Abstract
OBJECTIVE: To examine the association between levels of fasting plasma glucose (FPG) and incidence of stroke outcomes in a large cohort of asymptomatic men.
PATIENTS AND METHODS: Participants were 43,933 men (mean ± SD age, 44.3±9.9 years) who were free of known cardiovascular disease at baseline and whose FPG levels were assessed during a preventive medical examination at the Cooper Clinic, Dallas, TX, between January 7, 1971, and March 11, 2002. Patients with diagnosed diabetes mellitus (DM) or low FPG (<80 mg/dL [to convert to mmol/L, multiply by 0.0555]) were excluded. Fatal stroke was identified through the National Death Index, and nonfatal stroke was ascertained from mail-back surveys.
RESULTS: A total of 595 stroke events (156 fatal and 456 nonfatal strokes; 17 men reported a nonfatal stroke before they died of stroke) occurred during 702,928 person-years of exposure. Age-adjusted fatal, nonfatal, and total stroke event rates per 10,000 person-years for normal FPG (80-109 mg/dL), impaired fasting glucose (110-125 mg/dL), and undiagnosed DM (≥126 mg/dL) were 2.1, 3.4, and 4.0 (Ptrend=.002); 10.3, 11.8, and 18.0 (Ptrend=.008); and 8.2, 9.6, and 12.4 (Ptrend=.008), respectively. After further adjusting for potential confounders, the direct association between FPG and fatal, nonfatal, or total stroke events remained significant (Ptrend=.02, .03, and .01, respectively). For FPG levels of 110 mg/dL or greater, each 10-unit increment of FPG was associated with a 6% higher risk of total stroke events (P=.05).
CONCLUSION: Hyperglycemia (FPG, ≥110 mg/dL), even below the DM threshold (such as with impaired fasting glucose), was associated with a higher risk of fatal, nonfatal, or total stroke events in asymptomatic men.
ACLS = Aerobics Center Longitudinal Study; ADA = American Diabetes Association; BMI = body mass index; CI = confidence interval; CVD = cardiovascular disease; DM = diabetes mellitus; ECG = electrocardiography; FPG = fasting plasma glucose; ICD = International Classification of Diseases; IFG = impaired fasting glucose; MetS = metabolic syndrome; NDI = National Death Index
The primary causes of death and disability in patients with diabetes mellitus (DM) are cardiovascular and cerebrovascular complications.1-5 Previous studies have reported an independent and direct association of clinically diagnosed DM and stroke2,5-7; however, about half of all patients with type 2 DM are undiagnosed because they remain asymptomatic for long periods.8 Those with undiagnosed DM often have elevated levels of fasting plasma glucose (FPG) but no symptoms of DM. To date, few studies have examined the effect of FPG on stroke events, and the findings are inconclusive.6,9-13 Some have reported a positive association between elevated FPG levels and stroke,9,10 whereas others failed to identify impaired glucose levels as a significant risk predictor for stroke.6,11-13 The inconsistent findings may be due to differences in study populations, length of follow-up, stroke outcome definition (such as fatal, nonfatal, or a combination), confounders selection, or a combination of these factors.
For editorial comment, see page 1038
In 2003, the American Diabetes Association (ADA) recommended changing the lower limit for the diagnosis of impaired fasting glucose (IFG) from 110 to 100 mg/dL (to convert to mmol/L, multiply by 0.0555).14 However, the need for this change has since been questioned, and concerns have been raised regarding the potential public health implications.15-18 Therefore, we used the widely accepted 1997 ADA guidelines19 to define the IFG (FPG, 110-125 mg/dL) and DM (FPG, ≥126 mg/dL). We evaluated the association between FPG (including undiagnosed DM and IFG, as well as lower levels of FPG [100-109 mg/dL]) and fatal, nonfatal, and fatal/nonfatal combined stroke in a large cohort of men while controlling for cardiorespiratory fitness, an independent predictor of mortality and morbidity.20,21
PATIENTS AND METHODS
A total of 47,865 men from the Aerobics Center Longitudinal Study (ACLS) underwent a preventive medical examination at the Cooper Clinic in Dallas, TX, between January 7, 1971, and March 11, 2002. Patients came to the clinic for medical evaluation and health promotion counseling. Most of the study participants were college graduates from middle to upper socioeconomic strata and were employed in or retired from professional positions. More than 95% of them were non-Hispanic whites; they were referred by their employers or personal physicians or were self-referred. The study was approved annually by the Cooper Institute Institutional Review Board, and all participants gave written informed consent before data collection.
Participants were excluded from the current study if at baseline they were either younger than 20 years or older than 90 years (n=883); they had prevalent myocardial infarction (n=394), stroke (n=70), or cancer (n=705); they were missing the measurement of FPG (n=345); or they were missing any of the covariates (n=310). We also excluded men with an FPG of less than 80 mg/dL (n=369) and those who were previously diagnosed as having DM (n=582) or reported current therapy with insulin or hypoglycemic treatment (n=274) at baseline. These selection criteria resulted in 43,933 asymptomatic men aged between 20 and 87 years, who were followed up from their baseline date until the date of stroke death (identified from the National Death Index [NDI]), the date of a reported stroke event (identified from mail-back surveys), or the end of 2004. The men in the current analyses were similar to the overall ACLS cohort (data not shown), and the death rate for the men in the current study was not materially different from the age-adjusted rates for the overall cohort.
Clinical Examination
The baseline assessment, which was performed after an overnight fast of 12 hours, included a physical examination; clinical evaluations such as blood chemistry analyses and blood pressure measurements; questionnaires on personal and family medical history, smoking and drinking habits; and an exercise test. Briefly, body mass index (BMI) was calculated from measured weight and height (kg/m2), and alcohol consumption was quantified as drinks per week. One drink was standardized to 341 mL (12 oz) of beer, 142 mL (5 oz) of wine, or 43 mL (1.5 oz) of hard liquor. Resting blood pressure was measured by standard auscultatory methods after at least 5 minutes of seated rest and recorded as the average of at least 2 readings separated by 2 minutes.22 Hypertension was defined as systolic or diastolic blood pressure of 140/90 mm Hg or greater or a history of hypertension. Hypercholesterolemia was defined as serum total cholesterol of 240 mg/dL or greater (to convert to mmol/L, multiply by 0.0259). Smoking category (never, former, and current smokers), alcohol consumption (drinks/week), physical activity (sedentary or active), and family history of cardiovascular disease (CVD) were obtained from a standardized questionnaire. Cardiorespiratory fitness was defined as the total duration of a maximal tread-mill test using a modified Balke protocol.23 Abnormal findings on electrocardiography (ECG) were largely defined as rhythm and conduction disturbances and ischemic ST-T wave abnormalities, as described elsewhere.24
Serum samples were analyzed in a laboratory certified by the Centers for Disease Control and Prevention Lipid Standardization program. Undiagnosed type 2 DM and IFG were defined according to 1997 ADA criteria19: an FPG of 126 mg/dL or greater for type 2 DM and an FPG of 110 to 125 mg/dL for IFG. In addition, FPG levels were categorized into 6 ranges, several of which may be clinically relevant (80-89, 90-99, 100-109, 110-125, 126-150, and >150 mg/dL).
Stroke Outcomes Ascertainment
Total stroke (fatal and nonfatal stroke combined) was the primary outcome. Secondary outcomes were fatal and nonfatal stroke events, considered separately. The NDI was the primary source for identifying stroke death. The underlying cause of death was obtained using the International Classification of Diseases, Ninth Revision (ICD-9) codes 430 to 434 and 436 to 438 for deaths occurring before 1999 and the Tenth Revision (ICD-10) codes I60 to I69 for deaths occurring between 1999 and 2003. The accuracy of stroke mortality data from the NDI is similar to that of data from an Endpoints Review Committee that reviews both death certificates and relevant medical records to make its determination. Sesso et al25 compared causes of death determined by an Endpoints Committee with those from NDI in the Physicians Health study for deaths occurring between 1982 and 1998. For NDI, the sensitivity for stroke mortality was 89%, and the specificity was 100%. However, the classification and rule changes between ICD-9 and ICD-10 have resulted in the shifting of deaths away from some underlying cause-of-death categories into others, and the number of deaths due to stroke has increased with the implementation of ICD-10.26 Most of the deaths added to stroke in IDC-10 were classified as pneumonia in ICD-9.
The nonfatal stroke was confirmed from mail-back health surveys administered in 1982, 1986, 1990, 1995, 1999, and 2004. The cumulative survey response rate across all survey periods in the ACLS was approximately 70%. Nonresponse bias is a concern in epidemiological surveillance, and this issue has been investigated in the ACLS cohort.18 When data for decedents were excluded, the baseline health status and clinical measurements were similar between those who did and did not respond to the survey and those who responded early vs late to the survey. Total mortality rates were also similar between those who did and did not respond to the survey (unpublished data). These observations suggest that those who did and did not respond to the survey generally are more similar than not; however, it is impossible to completely rule out potential response bias.
On these surveys, participants were asked to report whether a health care professional had ever told them that they had a stroke. If they answered yes, they were then asked to write down the diagnosis year. No information was obtained on stroke subtype. For those who reported more than 1 stroke event, the first was used for this study. In the current analysis, there were 24,037 men with at least 1 mail-back survey. Seventeen participants died of stroke after they reported a nonfatal event. In a random sample of 50 reported stroke events to which we applied a standard definition for adjudicating stroke,27 the agreement between participant medical records and reported stroke events was close to 90%. We have used this method of case ascertainment in earlier ACLS articles.21,28,29 Other large epidemiological studies used the same method to identify stroke events.30
Statistical Analyses
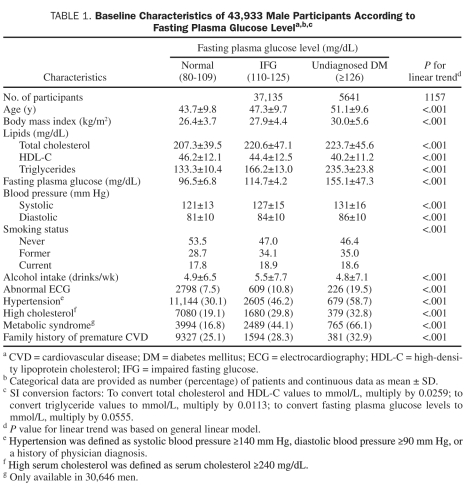
Baseline characteristics of the study population were stratified by FPG levels (Table 1). Tests for linear trends across FPG categories were calculated using general linear models. Person-time for each participant was computed from the baseline date to the date of stroke death, the date of a reported stroke event, or the end of 2004. The mean ± SD follow-up time was 15.3±8.9 years for fatal stroke and 17.7±8.8 years for nonfatal stroke. Stroke incident rates were calculated as person-time follow-up divided by the number of cases. Cox proportional hazards regression models were used to estimate hazard ratios, the associated 95% confidence intervals (CIs), and event rates (stroke outcomes per 10,000 person-years of follow-up). The proportional hazards assumption was examined and satisfied by comparing the log-log survival plots grouped on FPG categories.
TABLE 1.
Baseline Characteristics of 43,933 Male Participants According to Fasting Plasma Glucose Levela,b,c
Covariates included in the multivariable analyses were age, year of baseline examination, smoking habits (never, former, and current smoker), alcohol consumption (drinks per week), BMI (kg/m2), total serum cholesterol level (mg/dL), hypertension, abnormal findings on ECG, family history of CVD, and survey indicator (for nonfatal and total stroke outcomes). Indicator variables (did not respond/responded) for each of the 6 survey periods were constructed to account for differences in survey response frequency in order to standardize for surveillance period and follow-up time, therefore reducing the influence of ascertainment bias. This is a standard approach for taking into account the differences in survey response patterns among study participants.31 All statistical tests were 2-sided, and P<.05 was accepted to indicate statistical significance using SAS software, version 9.2 (SAS Institute, Cary, NC).
RESULTS
A total of 595 stroke events (156 fatal and 456 nonfatal strokes; 17 patients reported a nonfatal stroke event before they died of stroke) occurred during 702,928 person-years of follow-up. At baseline, 12.8% and 2.6% of men had IFG and undiagnosed DM, respectively. Levels of FPG were directly associated with age, BMI, and other clinical risk factors (Table 1). Also, FPG levels had an inverse association with never smokers and a positive association with alcohol intake (Table 1); however, because of the large sample size, the clinical relevance of these results needs to be considered cautiously
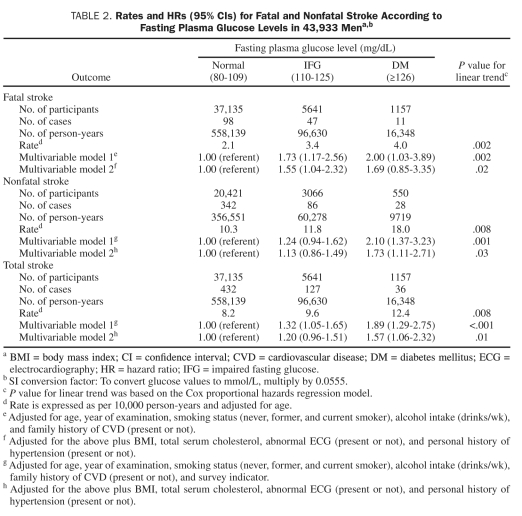
Age-adjusted fatal, nonfatal, and total stroke rates per 10,000 person-years across normal FPG, IFG, and undiagnosed DM were 2.1, 3.4, and 4.0 (Ptrend=.002); 10.3, 11.8, and 18.0 (Ptrend=.008); and 8.2, 9.6, and 12.4 (Ptrend=.008), respectively. After further adjustment for year of baseline examination, smoking, alcohol intake, total cholesterol, BMI, abnormal ECG, personal history of hypertension, family history of CVD, and survey indicator (for nonfatal and total stroke), the direct association between FPG levels and fatal, nonfatal, or total stroke remained significant (Ptrend=.02, .03, and .01, respectively) (Table 2). Additional adjustment for physical activity or cardiorespiratory fitness in the full model had little effect on the association between FPG levels and stroke risk (data not shown). Undiagnosed DM had a positive association with nonfatal and total stroke outcomes, respectively, but not with fatal stroke, likely because of the small number of deaths (n=11) in this group.
TABLE 2.
Rates and HRs (95% CIs) for Fatal and Nonfatal Stroke According to Fasting Plasma Glucose Levels in 43,933 Mena,b
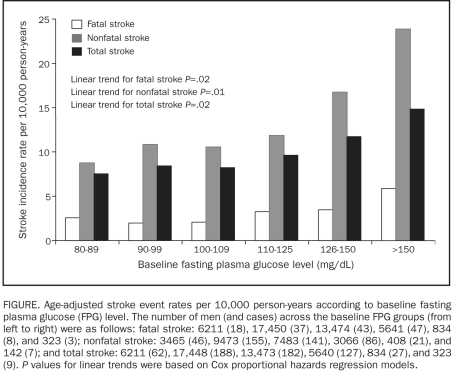
The Figure includes data on fatal, nonfatal, and total stroke according to baseline FPG levels. The age-adjusted event rates per 10,000 person-years were almost identical between groups with FPG levels of 80 to 89 mg/dL, 90 to 99 mg/dL, and 100 to 109 mg/dL. However, beginning at FPG levels of 110 mg/dL, significantly higher fatal (Ptrend=.02), nonfatal (Ptrend=.02), and total stroke (Ptrend=.01) rates were observed. Further adjustment of all the potential variables did not materially change the significant associations. In addition, we calculated the age-adjusted stroke rate among a subgroup of 30,646 men with baseline information available to determine metabolic syndrome (MetS) status. We found that the age-adjusted fatal stroke mortality rate was significantly higher in men with MetS compared with those without MetS (3.6 vs 2.4 per 10,000 person-years; P=.04). However, no difference was found for the other 2 stroke outcomes (data not shown).
FIGURE.
Age-adjusted stroke event rates per 10,000 person-years according to baseline fasting plasma glucose (FPG) level. The number of men (and cases) across the baseline FPG groups (from left to right) were as follows: fatal stroke: 6211 (18), 17,450 (37), 13,474 (43), 5641 (47), 834 (8), and 323 (3); nonfatal stroke: 3465 (46), 9473 (155), 7483 (141), 3066 (86), 408 (21), and 142 (7); and total stroke: 6211 (62), 17,448 (188), 13,473 (182), 5640 (127), 834 (27), and 323 (9). P values for linear trends were based on Cox proportional hazards regression models.
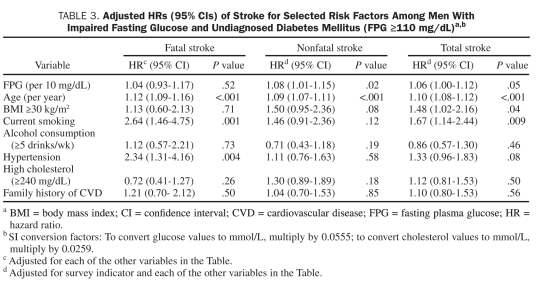
Because a dose-response association of FPG level to stroke outcomes appeared to begin at a level associated with IFG, we used FPG levels as a continuous variable to predict stroke in patients with FPG levels of 110 mg/dL or greater (ie, IFG and undiagnosed DM) (Table 3). After adjustment for conventional risk factors, each 10-unit elevation was associated with a 4% (95% CI, –7% to 17%) higher risk of fatal stroke, an 8% (95% CI, 1%-15%) higher risk of nonfatal stroke, and a 6% (95% CI, 0%-12%) higher risk of total stroke, respectively. Age was a significant predictor of fatal, nonfatal, and total stroke. In addition, current cigarette smoking, obesity, and a history of hypertension were also significant predictors of fatal or total stroke.
TABLE 3.
Adjusted HRs (95% CIs) of Stroke for Selected Risk Factors Among Men With Impaired Fasting Glucose and Undiagnosed Diabetes Mellitus (FPG ≥110 mg/dL)a,b
DISCUSSION
The main finding of the current study is that hyperglycemia is an independent predictor of stroke events in persons without a previous diagnosis of DM. We found an initial flat association that became linear at 110 mg/dL or greater between FPG level and fatal, nonfatal, and total stroke in asymptomatic men. Multivariable models showed that IFG was strongly associated with fatal stroke and that undiagnosed DM was strongly associated with nonfatal and total stroke. Importantly, a greater risk of stroke was detected at an FPG level of 110 mg/dL, and not at 100 mg/dL, which challenges the current ADA recommendations at least in terms of stroke risk.14 The World Health Organization expert group8 has not included FPG levels in the 100 to 109 mg/dL range in the definition of IFG and MetS.
Diabetes mellitus was the strongest single risk factor for stroke in a prospective study from Finland with a follow-up of 15 years.32 Of patients with an abnormal fasting glucose level (a predictor of type 2 DM), 20% to 50% develop type 2 DM within 10 years.33 In our cohort of asymptomatic men, IFG was present in 12.8%, which is close to the 11% from an earlier study.9 Overall, we found that more than 15% of asymptomatic men had IFG or DM. Such persons should be identified as high-risk and receive primary prevention for stroke.
Some,9,10 but not all,6,11,12 studies have reported an increased risk of stroke in those with elevated serum fasting glucose levels and in those with undiagnosed DM defined by FPG. In a study population of 13,999 patients from Israel with documented coronary heart disease, Tanne et al9 reported an 84% higher risk of incident nonfatal ischemic stroke or transient ischemic attack in patients with an FPG of 110 to 125 mg/dL but a nonsignificant higher risk for those with an FPG of 100 to 109 mg/dL. Recently, the DECODE study10 observed a significantly increased hazard ratio for both IFG (1.18 [95% CI, 1.00-1.40]) and for screen-detected DM (1.36 [95% CI, 1.02-1.80]). However, neither that study11,12 nor the Euro Heart Survey on Diabetes and the Heart6 showed an association between FPG levels and stroke mortality, perhaps indicating that the previous inconsistent findings between FPG levels and stroke events could be due to the different types of stroke outcomes. Therefore, to evaluate the precise role of FPG in the primary prevention of stroke, it is important to investigate various stroke outcomes in asymptomatic persons. In the current study, we found a strong association between FPG levels and stroke in men with IFG (for fatal stroke) and undiagnosed DM (for nonfatal or total stroke) after multivariable adjustment. When FPG was used as a continuous variable in the analysis, every 10-unit increment of FPG above 110 mg/dL was associated with an 8% and 6% higher risk of nonfatal and total stroke, respectively (Table 3).
Some previous studies have shown that the association between FPG levels and stroke outcomes differs by sex. For example, the DECODE Study Group reported a strong positive association between FPG and stroke mortality in women but not in men.12 However, most other studies did not separate men and women,6,9-11 and therefore sex-specific studies are needed to clarify this issue. We were unable to conduct such an analysis in women because of the small number of women and stroke deaths identified from the ACLS. As the ACLS cohort expands, we are hoping to update our results, especially in women, in the near future.
One of the potential biological mechanisms is that hyperglycemia may cause early atherosclerosis in the initial phases of DM.34 Atherosclerotic lesions often develop at an accelerated rate in the large arteries in people with undiagnosed DM.9 Many factors, such as hyperglycemia-induced endothelial cell dysfunction, procoagulation acceleration, fibrinolysis failure, and arterial remodeling dysfunction, may play a role in the prognosis of patients with higher levels of FPG.35,36 Laboratory data also suggest that increased levels of blood glucose may lead to infarct expansion through several maladaptive metabolic pathways.37
The principal strength of the current study is the large population and extensive database generated during the past 30 years. The primary limitation is the homogeneity of the cohort on socioeconomic variables: this study population comprises mainly non-Hispanic whites from middle to upper socioeconomic strata who are college graduates. However, the physiologic characteristics of our study population were similar to those of representative population groups.38 The homogeneity of the cohort on socioeconomic variables may reduce the possibility of confounding by education, occupation, and income. Because of their higher socioeconomic status, people in this cohort are likely to have better access to medical services than members of the general population, which may explain in part their lower stroke rate. The expected age-adjusted stroke mortality rate in the current cohort was 37.9 per 100,000 US standard population, which was slightly lower than the 2007 age-adjusted rate for US adults (42.2 per 100,000 US standard population).39 Another limitation of the current study is that we were unable to consider the stroke subtype. Given the size and nationwide distribution of our cohort, medical record reviewing is an ongoing process, and we have not yet completed this process on all reported cases. Therefore, we do not have sufficient information to identify ischemic or hemorrhagic stroke. Recent literature reported a J-shaped association between FPG levels and ischemic stroke in patients with preexisting atherothrombotic disease.9 More research is needed to better understand the specificity of associations between FPG levels and stroke subtypes. Regarding the exposure assessment, we only had a single measurement of FPG, which deviates from the ADA criteria for DM classification and might lead to misclassification of the exposure variable. In addition, men were classified at study enrollment, and so we were unable to assess the influence of follow-up changes in FPG levels on stroke end points. Men initially classified as having IFG could have had increases in FPG levels, developed DM, or been treated with glucose-lowing medications during the follow-up interval. Additionally, others with normal glucose levels could have transitioned to IFG, and some with IFG may have reverted to normal FPG levels. Such misclassification of exposure would likely affect the observed association in the current study. Other risk factors, such as screening guidelines, aggressiveness of DM interventions, and advanced imaging technology studies, also changed during the long period of follow-up, and the effect on results is unknown. It is also important to recognize that defining IFG by different criteria might result in other findings. Furthermore, we do not have complete data on some identified inflammatory markers (eg, C-reactive protein) to include in the current study. Other information such as aspirin use, dietary habits, glycated hemoglobin levels, and oral glucose tolerance testing was also unavailable.
CONCLUSION
Our findings suggest that higher FPG levels in people without a previous diagnosis of DM were associated with a higher risk of fatal, nonfatal, and total stroke. An FPG test is easy and inexpensive to perform and has good reproducibility and small variability in clinical practice. An early diagnosis of IFG as well as frank DM may lead to interventions, such as weight control, diet modification, and medication treatment, that optimize glycemic control and reduce stroke risk in asymptomatic patients with hyperglycemia. Clinicians should also carefully assess stroke risk in patients with FPG levels between 100 and 109 mg/dL.
Supplementary Material
Acknowledgments
We thank the Cooper Clinic physicians and technicians for collecting the baseline data and staff at the Cooper Institute for data entry and data management.
Footnotes
Supported by National Institutes of Health grants AG06945, HL62508, and R21DK088195 (to Dr Sui from the National Institute of Diabetes and Digestive and Kidney Diseases) and in part supported by an unrestricted research fund from The Coca-Cola Company.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
An earlier version of this article appeared Online First.
REFERENCES
- 1. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434-444 [DOI] [PubMed] [Google Scholar]
- 2. Tuomilehto J, Rastenyte D. Diabetes and glucose intolerance as risk factors for stroke. J Cardiovasc Risk. 1999;6(4):241-249 [DOI] [PubMed] [Google Scholar]
- 3. Tuomilehto J, Rastenyte D, Jousilahti P, Sarti C, Vartiainen E. Diabetes mellitus as a risk factor for death from stroke: prospective study of the middle-aged Finnish population. Stroke. 1996;27(2):210-215 [DOI] [PubMed] [Google Scholar]
- 4. Howard BV, Rodriguez BL, Bennett PH, et al. Prevention Conference VI: diabetes and cardiovascular disease; Writing Group I: epidemiology. Circulation. 2002;105(18):e132-e137 [DOI] [PubMed] [Google Scholar]
- 5. Goldstein LB, Adams R, Becker K, et al. Primary prevention of ischemic stroke: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Circulation. 2001;103(1):163-182 [DOI] [PubMed] [Google Scholar]
- 6. Lenzen M, Ryden L, Ohrvik J, et al. Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J. 2006;27(24):2969-2974 [DOI] [PubMed] [Google Scholar]
- 7. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229-234 [DOI] [PubMed] [Google Scholar]
- 8. Ryden L, Standl E, Bartnik M, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary: the Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J. 2007;28(1):88-136 [DOI] [PubMed] [Google Scholar]
- 9. Tanne D, Koren-Morag N, Goldbourt U. Fasting plasma glucose and risk of incident ischemic stroke or transient ischemic attacks: a prospective cohort study. Stroke. 2004;35(10):2351-2355 [DOI] [PubMed] [Google Scholar]
- 10. Hyvarinen M, Tuomilehto J, Mahonen M, et al. Hyperglycemia and incidence of ischemic and hemorrhagic stroke-comparison between fasting and 2-hour glucose criteria. Stroke. 2009;40(5):1633-1637 [DOI] [PubMed] [Google Scholar]
- 11. Hyvarinen M, Qiao Q, Tuomilehto J, et al. Hyperglycemia and stroke mortality: comparison between fasting and 2-h glucose criteria. Diabetes Care. 2009;32(2):348-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DECODE Study Group: the Eurpean Diabetes Epidemiology Group Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161(3):397-405 [DOI] [PubMed] [Google Scholar]
- 13. Preiss D, Welsh P, Murray HM, et al. Fasting plasma glucose in nondiabetic participants and the risk for incident cardiovascular events, diabetes, and mortality: results from WOSCOPS 15-year follow-up. Eur Heart J. 2010;31(10):1230-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(suppl 1):S5-S20 [DOI] [PubMed] [Google Scholar]
- 15. Tai ES, Goh SY, Lee JJ, et al. Lowering the criterion for impaired fasting glucose: impact on disease prevalence and associated risk of diabetes and ischemic heart disease. Diabetes Care. 2004;27(7):1728-1734 [DOI] [PubMed] [Google Scholar]
- 16. Davidson MB, Landsman PB, Alexander CM. Lowering the criterion for impaired fasting glucose will not provide clinical benefit. Diabetes Care. 2003;26(12):3329-3330 [DOI] [PubMed] [Google Scholar]
- 17. Borch-Johnsen K, Colagiuri S, Balkau B, et al. Creating a pandemic of prediabetes: the proposed new diagnostic criteria for impaired fasting glycaemia. Diabetologia. 2004;47(8):1396-1402 [DOI] [PubMed] [Google Scholar]
- 18. Schriger DL, Lorber B. Lowering the cut point for impaired fasting glucose: where is the evidence? Where is the logic? Diabetes Care. 2004; 27(2):592-601 [DOI] [PubMed] [Google Scholar]
- 19. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183-1197 [DOI] [PubMed] [Google Scholar]
- 20. Lyerly GW, Sui X, Lavie CJ, Church TS, Hand GA, Blair SN. The association between cardiorespiratory fitness and risk of all-cause mortality among women with impaired fasting glucose or undiagnosed diabetes mellitus. Mayo Clin Proc. 2009;84(9):780-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hooker SP, Sui X, Colabianchi N, et al. Cardiorespiratory fitness as a predictor of fatal and nonfatal stroke in asymptomatic women and men. Stroke. 2008;39(11):2950-2957 [DOI] [PubMed] [Google Scholar]
- 22. Shuger SL, Sui X, Church TS, Meriwether RA, Blair SN. Body mass index as a predictor of hypertension incidence among initially healthy normotensive women. Am J Hypertens. 2008;21(6):613-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. U S Armed Forces Med J. 1959;10:675-688 [PubMed] [Google Scholar]
- 24. Gibbons LW, Mitchell TL, Wei M, Blair SN, Cooper KH. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. Am J Cardiol. 2000;86(1):53-58 [DOI] [PubMed] [Google Scholar]
- 25. Sesso HD, Gaziano JM, Glynn RJ, Buring JE. Value of an endpoints committee versus the use of nosologists for validating cause of death. Contemp Clin Trials. 2006;27(4):333-339 [DOI] [PubMed] [Google Scholar]
- 26. Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;49(2):1-32 [PubMed] [Google Scholar]
- 27. Kelly-Hayes M, Robertson JT, Broderick JP, et al. The American Heart Association stroke outcome classification. Stroke. 1998;29(6):1274-1280 [DOI] [PubMed] [Google Scholar]
- 28. Blair SN, Goodyear NN, Gibbons LW, Cooper KH. Physical fitness and incidence of hypertension in healthy normotensive men and women. JAMA. 1984;252(4):487-490 [PubMed] [Google Scholar]
- 29. Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165(12):1413-1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu FB, Stampfer MJ, Colditz GA, et al. Physical activity and risk of stroke in women. JAMA. 2000;283(22):2961-2967 [DOI] [PubMed] [Google Scholar]
- 31. Barlow CE, Lamonte MJ, Fitzgerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol. 2006; 163(2):142-150 [DOI] [PubMed] [Google Scholar]
- 32. Adams HP, Jr, Putman SF, Kassell NF, Torner JC. Prevalence of diabetes mellitus among patients with subarachnoid hemorrhage. Arch Neurol. 1984;41(10):1033-1035 [DOI] [PubMed] [Google Scholar]
- 33. Barzilay JI, Spiekerman CF, Wahl PW, et al. Cardiovascular disease in older adults with glucose disorders: comparison of American Diabetes Association criteria for diabetes mellitus with WHO criteria. Lancet. 1999; 354(9179):622-625 [DOI] [PubMed] [Google Scholar]
- 34. Wagenknecht LE, D’Agostino R, Jr, Savage PJ, O’Leary DH, Saad MF, Haffner SM. Duration of diabetes and carotid wall thickness: the Insulin Resistance Atherosclerosis Study (IRAS). Stroke. 1997;28(5):999-1005 [DOI] [PubMed] [Google Scholar]
- 35. Chiquette E, Chilton R. Cardiovascular disease: much more aggressive in patients with type 2 diabetes. Curr Atheroscler Rep. 2002;4(2):134-142 [DOI] [PubMed] [Google Scholar]
- 36. Mudaliar S. Intense management of diabetes mellitus: role of glucose control and antiplatelet agents. J Clin Pharmacol. 2004;44(4):414-422 [DOI] [PubMed] [Google Scholar]
- 37. Gilmore RM, Stead LG. The role of hyperglycemia in acute ischemic stroke. Neurocrit Care. 2006;5(2):153-158 [DOI] [PubMed] [Google Scholar]
- 38. Blair SN, Kannel WB, Kohl HW, Goodyear N, Wilson PWF. Surrogate measures of physical activity and physical fitness: Evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am J Epidemiol. 1989;129(6):1145-1156 [DOI] [PubMed] [Google Scholar]
- 39. Xu JQ, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: final data for 2007. Natl Vital Stat Rep. 2010;58(19):1-136 http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_19.pdf Accessed July 1. 2011 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.