Abstract
OBJECTIVE: To investigate the association between serum vitamin D levels and depression in a large database of patients from the Cooper Clinic.
PATIENTS AND METHODS: We conducted a cross-sectional study of 12,594 participants seen at the Cooper Clinic from November 27, 2006, to October 4, 2010. Serum 25-hydroxyvitamin D [25(OH)D] was analyzed, and depression was defined as a Center for Epidemiologic Studies Depression Scale (CES-D) score of 10 or more. Those with and those without a history of depression represented 2 distinct populations with respect to CES-D scores; accordingly, they were analyzed separately.
RESULTS: In the total sample, higher vitamin D levels were associated with a significantly decreased risk [odds ratio, 0.92 (95% confidence interval, 0.87-0.97)] of current depression based on CES-D scores. The finding was stronger in those with a prior history of depression [odds ratio, 0.90 (95% confidence interval, 0.82-0.98)] and not significant in those without a history of depression [odds ratio, 0.95 (95% confidence interval, 0.89-1.02)].
CONCLUSION: We found that low vitamin D levels are associated with depressive symptoms, especially in persons with a history of depression. These findings suggest that primary care patients with a history of depression may be an important target for assessment of vitamin D levels.
BMI = body mass index; CCLS = Cooper Center Longitudinal Study; CES-D = Center for Epidemiologic Studies Depression Scale; 25(OH)D = serum 25-hydroxyvitamin D
Depression occurs in persons of all ages and backgrounds and both sexes and is a leading cause of disability worldwide.1 Depression affects overall health-related quality of life to an equal or greater degree than other chronic medical conditions.2 Therefore, identifying risk factors for depression or biomarkers associated with depressive symptoms is of considerable importance.
Low vitamin D level is implicated as a risk factor for numerous medical conditions, including autoimmune diseases, vascular disease, infectious diseases, osteoporosis, obesity, diabetes, cardiovascular disease, and certain cancers.3,4 More recently, low vitamin D level has also been associated with neurologic disorders such as multiple sclerosis, Alzheimer disease, Parkinson disease, and cognitive decline.3-7 Vitamin D receptors and the vitamin D–activating enzyme 1α–hydroxylase are found in most organ systems of the human body, including the brain.8 Within the hypothalamus and the dopaminergic neurons of the substantia nigra is found a high density of vitamin D receptors as well as the vitamin D–activating enzyme.8 Recent evidence suggests that damage to these aspects of the brain is associated with depression, at least in the elderly.9 In addition, vitamin D may have an effect on neurotransmitters, inflammatory markers, calcium homeostasis in the brain, and nerve growth factor synthesis.6,10-15 Vitamin D receptor knockout mice exhibit depression-like behaviors such as poorer performance on swim tests, less activity, and more anxiety than wild-type controls.16 Thus, these data on animals and humans suggest that vitamin D may have a role in depression.
Prior studies in humans have shown conflicting associations between vitamin D levels and depression. Several small clinical studies have found an association between low 25-hydroxyvitamin D [25(OH)D] levels and depression.17-19 To date, 5 population-based studies have explored the association between 25(OH)D and depression, with conflicting results.20-24 Hoogendijk et al20 examined 1282 people aged 65 to 95 years in Amsterdam and found 14% lower 25(OH)D levels in those with major and minor depression, defined by a Center for Epidemiologic Studies Depression Scale (CES-D) score of 16 or more, when compared with controls. Stewart and Hirani21 studied 2070 adults aged 65 years and older in England and found that depressive symptoms were associated with low vitamin D levels. However, negative studies have also been reported. Pan et al22 examined 3262 people aged 50 to 70 years in Beijing and Shanghai, China. Depressive symptoms were less prevalent in those in the top tertile of 25(OH)D concentrations compared with those in the lowest tertile, but this association disappeared after controlling for geographic location (Beijing vs Shanghai). Nanri et al23 examined 527 Japanese employees aged 21 to 67 years at 2 municipal offices and found no significant association between CES-D scores and 25(OH)D levels in the workers surveyed at either workplace. Finally, Zhao et al24 studied 3916 adults in the United States and found that 25(OH)D and parathyroid hormone levels were not significantly associated with depressive symptoms after adjusting for demographic variables, lifestyle factors, and existing chronic conditions.
In this report, we present data on 25(OH)D levels and depressive symptoms from the largest sample studied to date. The purpose of this project was to determine whether depressive symptoms, as defined by CES-D scores, are associated with 25(OH)D levels in a larger and generally healthy population. We hypothesized that vitamin D levels would be lower in the group with current depression as judged by CES-D score.
PATIENTS AND METHODS
Participants
The Cooper Center Longitudinal Study (CCLS) is a prospective study of patients who have completed a preventive medical examination at the Cooper Clinic in Dallas, TX.25 The Cooper Clinic is a fee-for-service preventive medicine clinic. Patients at the Cooper Clinic are generally well-educated and mostly non-Hispanic whites (95%) from middle to upper socioeconomic strata. We examined data from 12,594 patients seen from November 27, 2006, to October 4, 2010, who completed baseline examinations that included serum 25(OH)D levels and a CES-D score. In addition, demographic information was obtained, including age, body mass index (BMI), education level, smoking status, physical activity level, previous history of depression, antidepressant use, thyrotropin levels, and history of diabetes, heart attack, stroke, and/or cancer. Physicians reviewed and verified the questionnaires, but a formal diagnosis using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) (DSM-IV-TR) criteria was not made. The included patients signed an informed consent to allow the use of their data for research. The informed consent and the CCLS are reviewed and approved annually by The Cooper Institute’s Institutional Review Board. These data are maintained by The Cooper Institute in the CCLS database. Before analysis, all data were deidentified.
Assessments
Vitamin D Status. The blood chemistry was analyzed using automated techniques in the Cooper Clinic laboratory. This laboratory participates in and meets quality control standards of the Centers for Disease Control and Prevention Lipid Standardization Program. All assays were performed by trained technicians following standardized procedures. The 25(OH)D was chosen as the clinical measure of vitamin D status for Cooper Clinic patients because of its widespread clinical application, standardized ranges, and testing protocol. The 25(OH)D (D2 + D3) levels were measured on a DiaSorin Liaison Chemiluminescence Analyzer which uses a 1-step assay. The test-retest coefficient of variation for this assay was ±11%.
Depressive Symptoms. The 10-item CES-D was used to detect and quantify depressive symptoms experienced by participants during the previous week. This self-report scale assesses depressed affect, somatic retardation, and positive affect, generating a total severity score from 0 to 30, with a cutoff score of 10 or more indicating clinical depression in prior research.26-28 The CES-D has high reliability and validity in detecting symptoms of depression.27 In our population, participants with CES-D scores of 10 or more were considered to have “current depressive symptoms,” and those with scores below 10 were considered to “not have current depressive symptoms.” Previous history of depression was determined from self-report questionnaires that asked a yes/no question pertaining to past depression. Thus, current depression was defined entirely by a current CES-D score of 10 or more, whereas past depression was defined solely by patient self-report (yes/no).
Statistical Analyses
We used multiple logistic regression to predict CES-D scores of 10 or more on the basis of all the covariates presented in Table 1. We dichotomized age, BMI, education, and thyrotropin to construct clinically relevant odds ratios. In developing a model for all patients, we noticed that patients with a history of depression had markedly higher CES-D scores. Furthermore, history of depression interacted significantly with sex, education, and antidepressant use, although not with serum 25(OH)D. We fit one model to all patients and separate models to subgroups defined by history of depression. We report profile likelihood confidence intervals (CIs) for all odds ratios. To investigate the potential confounding role of outdoor exercise on the association between CES-D and serum 25(OH)D, we fit a multiple logistic regression model of CES-D scores of 10 or more, as previously described, but either (1) stratified by season or (2) included a season × regular exercise interaction effect. We also tested for a significant regular exercise × season interaction in a general linear regression model of serum 25(OH)D. We used χ2 tests for all Table 1 P values except for serum 25(OH)D, for which we used a t test. We used SAS/STAT 9.2 (SAS Institute Inc, Cary, NC) to fit the models as well as for summary statistics and hypothesis testing in Table 1.
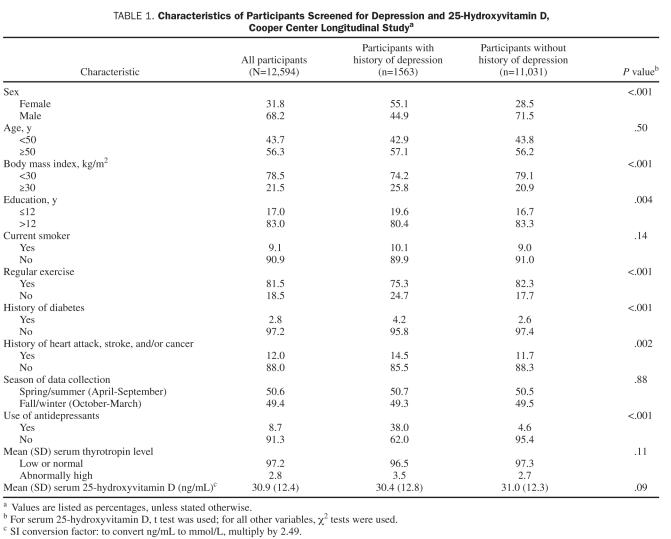
TABLE 1.
Characteristics of Participants Screened for Depression and 25-Hydroxyvitamin D, Cooper Center Longitudinal Studya
RESULTS
Participant characteristics are shown in Table 1. The study sample included 4005 women (31.8%) and 8589 men (68.2%) with a mean age of 51.7±11.0 years. Exploratory analysis showed that those with and those without a history of depression represented 2 distinct populations with respect to the CES-D score. Accordingly, in addition to analysis of the total sample, the 2 groups were analyzed separately. There were significantly higher percentages of women and participants with a history of diabetes, heart attack, stroke, and/or cancer in the group with a history of depression. In addition, participants in the group with a history of depression had less education, engaged in less regular physical activity, and had higher BMI, and a higher percentage used antidepressants. However, age, smoking status, season of data collection, and the mean serum 25(OH)D levels (P=.09) did not differ significantly between the 2 groups, those with and those without a history of depression.
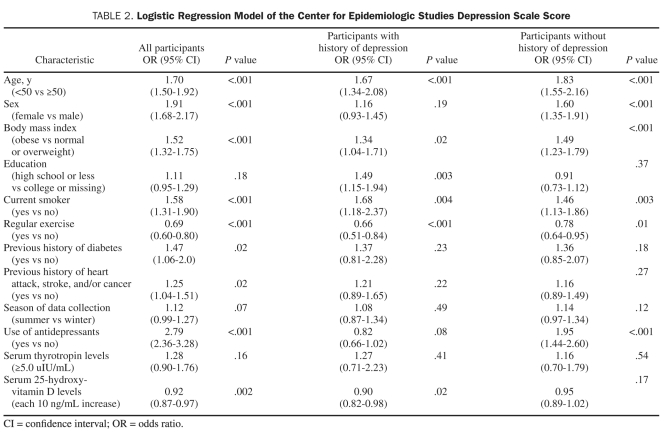
Table 2 shows the association between vitamin D and CES-D in the total sample and in those with and those without a history of depression. In the total sample, sex, age, BMI, current smoking, regular physical activity, antidepressant use, 25(OH)D level, and history of diabetes, heart attack, stroke, and/or cancer (P=.02) were significantly associated with current CES-D score. In those with a history of depression, age, BMI, current smoking, education, regular activity, and vitamin D level (P=.02) were significantly associated with current CES-D score. In those without a history of depression, sex, age, BMI, current smoking, regular activity, and antidepressant use were significantly associated with current CES-D score, whereas vitamin D level was not (P=.17). In the entire sample, low vitamin D levels were common, with 50.7% of participants having levels in either the deficient range [25(OH)D levels <20 ng/mL, [to convert ng/mL to mmol/L, multiply by 2.49], according to Institute of Medicine recommendations, or the insufficient range [25(OH)D levels <30 ng/mL].29,30 Those who did not engage in regular physical activity had significantly lower mean serum vitamin D levels (P<.001).
TABLE 2.
Logistic Regression Model of the Center for Epidemiologic Studies Depression Scale Score
Given the potential impact of sunlight on vitamin D levels, we also stratified by season (April to September and October to March). Vitamin D is significantly associated with less depression in October to March [odds ratio, 0.87 (95% CI, 0.80-0.95); P=.001] and not associated with depression in April to September [odds ratio, 0.96 (95% CI, 0.89-1.02); P=.2)], although the effect was in the same direction. In the model stratified by season, regular exercise was significantly associated with less depression. In the unstratified model, the season × regular exercise interaction effect was not significant (P=.4). The association between vitamin D and regular exercise did not vary by season (P=.9).
DISCUSSION
The association between vitamin D level and depression was analyzed in the largest data set to date, comprising generally healthy persons ranging from 20 to 90 years of age. Although mean vitamin D levels did not differ significantly between those with and those without a history of depression, there was a significant association between vitamin D levels and current “depressive symptoms” based on CES-D scores in those with a history of depression. This subset analysis may shed light on why there were conflicting results in earlier studies because the populations were not assessed on the basis of prior history of depression.20-24 These findings may potentially have clinical importance in the evaluation of patients with a prior history of depression.
Because of the cross-sectional design of our study, the causality of the association between vitamin D and depression cannot be determined. As previously discussed, vitamin D appears to be important for brain health and may be involved in the pathogenesis or prevention of depression. There is also emerging evidence of neuroprotective roles for vitamin D through antioxidant pathways, enhanced nerve conduction, neurotransmitter targets, neuronal calcium regulation, and effects on inflammation.6,10-15 Emerging data suggest that elevated levels of proinflammatory cytokines in the brain may be associated with depression.15 In human studies, an inverse correlation was found between vitamin D status and inflammation markers, and a positive correlation was found with anti-inflammatory cytokines in cord blood.13,14 Inflammation caused by increased cytokines can affect glial cell functions and damage neurons.15 Finally, 2 longitudinal studies have shown that vitamin D status at baseline is associated with the development of depression over time, suggesting that low vitamin D levels may predict the development of future depression.31,32 Thus, there is physiologic support for the role of vitamin D in prevention of depression.
The converse may be true as well. Depression may also be a risk factor for development of vitamin D deficiency. Depressed people may consume a less nutritious diet, stay indoors, and exercise less; all activities that contribute to lower serum vitamin D levels. In our sample, those who did not engage in regular physical activity had significantly lower mean serum vitamin D levels. Regular physical activity was also significantly associated with not having current symptoms of depression. Of note, a season-by-activity interaction was not observed.
The current study has several limitations. The sample was relatively homogenous in terms of race and ethnicity, potentially limiting the generalizability or external validity of the findings but enhancing the internal validity. Despite the homogeneity, the size of the population provides additional power to the internal validity. The study was diverse in other characteristics, including sex and age distribution. In addition, there are several methodological limitations of the current study. Because of data collection methods, as previously mentioned, we did not diagnose syndromal depression using DSM-IV criteria. The 10-item CES-D assesses participant mood for the past week, and its purpose is to measure the severity of depressive symptoms, not to diagnose depression as defined by DSM-IV criteria; however, a score of 10 has been established as an acceptable cutoff for clinical depression.26-28 Previous observational studies also used the CES-D to assess depressive symptoms in large populations.20-24,31 History of depression was based on patient report and confirmed by the physician but not further defined. In addition, although we controlled for many known confounds, some may have remained. For example, although we controlled for regular physical activity, differentiating between indoor and outdoor activity could have been more useful in ruling out the effects of sunlight exposure on vitamin D levels and possibly depressive symptoms. Of note, there were no significant associations between season of data collection and the prevalence of depressive symptoms, but this study was conducted in the southern region of the United States, where the amount of daylight and sun exposure does not vary as greatly between seasons as in more northern areas. Another important omission is the lack of dietary information, in particular nutrients such as vitamin D or omega-3 fatty acids, on the participants; nonetheless, we did have measured blood levels. Finally, because of our cross-sectional design, a causal association cannot be determined between vitamin D levels and depressive symptoms.
In this generally healthy sample, low vitamin D levels were associated with depressive symptoms in those with a history of depression. Given the inability of cross-sectional analysis to determine cause and effect, longitudinal studies are needed to provide the next step toward elucidating the association between vitamin D levels and depression. If vitamin D levels are a factor causing depression, then clinical trials of vitamin D supplementation for depression will be needed. To date, there have been only a few small trials of vitamin D supplementation in various depressed populations, with mixed results.33-35
CONCLUSION
Current depression as defined by the CES-D was associated with lower vitamin D levels in participants with prior history of depression by self-report in the largest sample investigated to date. The findings suggest that patients with a history of depression could be an important population to target for screening of vitamin D levels. Additional research is needed to determine the nature and direction of the association between vitamin D levels and depression.
Acknowledgments
We thank Dr Kenneth H. Cooper for establishing the Cooper Center Longitudinal Study, the Cooper Clinic staff for data collection, and The Cooper Institute data management staff for maintenance of the database. We also acknowledge support from the UT Southwestern Summer Medical Student Research Program.
Footnotes
Dr Brown has research support from the National Heart, Lung, and Blood Institute, National Institute of Mental Health, National Institute on Alcohol Abuse and Alcoholism, National Institute on Drug Abuse, the Stanley Medical Research Institute, and AstraZeneca.
REFERENCES
- 1. Bromet E, Andrade LH, Hwang I, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011July26;9:90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katon W. The impact of depression on workplace functioning and disability costs. Am J Manag Care. 2009;15:S322-S327 [PubMed] [Google Scholar]
- 3. Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010;340:b5664 [DOI] [PubMed] [Google Scholar]
- 4. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281 [DOI] [PubMed] [Google Scholar]
- 5. Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135-1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med. 2008;29:415-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Saaksjarvi K, Heliovaara M. Serum vitamin D and the risk of Parkinson disease. Arch Neurol. 2010;67:808-811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21-30 [DOI] [PubMed] [Google Scholar]
- 9. Tsopelas C, Stewart R, Savva GM, et al. Neuropathological correlates of late-life depression in older people. Br J Psychiatry. 2011;198:109-114 [DOI] [PubMed] [Google Scholar]
- 10. Stumpf WE. Vitamin D sites and mechanisms of action: a histochemical perspective: reflections on the utility of autoradiography and cytopharmacology for drug targeting. Histochem Cell Biol. 1995;104:417-427 [DOI] [PubMed] [Google Scholar]
- 11. Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074:261-271 [DOI] [PubMed] [Google Scholar]
- 12. McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982-1001 [DOI] [PubMed] [Google Scholar]
- 13. Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787-796 [DOI] [PubMed] [Google Scholar]
- 14. Zittermann A, Dembinski J, Stehle P. Low vitamin D status is associated with low cord blood levels of the immunosuppressive cytokine interleukin-10. Pediatr Allergy Immunol. 2004;15:242-246 [DOI] [PubMed] [Google Scholar]
- 15. Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):760-768 [DOI] [PubMed] [Google Scholar]
- 16. Burne TH, Johnston AN, McGrath JJ, Mackay-Sim A. Swimming behaviour and post-swimming activity in Vitamin D receptor knockout mice. Brain Res Bull. 2006;69:74-78 [DOI] [PubMed] [Google Scholar]
- 17. Armstrong DJ, Meenagh GK, Bickle I, Lee AS, Curran ES, Finch MB. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol. 2007;26:551-554 [DOI] [PubMed] [Google Scholar]
- 18. Schneider B, Weber B, Frensch A, Stein J, Fritz J. Vitamin D in schizophrenia, major depression and alcoholism. J Neural Transm. 2000;107:839-842 [DOI] [PubMed] [Google Scholar]
- 19. Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14:1032-1040 [DOI] [PubMed] [Google Scholar]
- 20. Hoogendijk WJ, Lips P, Dik MG, Deeg DJ, Beekman AT, Penninx BW. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65:508-512 [DOI] [PubMed] [Google Scholar]
- 21. Stewart R, Hirani V. Relationship between vitamin D levels and depressive symptoms in older residents from a national survey population. Psychosom Med. 2010;72(7):608-612 [DOI] [PubMed] [Google Scholar]
- 22. Pan A, Lu L, Franco OH, Yu Z, Li H, Lin X. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J Affect Disord. 2009;118:240-243 [DOI] [PubMed] [Google Scholar]
- 23. Nanri A, Mizoue T, Matsushita Y, et al. Association between serum 25-hydroxyvitamin D and depressive symptoms in Japanese: analysis by survey season. Eur J Clin Nutr. 2009;63:1444-1447 [DOI] [PubMed] [Google Scholar]
- 24. Zhao G, Ford ES, Li C, Balluz LS. No associations between serum concentrations of 25-hydroxyvitamin D and parathyroid hormone and depression among US adults. Br J Nutr. 2010;104(11):1696-1702 [DOI] [PubMed] [Google Scholar]
- 25. Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395-2401 [DOI] [PubMed] [Google Scholar]
- 26. Lee AE, Chokkanathan S. Factor structure of the 10-item CES-D scale among community dwelling older adults in Singapore. Int J Geriatr Psychiatry. 2008;23:592-597 [DOI] [PubMed] [Google Scholar]
- 27. Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77-84 [PubMed] [Google Scholar]
- 28. Lue BH, Chen LJ, Wu SC. Health, financial stresses, and life satisfaction affecting late-life depression among older adults: a nationwide, longitudinal survey in Taiwan. Arch Gerontol Geriatr. 2010;50(suppl 1):S34-S38 [DOI] [PubMed] [Google Scholar]
- 29. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S-1086S [DOI] [PubMed] [Google Scholar]
- 31. Milaneschi Y, Shardell M, Corsi AM, et al. Serum 25-Hydroxyvitamin D and depressive symptoms in older women and men. J Clin Endocrinol Metab. 2010;95(7):3225-3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. May HT, Bair TL, Lappe DL, et al. Association of vitamin D levels with incident depression among a general cardiovascular population. Am Heart J. 2010;159:1037-1043 [DOI] [PubMed] [Google Scholar]
- 33. Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264:599-609 [DOI] [PubMed] [Google Scholar]
- 34. Shipowick CD, Moore CB, Corbett C, Bindler R. Vitamin D and depressive symptoms in women during the winter: a pilot study. Appl Nurs Res. 2009;22:221-225 [DOI] [PubMed] [Google Scholar]
- 35. Dumville JC, Miles JN, Porthouse J, Cockayne S, Saxon L, King C. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. J Nutr Health Aging. 2006;10:151-153 [PubMed] [Google Scholar]