Li XA, Titlow WB, Jackson BA, Giltiay N, Nikolova-Karakashian M, Uittenbogaard A, Smart EJ. High density lipoprotein binding to scavenger receptor, Class B, type I activates endothelial nitric-oxide synthase in a ceramide-dependent manner. J Biol Chem. 2002;277:11058–11063. doi: 10.1074/jbc.M110985200. [DOI] [PubMed] [Google Scholar]
- 17.Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 18.Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High Density Lipoprotein-induced Endothelial Nitric-oxide Synthase Activation Is Mediated by Akt and MAP Kinases. J Biol Chem. 2003;278:9142–9149. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- 19.Gong M, Wilson M, Kelly T, Su W, Dressman J, Kincer J, Matveev SV, Guo L, Guerin T, Li XA, Zhu W, Uittenbogaard A, Smart EJ. HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner. J Clin Invest. 2003;111:1579–1587. doi: 10.1172/JCI16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li XA, Guo L, Dressman JL, Asmis R, Smart EJ. A Novel ligand-independent apoptotic pathway induced by scavenger receptor class B, type I and suppressed by endothelial nitric-oxide synthase and high density lipoprotein. J Biol Chem. 2005;280:19087–19096. doi: 10.1074/jbc.M500944200. [DOI] [PubMed] [Google Scholar]
- 21.Zhu P, Liu X, Treml LS, Cancro MP, Freedman BD. Mechanism and Regulatory Function of CpG Signaling via Scavenger Receptor B1 in Primary B Cells. Journal of Biological Chemistry. 2009;284:22878–22887. doi: 10.1074/jbc.M109.018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li XA, Guo L, Asmis R, Nikolova-Karakashian M, Smart EJ. Scavenger receptor BI prevents nitric oxide-induced cytotoxicity and endotoxin-induced death. Circ Res. 2006;98:e60–65. doi: 10.1161/01.RES.0000219310.00308.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai L, Ji A, de Beer FC, Tannock LR, van der Westhuyzen DR. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J Clin Invest. 2008;118:364–375. doi: 10.1172/JCI31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo L, Song Z, Li M, Wu Q, Wang D, Feng H, Bernard P, Daugherty A, Huang B, Li XA. Scavenger Receptor BI Protects against Septic Death through Its Role in Modulating Inflammatory Response. J Biol Chem. 2009;284:19826–19834. doi: 10.1074/jbc.M109.020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK, 3rd, Wu T, Li QZ, Davis LS, Mohan C, Perlman H. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Hsu KH, Hiramoto RN, Ghanta VK. Immunosuppressive effect of mouse serum lipoproteins. II. In vivo studies. J Immunol. 1982;128:2107–2110. [PubMed] [Google Scholar]
- 27.Hsu KH, Ghanta VK, Hiramoto RN. Immunosuppressive effect of mouse serum lipoproteins. I. In vitro studies. J Immunol. 1981;126:1909–1913. [PubMed] [Google Scholar]
- 28.Morse JH, Witte LD, Goodman DS. Inhibition of lymphocyte proliferation stimulated by lectins and allogeneic cells by normal plasma lipoproteins. J Exp Med. 1977;146:1791–1803. doi: 10.1084/jem.146.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Eck M, Hoekstra M, Hildebrand RB, Yaong Y, Stengel D, Kruijt JK, Sattler W, Tietge UJ, Ninio E, Van Berkel TJ, Pratico D. Increased oxidative stress in scavenger receptor BI knockout mice with dysfunctional HDL. Arterioscler Thromb Vasc Biol. 2007;27:2413–2419. doi: 10.1161/ATVBAHA.107.145474. [DOI] [PubMed] [Google Scholar]
- 30.Schulze-Luehrmann J, Ghosh S. Antigen-Receptor Signaling to Nuclear Factor [kappa]B. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Wilhelm AJ, Zabalawi M, Grayson JM, Weant AE, Major AS, Owen J, Bharadwaj M, Walzem R, Chan L, Oka K, Thomas MJ, Sorci-Thomas MG. Apolipoprotein A-I and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arterioscler Thromb Vasc Biol. 2009;29:843–849. doi: 10.1161/ATVBAHA.108.183442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 33.Manson J, Rahman A. Systemic lupus erythematosus. Orphanet Journal of Rare Diseases. 2006;1:6. doi: 10.1186/1750-1172-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoekstra M, Meurs I, Koenders M, Out R, Hildebrand RB, Kruijt JK, Van Eck M, Van Berkel TJ. Absence of HDL cholesteryl ester uptake in mice via SR-BI impairs an adequate adrenal glucocorticoid-mediated stress response to fasting. J Lipid Res. 2008;49:738–745. doi: 10.1194/jlr.M700475-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong AJ, Gebre AK, Parks JS, Hedrick CC. ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation. J Immunol. 2010;184:173–183. doi: 10.4049/jimmunol.0902372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilhelm AJ, Zabalawi M, Owen JS, Shah D, Grayson JM, Major AS, Bhat S, Gibbs DP, Jr, Thomas MJ, Sorci-Thomas MG. Apolipoprotein A-I Modulates Regulatory T Cells in Autoimmune LDLr−/−, ApoA-I−/− Mice. J Biol Chem. 2010;285:36158–36169. doi: 10.1074/jbc.M110.134130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-Binding Cassette Transporters and HDL Suppress Hematopoietic Stem Cell Proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji A, Meyer JM, Cai L, Akinmusire A, de Beer MC, Webb NR, van der Westhuyzen DR. Scavenger receptor SR-BI in macrophage lipid metabolism. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holm TM, Braun A, Trigatti BL, Brugnara C, Sakamoto M, Krieger M, Andrews NC. Failure of red blood cell maturation in mice with defects in the high-density lipoprotein receptor SR-BI. Blood. 2002;99:1817–1824. doi: 10.1182/blood.v99.5.1817. [DOI] [PubMed] [Google Scholar]
- 41.Meurs I, Hoekstra M, van Wanrooij EJ, Hildebrand RB, Kuiper J, Kuipers F, Hardeman MR, Van Berkel TJ, Van Eck M. HDL cholesterol levels are an important factor for determining the lifespan of erythrocytes. Exp Hematol. 2005;33:1309–1319. doi: 10.1016/j.exphem.2005.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.