Abstract
Background
Fatty acid absorption patterns can have a major impact on the fatty acid composition in the portal, intestinal lymph, and systemic circulation. This study sought to determine the effects of long-chain triglycerides (LCT), medium-chain triglycerides (MCT), and 2-monododecanoin (2mono) on intestinal fatty acid composition during continuous feeding over a brief period.
Methods
The lipid sources were 100% LCT, 100% MCT, a 50:50 mixture of LCT and MCT (LCT/MCT), and a 50:50 mixture of LCT and 2mono (LCT/2mono). A total of 27 rats were randomly given 1 of the 4 diets at 200 kcal/kg/d, with 30% of total calories from lipids over 3 hours.
Results
MCT significantly increased each of the medium-chain fatty acids (C6:0, C8:0, and C10:0) as free fatty acids in the portal vein and about 10%/mol of C10:0 as triglycerides in the lymph compared with the other groups. There was significantly less C10:0 in lymphatic triglycerides with LCT/MCT than with MCT, but more than in the LCT and LCT/2mono diets. MCT also significantly increased the contents of C16:0, C18:0, C18:1, and C20:4 in the lymphatic triglycerides compared with all other groups including LCT/MCT. The amount of linoleic acid (C18:2) in lymphatic triglycerides followed the relative amounts of this fatty acid in the diet, with the greatest in LCT followed by LCT/MCT and LCT/2mono and least in MCT. A so-called structured lipid composed of the medium-chain fatty acid dodecanoic acid on the 2 position and long-chain fatty acids on the 1 and 3 positions appeared to be endogenously synthesized in response to the LCT/2mono diet.
Conclusions
The original differences in MCT and LCT content in the diets were preserved in the fatty acid composition in the intestinal free fatty acids and triglycerides during feeding. In addition, the duration of lipid administration can play a role in altering fatty acid composition in the intestine.
Keywords: long-chain fatty acids (LCFA), medium-chain fatty acids (MCFA), 2-monododecanoin
The hydrolysis of dietary triglycerides starts in the stomach by lingual lipase and continues in the duodenum with gastric and pancreatic lipases. All these lipases preferentially hydrolyze the 1 and 3 ester bonds of the triglycerides and also show higher activity for short- and medium-chain fatty acids (MCFA) than for long-chain fatty acids (LCFA), producing 2 free fatty acids and one 2-monoglyceride per triglyceride acted upon.1–4 Therefore, dietary medium-chain triglycerides (MCT) and long-chain triglycerides (LCT) have very different metabolic pathways in digestion and absorption.
MCT are more likely to be degraded into 3 fatty acids and the simple glycerol backbone, or to be absorbed intact, and are also more readily oxidized to carbon dioxide or converted to 2 carbon fragments.1–4 MCFA absorbed from the small intestinal cells are transferred directly into the portal vein for transport to the liver for hepatic metabolism. In contrast, LCFA are usually reesterified into LCT from the absorbed LCFA and 2-monoglycerides, and incorporated into chylomicrons that then enter the lymphatic system. The chylomicron triglycerides largely retain the original LCFA on the 2 position.5 Moreover, LCFA that are reesterified to triacylglycerols are either oxidized for energy or stored in adipose tissue, mostly retaining their original identity in the 2 position. Such very different absorption patterns for MCT and LCT certainly can have a major impact on the fatty acid composition of various lipid components in the portal, intestinal lymph, and systemic circulation.
Bolus injections of pure oils or isotope-labeled fatty acids have been widely used to trace the changes in the metabolism of specific fatty acids. During more normal feeding practice, however, the lipids are mixed with other nutrients and given constantly over a period of time. In addition, the amount of lipids would be relatively smaller than those used by bolus injection. One study from our laboratory showed that the continuous feeding of 100% MCT at 250 kcal/kg/d as the principal lipid source (30% of total energy from MCT) in nutrition solution for 20 hours did not lead to the appearance of MCFA in portal vein free fatty acids and plasma triglycerides.6 Thus, the impact of various dietary lipids on fatty acid absorption and metabolism is still not clearly defined and deserves further investigation.
We undertook the present study to determine the effects of different lipids in nutrition solutions on intestinal fatty acid composition during continuous feeding over a brief period. The feeding method used in this study was the same as used in our previous feeding study,6 except the feeding lasted only over 3 hours in this study. Free fatty acids were determined in the portal vein and triglycerides in the intestinal lymph and systemic circulation because free fatty acids are the principal mode of lipid transport in the portal vein while triglycerides are the principal lipid component in the lymph and systemic circulation. A short collection time was used to minimize endogenous contributions to fatty acid composition. The results were compared with those published previously.6 In addition, we have also evaluated the stability of the 2 position of glycerides using a transition fatty acid, dodecanoic acid (C12:0), between MCFA and LCFA, as a factor in the digestion and absorption of lipids.
Materials and Methods
Animals and Sample Collection
Male Sprague-Dawley rats were purchased from Taconic Farms (Germantown, New York) at a weight of 250–270 g. Rats were housed in individual cages and exposed to a 12-hour light-dark cycle under controlled temperature (21 °C ± 2 °C) for 5 days. During this adaptation period, a standard laboratory rat chow diet and water were provided.
A total of 27 rats had silastic catheters (0.025 ID × 0.047 OD; Dow Corning Laboratory, Corning, New York) surgically implanted into the antrum of the stomach and advanced ~1 cm beyond the pylorus into the small intestine under ethyl ether anesthesia. The catheter was extended subcutaneously and exteriorized from the midscapular region, then connected to a flow-through swivel (Instech Laboratories, Philadelphia, Pennsylvania) so that the catheter was protected from rat movements and allowed for infusion. After surgery, rats were returned to individual cages for recovery. During the recovery, saline was constantly infused into the small intestinal cannula at 2 mL/h, and rat chow and water were provided. After 24 hours of recovery from the surgery, the rat chow was removed, but rats were continuously infused with saline while being fasted.
On the following morning, after at least 16 hours of fasting, rats were randomly given 4 nutrition solutions with different lipid sources through their small intestine cannula. According to rat sizes, rats were given 200 kcal/kg/d, with 30% of total calories from lipid sources. L-Emental enteral nutrition (Nutrition Medical, Buffalo, Minnesota) was used as a base formula. L-Emental enteral nutrition is a chemically defined diet intended for clinical use, providing adequate amounts of all essential nutrients and containing protein as amino acids, carbohydrate as glucose, and very low fat. One hundred grams of L-Emental enteral nutrition powder contains only 0.3 g of sunflower oil. Four different lipids were then added to this base formula. As in our previous study,6 1 diet had 100% of LCT as sunflower oil, which is largely composed of linoleic acid (Sunfresh Inc, West Seneca, New York; LCT, n = 6). Based on the molar quantity of LCFA in the LCT diet, other diets contained isomolar amounts of 100% of MCT (Mead Johnson, Evansville, Indiana; MCT, n = 7), 50:50 by moles of fatty acids of LCT and MCT (LCT/MCT, n = 6), or 50:50 by moles of fatty acids of LCT and 2-monododecanoin (Stepan Company, Northfield, Illinois; LCT/2mono, n = 8). All 4 diets thus contained the same number of moles of fatty acids but slightly different calories because of the different energy contents of the lipid sources (eg, LCT contained 8.57 kcal/g, while MCT contained 8.1 kcal/g). Tables 1 and 2 list the detailed dietary information.
Table 1.
Dietary Compositions in the Experimental Diets
| Diet, g/100 mL | ||||
|---|---|---|---|---|
| LCT | MCT | LCT/MCT | LCT/2mono | |
| L-Emental nutrition | 20.08 | 24.09 | 22.07 | 20.55 |
| LCT oil | 3.75 | 0.00 | 1.88 | 1.88 |
| MCT oil | 0.00 | 2.12 | 1.07 | 0.00 |
| 2-monododecanoin | 0.00 | 0.00 | 0.00 | 1.76 |
L-Emental (Nutrition Medical, Buffalo, MN) nutrition is chemically defined diet intended for clinical use providing adequate amounts of all essential nutrients and containing protein as amino acids, carbohydrate as glucose, and which is very low in fat (100 g of L-Emental nutrition powder contains 0.3 g of sunflower oil). LCT, a diet containing 100% of long-chain triglycerides as lipid source; MCT, a diet containing 100% of medium-chain triglycerides as lipid source; LCT/MCT, a diet containing lipid source from 50:50 of LCT and MCT; LCT/2mono, a diet containing lipid source from 50:50 of LCT and 2-monododecanoin.
Table 2.
Percentage of the Major Fatty Acids in LCT Oil, MCT Oil, and 2-Monododecanoin
| Fatty Acids | LCT Oil | MCT Oil | 2-mono |
|---|---|---|---|
| 8:0 | — | 67 | — |
| 10:0 | — | 23 | — |
| 12:0 | — | — | 100 |
| 16:0 | 6 | — | — |
| 18:0 | 4 | — | — |
| 18:1 | 24 | — | — |
| 18:2 | 65 | — | — |
| Unknown | 1 | — | — |
Information was provided by the manufacturers: LCT oil from Sunfresh Inc (West Seneca, New York), MCT oil from Mead Johnson (Evansville, Indiana), and 2-monododecanoin from Stepan Company (Northfield, Illinois). LCT, long-chain triglycerides; MCT, medium-chain triglycerides; 2-mono, 2-mondodecanoin.
The nutrition solutions were continuously infused at 6.9 mL/h over 3 hours. After 1½ hours of feeding, all animals were anesthetized under ethyl ether while a silicone tube was inserted into the lymphatic duct. Since the maximum lymphatic transport of fatty acids is normally found between 2 and 4 hours after lipid administration,6–8 the collection of lymphatic fluid was started after 2 hours of feeding, as in our previous study. Under light ethyl ether anesthesia, the lymph fluid was collected (1–1.5 mL) in a heparin-coated syringe. At the end of 3 hours of feeding, a portal blood sample (1–1.5 mL) was collected by direct venipuncture of the portal vein. Mixed systemic blood was collected in EDTA-containing tubes at decapitation. Plasma was then separated. All the plasma and lymph samples were stored at −20°C until assay.
The experimental protocol was approved by the Animal Care Committee of the Beth Israel Deaconess Medical Center, which follows guidelines prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources of the National Research Council.
Analysis
The components of free fatty acids and triglycerides in plasma or lymphatic fluid were extracted according to the method reported by Folch et al.9 Briefly, 0.5 mL of systemic plasma, 0.5 mL of portal plasma, and 0.2 mL of lymph fluid were extracted with 8 mL chloroform and methanol (2:1 by volume) solution. Free tridecanoic (C13:0) and triglyceride esterified with tridecanoic (NuChek Prep, Elysian, Minnesota) were added as internal standards before the extractions. Thin-layer chromatography by use of Whatman LK5D silica-gel plates phase (Whatman Chemical Separations, Inc, Clifton, New Jersey) was performed, with a solvent system of 80:20:1 in petroleum ether:diethylether: glacial acetic acid to separate triglycerides and free fatty acids. The bands of triglycerides and free fatty acids were identified relative to the migration of standards using dichlorofluorescein spray. The bands were then isolated, scraped into Teflon screw-cap glass tubes lined with nonstick coating, and hydrolyzed and methylated under nitrogen with 14% boron trifluoride/methanol and methanol in benzene for 45 minutes in a steam bath (~45°C) in a closed system. Liquid-gas chromatography (Hewlett Packard 5890, Palo Alto, California) was finally performed to separate and quantify fatty acid species in each sample. The column used was a 50-m SP-2330 fused silica capillary column with 0.32-mm ID and 0.20-µm film thickness (Supelco, Bellefonte, Pennsylvania) and split ratio of 1.03 mL/min. Helium was used as the carrier gas, with a rate of 1.03 mL/min. The identification of fatty acid esters in the samples was obtained by comparison with the relative retention times of pure standard mixtures. Response factors for individual fatty acids in the flame ionization detector were not made. Quantitation of the fatty acids was determined by the comparison of different responses of internal standards and unknown samples with the external standards. The recovery rate of known amounts (from approximately 0.005 to 0.04 µmol/mL) of C10:0 and C18:0 under our assay conditions was 86.5% ±4.5% and 102.9% ±4.8% (mean ±SEM [standard error of mean]), respectively.6
Statistical Analysis
Data are presented as the mean ± SEM. Data from the 4 dietary groups were compared by 1-way ANOVA to identify significant differences at P < .05. The least significant difference test (LSD) was used to compare the differences among the groups when the ANOVA was found to be significant at the 95% confidence level.
Results
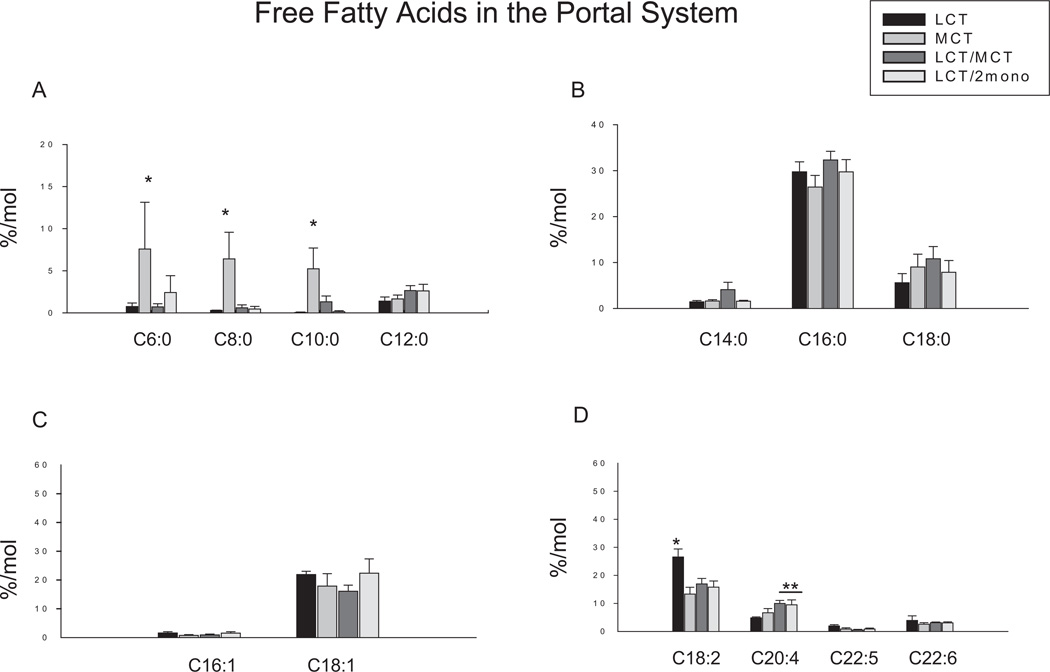
Figure 1 shows the levels of free fatty acids in the portal plasma in the different dietary groups. Trace amounts of MCFA, including caproic acid (C6:0), octanoic acid (C8:0), and decanoic acid (C10:0), were detected in the portal vein in groups fed by diets containing LCT (ie, the LCT, LCT/MCT, and LCT/2mono groups). However, significantly greater amounts of these MCFA were found in the portal vein in the MCT group. No differences were found in dodecanoic acid (C12:0); long-chain saturated fatty acids including myristic acid (C14:0), palmitic acid (C16:0), and stearic acid (C18:0); and monounsaturated fatty acids including palmitoleic acid (C16:1) and oleic acid (C18:1), among all groups. The highest levels of linoleic acid (C18:2) were found in the LCT group compared with the other 3 groups. Although this fatty acid was slightly lower in the MCT group, no statistical significances were noted among the MCT, LCT/MCT, and LCT/2mono groups. The level of arachidonic acid (C20:4) was significantly lower in the LCT group compared with the LCT/MCT and LCT/2mono groups but not the MCT group. There was no difference in C20:4 between the LCT and LCT/MCT groups. There were no differences in eicosapentaenoic acid (C20:5) and docosahexaenoic acid (C22:6) among the 4 dietary groups.
Figure 1.
Effects of different diets on fatty acid profiles in free fatty acid fraction in the portal vein plasma. Results are depicted in the bar graphs, where the data represent the mean ± SEM for 6–8 rats per group. Each bar corresponds to the group indicated by different diets. LCT, a diet containing lipid source with 100% of long-chain triglycerides (n = 6); MCT, a diet containing lipid source with 100% of medium-chain triglycerides (n = 7); LCT/MCT, a diet containing lipid source with 50:50 of long-chain triglycerides and medium-chain triglycerides (n = 6); LCT/2mono, a diet containing lipid source with 50:50 of long-chain triglycerides and 2-monododecanoin (n = 8). (A) Fatty acids with carbons ranging from 6 to 12. C6:0, caproic acid; C8:0, octanoic acid; C10:0, decanoic acid; C12:0, dodecanoic acid. (B) Saturated fatty acids. C14:0, myristic acid; C16:0, palmitic aicd; C18:0, stearic acid. (C) Monounsaturated fatty acids. C16:1, palmitoleic acid; C18:1, oleic acid. (D) Polyunsaturated fatty acids. C18:2, linoleic acid; C20:4, arachidonic acid; C20:5, eicosapentaenoic acid; C22:6, docosahexaenoic acid. *P < .05 vs all other groups; **P < .05 vs LCT group by 1-way ANOVA with least significant difference test.
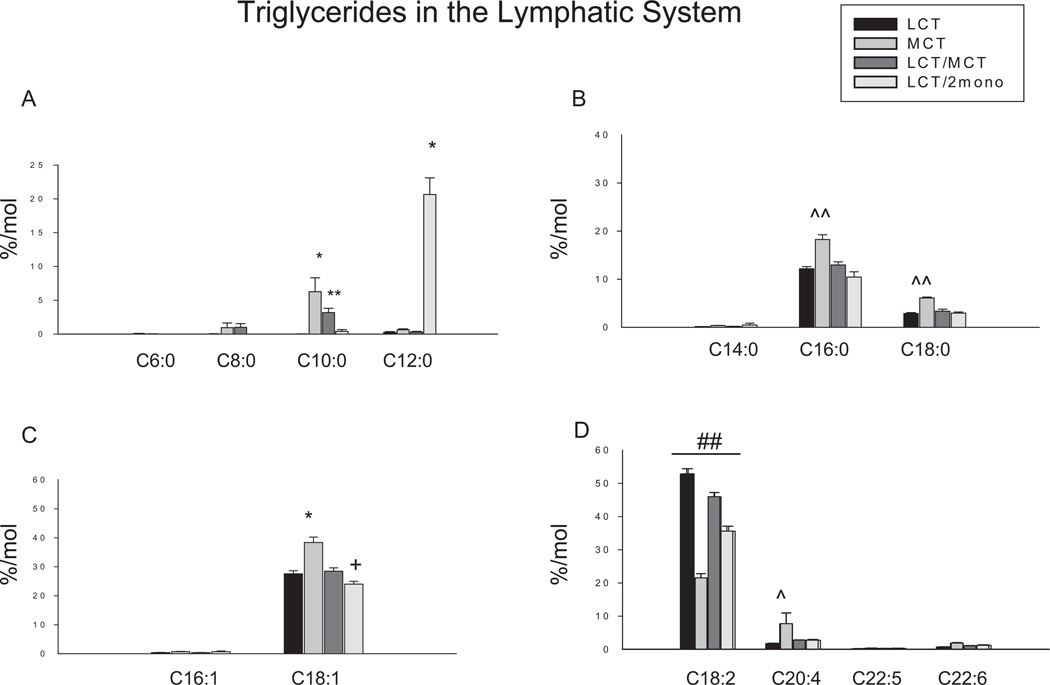
The triglyceride profiles in the lymphatic fluid are shown in Figure 2. Caproic acid (C6:0) was not detected in the lymph fluid. However, trace amounts of octanoic acid (C8:0) and < 10 mol % of decanoic acid (C10:0) in triglyceride fraction was found in lymphatic fluid in the MCT and the LCT/MCT groups with significantly more in the MCT group, but none was found in the LCT and the LCT/2mono groups. Significantly greater amounts of dodecanoic acid (C12:0) were only found in the LCT/2mono group but not in the other groups. The MCT group had the highest levels of palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1) and arachidonic acid (C20:4) compared to other 3 groups. Among LCT, LCT/MCT, and LCT/2mono groups, the amounts of these fatty acids were not different in the lymphatic triglycerides. In contrast, the MCT group had the lowest levels of linoleic acid (C18:2) compared to the other groups. Among the other 3 groups, the highest amount of linoleic acid (C18:2) in lymphatic triglycerides was found in the LCT group, followed by the LCT/MCT and the LCT/2mono groups. Although there were no differences in C18:1 between the LCT and LCT/MCT group, the levels of C18:1 in the LCT/MCT group were significantly higher than those in the LCT/2mono group. There were very small amounts of myristic acid (C14:0), palmitoleic acid (C16:1), eicosapentaenoic acid (C20:5) and docosahexaenoic acid (C22:6) in the lymphatic triglycerides and no differences were found in these fatty acids among the groups.
Figure 2.
Effects of different diets on fatty acid profiles in triglyceride fraction in the lymphatic fluid. Results are depicted in the bar graphs, where the data represent the mean ±SEM for 6–8 rats per group. Each bar corresponds to the group indicated by different diets. LCT, a diet containing lipid source with 100% of long-chain triglycerides (n = 6); MCT, a diet containing lipid source with 100% of medium-chain triglycerides (n = 7); LCT/MCT, a diet containing lipid source with 50:50 of long-chain triglycerides and medium-chain triglycerides (n = 6); LCT/2mono: a diet containing lipid source with 50:50 of long-chain triglycerides and 2-monododecanoin (n = 8). (A) Fatty acids with carbons ranging from 6 to 12. C6:0, caproic acid; C8:0, octanoic acid; C10:0, decanoic acid; C12:0, dodecanoic acid. (B) Saturated fatty acids. C14:0, myristic acid; C16:0, palmitic acid; C18:0, stearic acid. (C) Monounsaturated fatty acids. C16:1, palmitoleic acid; C18:1, oleic acid. (D) Polyunsaturated fatty acids. C18:2, linoleic acid; C20:4, arachidonic acid; C20:5, eicosapentaenoic acid; C22:6, docosahexaenoic acid. *P < .001 and **P < .001 vs all other groups; ^^P < .05 and ^P < .05 vs all other groups; +P < .05 vs LCT/MCT and ##P < .001 vs each other by 1-way ANOVA with least significant difference test.
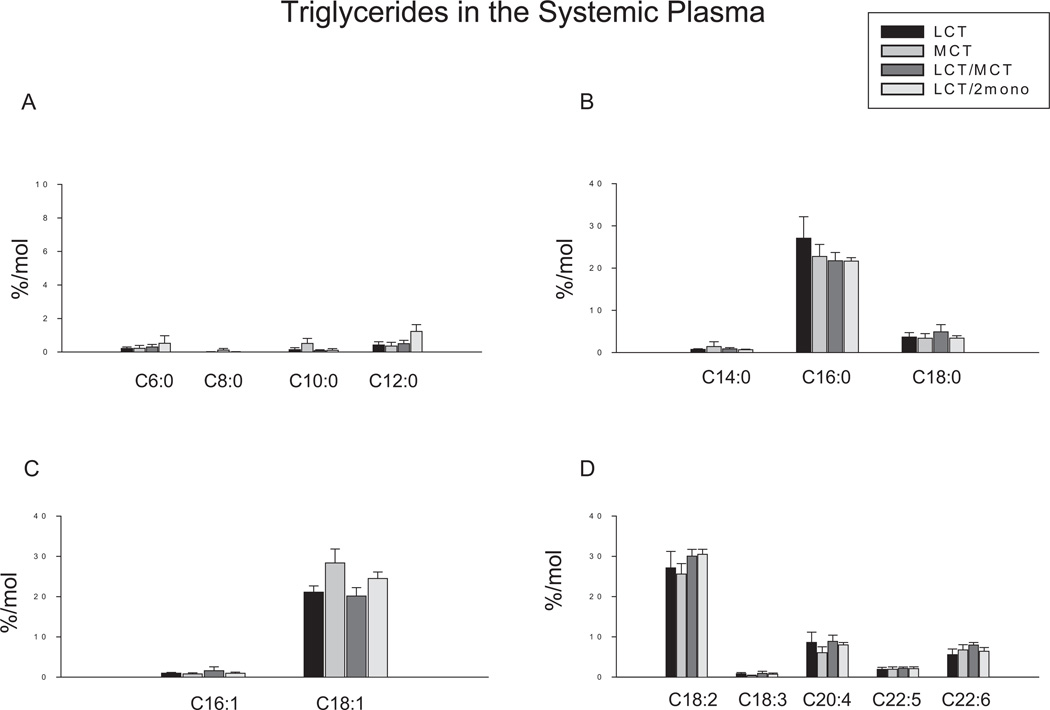
No significant differences were found in all the measured fatty acids in the triglyceride fraction in the systemic circulation (Figure 3).
Figure 3.
Effects of different diets on fatty acid profiles in triglyceride fraction in systemic plasma. Results are depicted in the bar graphs, where the data represent the mean ± SEM for 6–8 rats per group. Each bar corresponds to the group indicated by different diets. LCT, a diet containing lipid source with 100% of long-chain triglycerides (n = 6); MCT, a diet containing lipid source with 100% of medium-chain triglycerides (n = 7); LCT/MCT, a diet containing lipid source with 50:50 of long-chain triglycerides and medium-chain triglycerides (n = 6); LCT/2mono, a diet containing lipid source with 50:50 of long-chain triglycerides and 2-monododecanoin (n = 8). (A) Fatty acids with carbons ranging from 6 to 12. C6:0, caproic acid; C8:0, octanoic acid; C10:0, decanoic acid; C12:0, dodecanoic acid. (B) Saturated fatty acids. C14:0, myristic acid; C16:0, palmitic acid; C18:0, stearic acid. (C) Monounsaturated fatty acids. C16:1, palmitoleic acid; C18:1, oleic acid. (D) Polyunsaturated fatty acids. C18:2, linoleic acid; C18:3, α-linolenic acid; C20:4, arachidonic acid; C20:5, eicosapentaenoic acid; C22:6, docosahexaenoic acid.
Discussion
The present study showed that 3 hours of feeding 100% MCT as a principal lipid source significantly increased caproic acid (C6:0), octanoic acid (C8:0), and decanoic acid (C10:0) in the portal vein free fatty acids, and also significantly increased decanoic acid (C10:0) in the lymphatic triglycerides. These findings are consistent with previous published reports, using bolus administration methods, that dietary MCT are more likely to be taken up into the portal system as their constituent free fatty acids.1–4 In addition, small amounts of MCFA, particularly the longer chain ones, are directly taken up into the lymphatic system as triglycerides.1–4 Because the LCT/MCT diet contained half the amount of MCFA as in the MCT diet, less decanoic acid (10:0) was found in both the portal and lymphatic systems, suggesting that the greater the amount of MCFA in the diets, the greater the amount in the portal and lymphatic systems (Figures 1 and 2A). However, the present findings were different from those observed in our previous study.6 In that study, no MCFA were detected in the portal and lymph systems after 20 hours of feeding with similar MCT and LCT/MCT diets. Taking our 2 studies together, these findings suggest that feeding MCT may foster different pathways over time rather than traditional portal transport, perhaps related to induction of pathways such as de novo lipogenesis.
In our previous study, 20 hours of feeding with the MCT diet significantly reduced the level of linoleic acid (C18:2) in the portal fatty acids as well as in the lymphatic and plasma triglycerides to a level one-half or one-third that observed in LCT, reflecting the deficiency or absence of this essential fatty acid in the MCT diet. The significant reduction of linoleic acid (C18:2) was also found in the portal fatty acids and in the lymphatic triglycerides after 3 hours of feeding with the MCT diet in the current study, although differences in this fatty acid were not found in plasma triglycerides between the MCT and LCT groups.
The present study also showed that 3 hours of MCT feeding significantly increased the percentage of C16:0, C18:0, C18:1, and C20:4 in the lymphatic triglycerides. Because the MCT feeding did not provide these specific LCFA and the study was conducted in the fed state where feeding was continuous, the higher levels of these fatty acids—C16:0, C18:0, and C18:1—were likely to be the result of MCT-enhanced de novo lipogenesis. Although it has been well documented that MCT increase longer-chain fatty acids such as C16:0, C18:0, and C18:1 by using radiolabeled fatty acid methods,10–13 the current findings might further suggest that the process of MCT-enhanced de novo lipogenesis can be observed under the condition in which MCT was constantly provided from the diet, and the diet itself was lipogenic related to the high glucose content and limited amount of LCT. The total amount of lymphatic triglycerides in the MCT group was the lowest among all groups in the current study (data not shown), presumably reflecting the principal route of MCFA transport by the portal vein. The increase of C20:4 in lymphatic triglycerides in the MCT group along with the significantly lower content of C18:2 presumably reflected the enhanced elongation and desaturation of C18:2.
Interestingly, the levels of C16:0, C18:0, C18:1, and C20:4 in the lymphatic triglycerides in the LCT/MCT were not significantly different from those in the LCT group, suggesting the processes of increased desaturation and elongation and de novo lipogenesis from the MCT diet are modulated and/or inhibited by adding LCT into the diet.
The present results also showed that there were no substantial differences found between LCT/MCT and LCT/2mono, except for the levels of dodecanoic acid (C12:0), oleic acid (C18:1), and linoleic acid (C18:2) in the lymphatic triglycerides (Figures 2A, 2C, and 2D). The elevated level of dodecanoic acid (C12:0) presumably reflects the presence of this fatty acid in the LCT/2 mono diet. However, the C12:0 was located only on the 2 position as 2-monododecanoin in the LCT/2mono diet. Thus, the precise mechanism of this elevation is not known, but a consideration of pathways of fatty acid absorption yields a possible explanation. Early studies have reported that approximately 75% of C12:0 initially located on the 2 position is conserved on this position during hydrolysis, suggesting that dodecanoic acid acts somewhat like a LCFA in this regard.14,15 In addition, it has also been reported that LCFA (chain length >12 carbons) released by hydrolysis in the intestine are reesterified in the 1 and 3 positions by the enterocyte to the preserved 2-monoglyceride absorbed from the intestine as the principal pathway of intestinal triglyceride production.5,16,17 It is likely that only a limited amount of the dodecanoic acid from the LCT/2mono diet is released by hydrolysis and absorbed into the portal vein as free fatty acid because there were no differences noted among the 4 groups in dodecanoic acid in the portal vein. Thus, the 2-monododecanoic acid appears to serve as the template for the LCFA from the LCT to occupy the 1 and 3 positions of the triglycerides.18 In such a manner, a so-called structured triglyceride composed of a transitional MCFA, dodecanoic acid, on the 2 position and LCFA on the 1 and 3 position can be endogenously synthesized in the LCT/2mono group. This interpretation is supported by the evidence that the amounts of linoleic acid (18:2) and oleic acid (18:1) in the lymphatic triglycerides were significantly lower in the LCT/2mono group compared with the LCT/MCT group. Although these 2 diets had the same molar amounts of LCFA, the MCFA from the LCT/MCT diet do not serve as a source of 2-monoglycerides to form lymphatic triglycerides. Therefore, the LCFA from the LCT/MCT diet would dominate the composition of lymphatic triglycerides. Certainly, more studies are needed to define these possibilities.
In conclusion, the original differences in MCT and LCT content in the diets were preserved in the fatty acid composition of the intestinal free fatty acids and triglycerides during feeding. Although the amount and duration of lipid administration can play a role as well in altering fatty acid composition in the intestine, it must be confirmed by a crossover study or a study with a longer period of feeding with different times of sampling. Because the flow rates of portal blood and lymphatic fluid were not determined in this study, the absolute amounts of fatty acid absorbed in different sites cannot be quantified in this study. Moreover, de novo lipogenesis may be an obligatory metabolic pathway in response to dietary MCT in the setting of high carbohydrate feeding, but the contribution of endogenous de novo lipogenesis occurring in the intestine to overall triglyceride production could not be defined, at least under the current experimental conditions.
Footnotes
Financial disclosure: none declared.
References
- 1.Bloom B, Chaikoff IL, Reinhardt WO. Intestinal lymph as pathway for transport of absorbed fatty acids of different chain lengths. Am J Physiol. 1951;166:451–455. doi: 10.1152/ajplegacy.1951.166.2.451. [DOI] [PubMed] [Google Scholar]
- 2.Carey MC, Small DM, Bliss CM. Lipid digestion and absorption. Annu Rev Physiol. 1983;45:651–677. doi: 10.1146/annurev.ph.45.030183.003251. [DOI] [PubMed] [Google Scholar]
- 3.Mattson FH, Volpenhein RA. The digestion and absorption of triglycerides. J Biol Chem. 1964;239:2772–2777. [PubMed] [Google Scholar]
- 4.Bach AC, Babayan VK. Medium-chain triglycerides: an update. Am J Clin Nutr. 1982;36:950–962. doi: 10.1093/ajcn/36.5.950. [DOI] [PubMed] [Google Scholar]
- 5.Kayden HJ, Senior JR, Mattson FH. The monoglyceride pathway of fat absorption in man. J Clin Invest. 1967;46:1695–1703. doi: 10.1172/JCI105660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.You YQ, Ling PR, Qu Z, Bistrian BR. Effects of continuous enteral medium-chain fatty acid infusion on lipid metabolism in rats. Lipids. 1998;33:261–266. doi: 10.1007/s11745-998-0204-z. [DOI] [PubMed] [Google Scholar]
- 7.Porsgaard T, Straarup EM, Hoy CE. Gastric emptying in rats following administration of a range of different fats measured as acetaminophen concentration in plasma. Ann Nutr Metab. 2003;47:132–138. doi: 10.1159/000070035. [DOI] [PubMed] [Google Scholar]
- 8.Posgaard T, Straarup EM, Hoy CE. Lymphatic fatty acid absorption profile during 24 hours after administration of triglycerides to rats. Lipids. 1999;34:103–107. doi: 10.1007/s11745-999-0342-3. [DOI] [PubMed] [Google Scholar]
- 9.Folch J, Lees M, Stoane SGH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 10.Leveille GA, Pardini RS, Tillotson JA. Influence of medium-chain triglycerides on lipid metabolism in rat. Lipids. 1967;2:287–294. doi: 10.1007/BF02532113. [DOI] [PubMed] [Google Scholar]
- 11.Kritchevsky D, Tepper SA. Influence of medium-chain triglycerides (MCT) on cholesterol metabolism in rats. J Nutr. 1965;86:67–72. doi: 10.1093/jn/86.1.67. [DOI] [PubMed] [Google Scholar]
- 12.Crozier GL. Medium-chain triglyceride feeding over the long term: the metabolic fate of [14C]octanoate and [14C]oleate in isolated rat hepatocytes. J Nutr. 1988;118:297–304. doi: 10.1093/jn/118.3.297. [DOI] [PubMed] [Google Scholar]
- 13.Hill JO, Peters JC, Swift LL, et al. Changes in blood lipids during six days of overfeeding with medium or long chain triglycerides. J Lipids Res. 1990;31:407–416. [PubMed] [Google Scholar]
- 14.Jensen MM, Christensen MS, Hoy CE. Intestinal absorption of octanoic, decanoic, and linoleic acids:effect of triglyceride structure. Ann Nutr Metab. 1994;38:104–116. doi: 10.1159/000177799. [DOI] [PubMed] [Google Scholar]
- 15.Akessom B, Gronowitz S, Herslof B, Ohlson R. Absorption of synthetic, stereochemically defined acylglycerols in the rat. Lipids. 1978;13:338–343. doi: 10.1007/BF02533725. [DOI] [PubMed] [Google Scholar]
- 16.Christiansen EN, Rortveit T, Norum KR, Thomassen MS. Fatty acid chain elongation in rat small intestine. Biochem J. 1986;237:293–295. doi: 10.1042/bj2370293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayden HJ, Senior JR, Mattson FH. The monoglyceride pathway of fat absorption in man. J Clin Invest. 1967;46:1695–1703. doi: 10.1172/JCI105660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen GL, McGarvey N, Taraszewski R, et al. Lymphatic absorption of enterally fed structured triacylglycerol vs physical mix in a canine model. Am J Clin Nutr. 1994;60:518–524. doi: 10.1093/ajcn/60.4.518. [DOI] [PubMed] [Google Scholar]