Abstract
OBJECTIVE: To ascertain the impact of prior antiplatelet and statin therapy on symptomatic embolic events in native valve infective endocarditis (IE).
PATIENTS AND METHODS: We studied a retrospective cohort of adult patients with a diagnosis of IE who presented to Mayo Clinic (Rochester, MN) from January 1, 2003, to December 31, 2006. Patients were grouped into those who received treatment before infection or controls who did not receive treatment for both antiplatelet therapy and, separately, statin therapy. Because of the retrospective study design and thus the nonrandomized treatment groups, a propensity score approach was used to account for the confounding factors that may have influenced treatment allocation. Antiplatelet therapy included aspirin, dipyridamole, clopidogrel, ticlopidine or any combination of these agents. Statin therapy included atorvastatin, simvastatin, pravastatin, lovastatin, rosuvastatin, or fluvastatin. The primary end point was a symptomatic embolic event that occurred before or during hospitalization. Multivariable logistic regression was used to assess the propensity-adjusted effects of continuous daily therapy with antiplatelet and statin agents on risk of symptomatic emboli. Likewise, Cox proportional hazards regression was used to test for an independent association with 6-month mortality for each of the treatments.
RESULTS: The study cohort comprised 283 patients with native valve IE. Twenty-eight patients (24.1%) who received prior continuous antiplatelet therapy developed a symptomatic embolic event compared with 66 (39.5%) who did not receive such treatment. After adjusting for propensity to treat, the effect of antiplatelet therapy on embolic risk was not statistically significant (odds ratio, 0.71; 95% confidence interval [CI], 0.37-1.36; P=.30). Only 14 patients (18.2%) who received prior continuous statin therapy developed a symptomatic embolic event compared with 80 (39.4%) of the 203 patients who did not. After adjusting for propensity to treat with statin therapy, the benefit attributable to statins was significant (odds ratio, 0.30; 95% CI, 0.14-0.62; P=.001). The 6-month mortality rate of the entire cohort was 28% (95% CI, 23%-34%). No significant difference was found in the propensity-adjusted rate of 6-month mortality between patients who had and had not undergone prior antiplatelet therapy (P=.91) or those who had and had not undergone prior statin therapy (P=.87).
CONCLUSION: The rate of symptomatic emboli associated with IE was reduced in patients who received continuous daily statin therapy before onset of IE. Despite fewer embolic events observed in patients who received antiplatelet agents, a significant association was not found after adjusting for propensity factors. A continued evaluation of these drugs and their potential impact on subsequent embolism among IE patients is warranted.
CI = confidence interval; HR = hazard ratio; IE = infective endocarditis; OR = odds ratio; PAR-1 = protease-activated receptor 1
Symptomatic embolization occurs in 22% to 50% of patients with infective endocarditis (IE) and results in death in 24% to 50%.1-4 Although valve surgery can reduce embolic risk, antimicrobial therapy is the only proven medical intervention that can decrease this risk once the diagnosis of IE is established.
Antiplatelet agents have received considerable attention in the past decade as potential preemptive medical therapies to reduce subsequent embolic risk in IE.5-11 Results of these studies have suggested that, although antiplatelet therapy initiated after the diagnosis of IE is not helpful and potentially harmful due to bleeding risk,6,7 antiplatelet therapy administered in the long term before the onset of valvular infection may reduce symptomatic embolism in IE.5 In addition, the rate of embolism may be pathogen dependent, although results in this regard have been mixed.5,11 Because most patients undergoing long-term antiplatelet therapies for non-IE reasons currently receive both antiplatelets and statins and contemporary data indicate that statin therapy, which inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase, can have pleiotropic effects,12,13 including inhibition of platelet function and immunomodulatory effects, further examination of the role of preemptive medical therapies in IE is warranted.
In our previous work,5 we demonstrated a reduction in the rate of symptomatic emboli in a subset of IE patients who had received long-term antiplatelet therapy before endocarditis onset. However, the cohort in that retrospective survey of patients between 1980 and 1998 was not likely to have received statin therapy, the use of which substantially increased after a 1994 publication14 that demonstrated its benefit in reducing subsequent cardiac complications in patients with coronary artery disease and elevated cholesterol levels. Therefore, we examined a more contemporary population of IE patients to determine the rate of symptomatic emboli and what role, if any, antiplatelet and statin therapies had on this rate.
PATIENTS AND METHODS
The study cohort included all patients with a confirmed diagnosis of IE treated at Mayo Clinic (Rochester, MN) from January 1, 2003, to December 31, 2006. Prospective records maintained by the Division of Infectious Diseases of all patients treated for IE and the diagnostic codes for IE used by the Medical Index Retrievals–Health Sciences Research were used to obtain a list of patients with a probable diagnosis of IE. The medical records were screened by physician reviewers (J.C.S., D.D.C., J.M.T.) to confirm a diagnosis of IE, using the modified Duke criteria. The study was approved by the Mayo Clinic Institutional Review Board.
Data Collection
Standardized data abstraction forms with detailed definitions of variables were used. Clinical, laboratory, and outcome data were obtained from the medical records. These data were abstracted by thorough review of medical records, including daily physicians’ progress notes and all subspecialty consultations. All patients were observed daily in the hospital by infectious diseases specialists and cardiology subspecialists, and additional consultations were secured when indicated. Any uncertainties in data abstraction were discussed with one of the experienced investigators (J.M.S., W.R.W., and L.M.B.)
Antiplatelet and 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Therapy
Antiplatelet therapy was defined as aspirin, dipyridamole, clopidogrel, ticlopidine, or any combination of these agents. 3-Hydroxy-3-methylglutaryl coenzyme A reductase therapy (statin therapy) was defined as atorvastatin, pravastatin, lovastatin, simvastatin, fluvastatin, or rosuvastatin. Patients who had been receiving antiplatelet or statin therapy at the time of hospital admission were first identified. Their medical records before admission were then scrutinized for previous use of antiplatelet and/or statin agents in their daily medical regimen. Patients were considered to have received continuous daily antiplatelet or statin therapy if their exposure to these agents occurred for at least 6 months before the diagnosis of IE and if interim medical patient records revealed the continued presence of antiplatelet and/or statin agents in the medical regimen. The rationale for the prior 6-month duration was to ensure that this therapy preceded infection onset and to remain consistent with our prior study in the field.
End Points
The primary end point was a symptomatic embolic event that occurred before or during hospitalization for IE. Again, consistent with our prior work, we included only symptomatic emboli to avoid outcome ascertainment bias as a result of a varied diagnostic evaluation for emboli. Embolic events were identified by physical examination in cases of peripheral limb involvement. All other embolic events were identified by specific imaging modalities that were performed in accordance with the patient’s clinical symptom. Embolic events were represented by stroke, major artery emboli (extremity and coronary with myocardial infarction), as well as spleen, kidney, and skin emboli. Stroke was defined by the World Health Organization as a neurologic deficit that lasted more than 24 hours and was of presumed vascular origin.15 The secondary end point was all-cause mortality within the first 6 months of diagnosis of IE.
Statistical Analyses
Descriptive statistics were used to summarize the data: medians and interquartile ranges for continuous variables and counts and percentages for categorical variables. Patients were grouped into those who received treatment before infection and controls who did not receive treatment for both antiplatelet therapy and, separately, statin therapy. Because each of these treatment groups was assembled from retrospective data and not randomized, a propensity score approach was used to identify factors that may have contributed to the propensity of treatment allocation. Therefore, patients who received antiplatelet therapy were compared with those who did not receive antiplatelet therapy based on patient characteristics and clinical factors, as were patients who received statin therapy vs those who did not receive statin therapy. Included among these factors was the Charlson comorbidity index, a severity-weighted score based on 19 categories of chronic comorbid conditions defined by diagnostic codes,16,17 which has been previously validated as an independent predictor of 6-month mortality in patients with left-sided IE.18 Logistic regression was used to identify all factors that contributed to the propensity to treat with antiplatelet and statin therapies. For both types of therapy, propensity scores were computed as the logit-transformed predicted probabilities from a multivariable logistic model of all respective factors and all 2-way interactions.
The primary objective of this study was to compare the embolic rate around the time of diagnosis between those who received treatment before infection vs those who did not receive treatment for both antiplatelet therapy and statin therapy. Multivariable logistic regression was used to test for a difference in embolic rates between treatment and control groups, while controlling for the propensity to be treated. As aforementioned, the propensity of being treated was estimated using multivariable logistic regression comparing each of the antiplatelet and statin therapy groups to their respective control groups. The logit-transformed predicted probabilities of being treated with antiplatelets, as obtained from this model, were used to represent the antiplatelet propensity score, which was included as a covariate in the embolism model along with the indicator variable for antiplatelet therapy. Likewise, the statin propensity score constructed from the logit-transformed predicted probabilities of the statin multivariable model used as an adjusting covariate in the embolism model comparing those previously treated vs not treated with statin therapy.
As a secondary end point, the rate of 6-month mortality was compared between treatment groups using the Cox proportional hazards regression, adjusting for the propensity to be treated. In addition, for both study end points, hypothesized risk factors were evaluated via multivariable regression, with those detected from univariate modeling as having a significant to marginal association (P<.10) included as adjusting covariates along with the propensity score in the treatment-outcome models. All analyses were performed using the SAS statistical software package, version 9.1 (SAS Institute Inc, Cary, NC). P<.05 was considered statistically significant.
RESULTS
The study cohort comprised 283 patients with IE diagnosed between January 1, 2003, and December 31, 2006, of whom 116 (41.0%) had received continuous antiplatelet therapy and 77 (27.2%) had received continuous statin therapy (Table 1). A total of 59 patients (20.8%) had received both prior antiplatelet and statin therapy, whereas 149 (52.6%) had received neither. The predominant agents used were aspirin (96.5%) for antiplatelet therapy and atorvastatin (52.6%) followed by simvastatin (38.2%) for statin therapy. Among patients who received aspirin, most received a dosage of 81 mg/d.
TABLE 1.
Characteristics of the Study Cohort
In all, 94 (33.2%) of the 283 patients developed symptomatic embolic events, including 31 patients who died within 6 months. The predominant embolic events were stroke (56.4%), splenic embolism (26.6%), and renal embolism (22.3%). To understand the effect of antiplatelet therapy and statin therapy on embolic risk, the nonrandomized treatment groups were first studied on the basis of the following separate comparisons: (1) antiplatelet-treated patients vs non–antiplatelet-treated patients and (2) statin-treated patients vs non–statin-treated patients. A 2-staged propensity score design was used to control for any nonrandom factors that may have prompted either treatment to be prescribed.
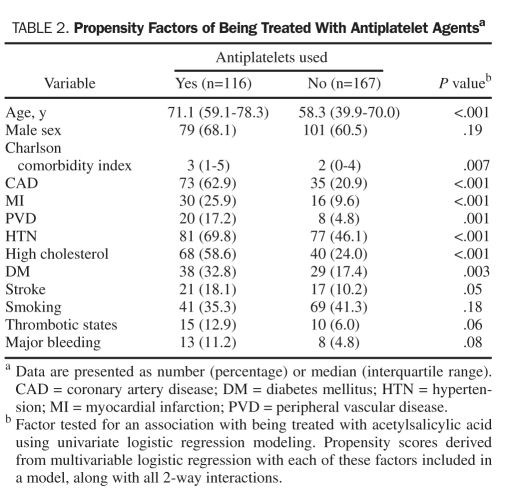
Patients receiving antiplatelet therapy were generally older and had increased comorbidity compared with patients not taking such agents (Table 2). From univariate analysis, the comorbid conditions associated with receiving antiplatelet therapy were primarily cardiovascular related, including hypertension, hyperlipidemia, coronary artery disease, peripheral vascular disease, prior myocardial infarction, diabetes mellitus, and prior stroke. These factors, as well as age, male sex, Charlson comorbidity index, smoking status, thrombotic states, and a history of major bleeding, were considered to contribute to the propensity to treat with antiplatelet medicines and thus used to calculate the antiplatelet propensity score (Table 2).
TABLE 2.
Propensity Factors of Being Treated With Antiplatelet Agentsa
Similar to those undergoing antiplatelet therapy, patients who received statin therapy were more likely to be older, to be male, and to have increased cardiovascular comorbidity, particularly prior hyperlipidemia (Table 3). Other comorbid conditions associated with receiving statin therapy included prior hypertension, coronary artery disease, past myocardial infarction, and diabetes mellitus. Although not statistically significant by univariate analysis, peripheral vascular disease, thrombotic states, and prior anticoagulation were considered factors that may have contributed to the propensity to treat with statin therapy and were included in the propensity model.
TABLE 3.
Propensity Factors of Being Treated With Statinsa
In all, 28 (24.1%) of the 116 patients who had received prior continuous antiplatelet therapy developed a symptomatic embolic event compared with 66 (39.5%) of the 167 patients who had not received prior antiplatelet therapy. The apparent benefit of prior antiplatelet therapy resulted in a 2-fold reduction in risk of symptomatic emboli (odds ratio [OR], 0.49; 95% confidence interval [CI], 0.29-0.82; P=.007). However, after adjusting for propensity to treat with antiplatelet therapy, the benefit attributable to antiplatelet agents was attenuated and not statistically significant (OR, 0.71; 95% CI, 0.37-1.36; P=.30). The effect was further attenuated with additional adjustment for symptomatic emboli risk factors (OR, 0.82; 95% CI, 0.41-1.65; P=.58) and also for statin therapy and propensity (OR, 0.94; 95% CI, 0.46-1.92; P=.86) (Table 4).
TABLE 4.
Association of Antiplatelet and Statin Therapy With Outcomes
Only 14 patients (18.2%) receiving prior continuous statin therapy developed a symptomatic embolic event compared with 80 (39.4%) of the 203 patients who did not receive prior continuous statin therapy. The unadjusted reduction in risk of symptomatic emboli from use of prior statin therapy was nearly 3-fold (OR, 0.35; 95% CI, 0.18-0.67; P=.001). After adjusting for propensity to treat with statin therapy, the benefit attributable to statins was slightly stronger (OR, 0.30; 95% CI, 0.14-0.62; P=.001). Further adjustment for symptomatic emboli risk factors slightly attenuated the effect of statin therapy on embolism (OR, 0.40; 95% CI, 0.15-1.04; P=.06), as did controlling for the additional covariates of antiplatelet therapy and propensity (OR, 0.42; 95% CI, 0.16-1.13; P=.08). Thus, we found that statin use corresponded to a significant reduction in embolic risk independent of propensity factors, as well as a borderline association independent of symptomatic risk factors and of antiplatelet therapy and propensity.
The 6-month mortality rate of the entire cohort after IE diagnosis was 28% (95% CI, 23%-34%). No significant difference was found in the unadjusted rate of 6-month mortality between patients with and without prior antiplatelet therapy (P=.87) or between those with and without prior statin therapy (P=.85; Table 4). Adjusted for their respective propensity scores, the protection against 6-month mortality was also not significant for patients undergoing prior antiplatelet therapy (hazard ratio [HR], 0.97; 95% CI, 0.55-1.69; P=.91) compared with those not undergoing therapy or for those undergoing prior statin therapy (HR, 1.05; 95% CI, 0.57-1.93; P=.87) compared with those not undergoing prior statin therapy. Further adjustment for 6-month mortality risk factors and for the other treatment group and propensity covariates yielded a nonassociation for prior statin therapy and a nonsignificant protective trend against 6-month mortality for prior antiplatelet use (HR, 0.64; 95% CI, 0.33-1.24; P=.18).
DISCUSSION
The current work was not designed to define purported risk factors associated with embolism in IE. It represents an initial investigation of the potential role of prior statin therapy as preemptive therapy that may affect the rate of symptomatic emboli in IE. Our primary aim was to assess the occurrence of symptomatic embolic events in patients who developed IE after having received prior long-term statin and antiplatelet therapies or after having received no such therapies. An evaluation of statin therapy was not feasible in our prior retrospective investigation5 because the period (1980-1998) of patient inclusion largely covered a prestatin era of antihyperlipidemic therapy. Although the exact mechanism(s) involved in the perceived benefit of prior statin therapy in symptomatic embolic risk reduction in patients who subsequently develop IE remains to be defined, it could be attributed to several factors, including immunomodulatory, anti-inflammatory, and antiplatelet effects.12,13
The immunomodulatory properties of statins may explain the observed preventive effects against infection in a number of observational studies.13 Statins decrease levels of intermediate products of cholesterol synthesis, including mevalonate, farnesyl pyrophosphate, and geranylgeranyl pyrophosphate, which play a crucial role in several intracellular signaling pathways involved in the innate and adaptive immune systems and anti-inflammatory effects.19,20 A number of clinical studies in different settings, including atherosclerosis, organ transplant, osteoporosis, dementia, multiple sclerosis, and rheumatoid arthritis, provide evidence of the immunomodulatory effects of statin use that are independent of its lipid-lowering effect.19-21 Statins appear to reduce interferon γ–induced major histocompatibility complex class II expression, which is involved in controlling immune responses such as T-cell activation.19 Statins also exert an anti-inflammatory response independent of their lipid-lowering properties.21 Simvastatin has been shown to inhibit the inflammatory response to Staphylococcus aureus α-toxin in rats,22 and clinical trials in atherosclerosis have demonstrated a positive influence of statins on endothelial function.23,24
Statins may also exert a direct effect on pathogenic microorganisms. Lovastatin has been found to reduce the intracellular growth of Salmonella Typhimurium in cultured macrophages25 and also reduces human immunodeficiency virus load and increases CD4 T-cell counts in acute human immunodeficiency virus infection in animal models and in patients with chronic human immunodeficiency virus infection.26
A recent systematic review and meta-analysis suggested that statin use may be associated with a beneficial effect in treating and preventing a variety of infections.13 The pooled adjusted estimate from observational studies suggested that patients who developed infection and were taking statins had improved outcomes.
It is well established that statins, as a manifestation of their pleiotropic effects, exert an antiplatelet effect,12 which could account for the observations described in our study. Statin use is associated with a reduced thrombosis burden and diminished platelet activity, which have been demonstrated in both animal models and in vitro studies.27,28 Statins, in general, and atorvastatin, in particular, inhibit platelet expression of the protease-activated receptor 1 (PAR-1) thrombin receptor.29 The PAR-1 Inhibition by Statins12 study showed that treatment with all statins, regardless of class, was associated with significant inhibition of activated and, later, intact platelet thrombin PAR-1 receptor for primary prevention in patients with documented metabolic syndrome. The PAR-1 Inhibition by Statins12 study demonstrated that statins specifically target the platelet thrombin receptor and therefore have modulating antiplatelet and antithrombotic properties. Notably, the observed effect of statins on PAR-1 is drug class specific and is not affected by aspirin or clopidogrel.12,29 Thrombin is a potent serine protease that is a cornerstone in hemostasis after tissue injury by converting soluble plasma fibrinogen into an insoluble fibrin clot and by promoting irreversible platelet aggregation.30 Thrombin enhances the recruitment and trafficking of inflammatory cells, also serving as a potent mitogen to endothelial cells, fibroblasts, and smooth muscle cells, promoting platelet-vessel wall crosstalk, and remodeling vascular endothelium,31 all mechanisms thought to be involved in vegetation formation and progression. In summary, although not definitely proven to be effective in patients with IE, the likely mechanisms of the perceived benefits of statin therapy include a combination of immunomodulatory, anti-inflammatory, and antiplatelet effects.
Although on univariate analysis antiplatelet therapy with aspirin was associated with reduced symptomatic embolic events, on multivariate analysis a statistical significance was not maintained. Our group previously reported5 a reduction in symptomatic embolic events among patients receiving continuous antiplatelet therapy (OR, 0.36; 95% CI, 0.19-0.68; P=.02) before the development of IE.5 These results paralleled observations in experimental models of endocarditis.32-37 When considering both the current and the previous findings from our 2 investigations, a critical difference exists between the 2 study cohorts in the frequency of cardiovascular risk factors for which antiplatelet agents were prescribed. In our initial study,5 patients who received prior daily antiplatelet therapy for cardiovascular comorbid conditions, including hypertension, coronary artery disease, and prior myocardial infarction, did not experience the same benefit of symptomatic embolic risk reduction as did those without these conditions; the hypothesis was that the lack of benefit could have been attributed to several factors, including the phenomenon of aspirin resistance, that may attenuate the beneficial effects of aspirin in this setting. In the current, contemporary cohort, more patients received antiplatelet therapy, which was influenced by the presence of traditional cardiovascular risk factors. When compared with our earlier cohort,5 there is a higher proportion of patients with a history of coronary artery disease (62.9% vs 43.8%) and hypertension (72.9% vs 48.5%) in the contemporary study. The preponderance of patients with these risk factors in our current study may have contributed to an attenuation of a true benefit of aspirin therapy in symptomatic embolic risk reduction.
Congruent with findings in our prior study,5 there was no difference in 6-month mortality between patients who received continuous daily antiplatelet (or statin therapy) compared with those who did not. This can be explained, in part, by an increased number of comorbid conditions and confounding factors that characterized patients who received either antiplatelet or statin therapy. For instance, the median age of patients who received antiplatelet and statin therapies was 13 years and 12 years older, respectively, than those who did not receive such therapies. Also, patients who received either therapy were, in general, more likely to have coronary artery disease, hyperlipidemia, and higher Charlson comorbidity index scores. Although the propensity design and regression adjustment attempted to control for treatment group imbalances, the preponderance of comorbidity and confounding may have rendered insufficient statistical power to detect an association after this adjustment. In particular, after controlling for the antiplatelet propensity score, several mortality risk factors, and prior statin therapy and propensity, there was a 35% reduction in hazard of 6-month mortality for those with prior continuous antiplatelet therapy vs those without. Although this was not statistically significant, one could argue this finding represents a clinically important benefit and should not be overlooked.
Our study has several limitations. Mayo Clinic is a large tertiary care facility, and there is potential for referral bias among patients who are referred for admission to these larger facilities. Our findings are based on data collected in a retrospective study design; although all medical records were available for review, completeness and accuracy of the patient records cannot be guaranteed. Ideally, a prospective, randomized trial would be conducted to evaluate the effects of therapies on prevention of a disease or its complications. This method of study is difficult to perform in the examination of IE because of the low incidence of disease and the associated financial costs of conducting an extremely large prospective, randomized trial to determine the impact, if any, of adjunctive “preventive” therapies including antiplatelet agents on disease complications. In fact, the largest prospective, randomized trial in IE enrolled only 115 patients from 19 centers during 4 years,6 thus highlighting the aforementioned challenges. These factors have been operative in our inability to conduct a trial to determine whether antibiotic prophylaxis in the setting of dental procedures is efficacious in preventing IE, for example. We did not collect data on complications involving bleeding in our study. We did not review the medical records of patients included in our earlier study for statin use. Because statin therapy became more common after the publication of a pivotal investigation in 1994,14 we do not believe that statin use was prevalent in the earlier cohort that had been seen between 1980 and 1998.
Continuous daily statin therapy administered before infection onset is associated with a decreased rate of symptomatic embolic events in IE patients. Thus, it is conceivable that continuous daily statin therapy could prove to be beneficial in selected patients with known underlying valvular abnormalities who are at increased risk of developing IE. Although a similar benefit was not observed with prior aspirin therapy, the absence of a beneficial effect may be attributed to patient factors that were previously described, and as such further study in appropriately selected patients without traditional cardiovascular risk factors may allude to the true presence or absence of effect.
CONCLUSION
The current investigation provides findings that are sentinel and support the notion that adjunctive therapies could conceivably play a role in the current medical approach in the treatment of IE. Although it would be premature to advocate such therapies to decrease the rate of symptomatic embolic events in patients at increased risk of developing IE, it would appear that such therapy influences the pathogenesis of the disease, and therefore continued study of these therapies seems prudent.
REFERENCES
- 1. Sanfilippo AJ, Picard MH, Newell JB, et al. Echocardiographic assessment of patients with infectious endocarditis: prediction of risk for complications. J Am Coll Cardiol. 1991;18:1191-1199 [DOI] [PubMed] [Google Scholar]
- 2. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association. Circulation. 2005;111:e394-e434 [DOI] [PubMed] [Google Scholar]
- 3. Delahaye JP, Poncet P, Malquarti V, Beaune J, Gare JP, Mann JM. Cerebrovascular accidents in infective endocarditis: role of anticoagulation. Eur Heart J. 1990;11:1074-1078 [DOI] [PubMed] [Google Scholar]
- 4. Heiro M, Nikoskelainen J, Engblom E, Kotilainen E, Marttila R, Kotilainen P. Neurologic manifestations of infective endocarditis: a 17-year experience in a teaching hospital in Finland. Arch Intern Med. 2000;160:2781-2787 [DOI] [PubMed] [Google Scholar]
- 5. Anavekar NS, Tleyjeh IM, Anavekar NS, et al. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin Infect Dis. 2007;44:1180-1186 [DOI] [PubMed] [Google Scholar]
- 6. Chan KL, Dumesnil JG, Cujec B, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol. 2003;42:775-780 [DOI] [PubMed] [Google Scholar]
- 7. Chan KL, Tam J, Dumesnil JG, et al. Effect of long-term aspirin use on embolic events in infective endocarditis. Clin Infect Dis. 2008;46:37-41 [DOI] [PubMed] [Google Scholar]
- 8. Eisen DP, Corey GR, McBryde ES, et al. Reduced valve replacement surgery and complication rate in Staphylococcus aureus endocarditis patients receiving acetyl-salicylic acid. J Infect. 2009;58:332-338 [DOI] [PubMed] [Google Scholar]
- 9. Taha TH, Durrant SS, Mazeika PK, Nihoyannopoulos P, Oakley CM. Aspirin to prevent growth of vegetations and cerebral emboli in infective endocarditis. J Intern Med. 1992;231:543-546 [DOI] [PubMed] [Google Scholar]
- 10. Pepin J, Tremblay V, Bechard D, et al. Chronic antiplatelet therapy and mortality among patients with infective endocarditis. Clin Microbiol Infect. 2009;15:193-199 [DOI] [PubMed] [Google Scholar]
- 11. Sedlacek M, Gemery JM, Cheung AL, Bayer AS, Remillard BD. Aspirin treatment is associated with a significantly decreased risk of Staphylococcus aureus bacteremia in hemodialysis patients with tunneled catheters. Am J Kidney Dis. 2007;49:401-408 [DOI] [PubMed] [Google Scholar]
- 12. Serebruany VL, Miller M, Pokov AN, et al. Effect of statins on platelet PAR-1 thrombin receptor in patients with the metabolic syndrome (from the PAR-1 inhibition by statins [PARIS] study). Am J Cardiol. 2006;97:1332-1336 [DOI] [PubMed] [Google Scholar]
- 13. Tleyjeh IM, Kashour T, Hakim FA, et al. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med. 2009;169:1658-1667 [DOI] [PubMed] [Google Scholar]
- 14. Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383-1389 [PubMed] [Google Scholar]
- 15. Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ. 1976;54:541-553 [PMC free article] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383 [DOI] [PubMed] [Google Scholar]
- 17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619 [DOI] [PubMed] [Google Scholar]
- 18. Hasbun R, Vikram HR, Barakat LA, Buenconsejo J, Quagliarello VJ. Complicated left-sided native valve endocarditis in adults: risk classification for mortality. JAMA. 2003;289(50):1933-1940 [DOI] [PubMed] [Google Scholar]
- 19. Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160-1164 [DOI] [PubMed] [Google Scholar]
- 20. Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399-1402 [DOI] [PubMed] [Google Scholar]
- 21. Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977-987 [DOI] [PubMed] [Google Scholar]
- 22. Pruefer D, Makowski J, Schnell M, et al. Simvastatin inhibits inflammatory properties of Staphylococcus aureus alpha-toxin. Circulation. 2002;106:2104-2110 [DOI] [PubMed] [Google Scholar]
- 23. Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527-3561 [PubMed] [Google Scholar]
- 24. Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266-24271 [DOI] [PubMed] [Google Scholar]
- 25. Catron DM, Lange Y, Borensztajn J, Sylvester MD, Jones BD, Haldar K. Salmonella enterica serovar typhimurium requires nonsterol precursors of the cholesterol biosynthetic pathway for intracellular proliferation. Infect Immun. 2004;72:1036-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. del Real G, Jimenez-Baranda S, Mira E, et al. Statins inhibit HIV-1 infection by down-regulating Rho activity. J Exp Med. 2004;200:541-547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schafer K, Kaiser K, Konstantinides S. Rosuvastatin exerts favourable effects on thrombosis and neointimal growth in a mouse model of endothelial injury. Thromb Haemost. 2005;93:145-152 [DOI] [PubMed] [Google Scholar]
- 28. Undas A, Brozek J, Musial J. Anti-inflammatory and antithrombotic effects of statins in the management of coronary artery disease. Clin Lab. 2002;48:287-296 [PubMed] [Google Scholar]
- 29. Serebruany VL, Midei MG, Malinin AI, et al. Absence of interaction between atorvastatin or other statins and clopidogrel: results from the interaction study. Arch Intern Med. 2004;164:2051-2057 [DOI] [PubMed] [Google Scholar]
- 30. De Cristofaro R, De Candia E. Thrombin domains: structure, function and interaction with platelet receptors. J Thromb Thrombolysis. 2003;15:151-163 [DOI] [PubMed] [Google Scholar]
- 31. Patterson C, Stouffer GA, Madamanchi N, Runge MS. New tricks for old dogs: nonthrombotic effects of thrombin in vessel wall biology. Circ Res. 2001;88:987-997 [DOI] [PubMed] [Google Scholar]
- 32. Kupferwasser LI, Yeaman MR, Nast CC, et al. Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J Clin Invest. 2003;112:222-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kupferwasser LI, Yeaman MR, Shapiro SM, et al. Acetylsalicylic acid reduces vegetation bacterial density, hematogenous bacterial dissemination, and frequency of embolic events in experimental Staphylococcus aureus endocarditis through antiplatelet and antibacterial effects. Circulation. 1999;99:2791-2797 [DOI] [PubMed] [Google Scholar]
- 34. Nicolau DP, Freeman CD, Nightingale CH, et al. Reduction of bacterial titers by low-dose aspirin in experimental aortic valve endocarditis. Infect Immun. 1993;61:1593-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nicolau DP, Marangos MN, Nightingale CH, Quintiliani R. Influence of aspirin on development and treatment of experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1995;39:1748-1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nicolau DP, Tessier PR, Nightingale CH. Beneficial effect of combination antiplatelet therapy on the development of experimental Staphylococcus aureus endocarditis. Int J Antimicrob Agents. 1999;11:159-161 [DOI] [PubMed] [Google Scholar]
- 37. Nicolau DP, Tessier PR, Nightingale CH, Quintiliani R. Influence of adjunctive ticlopidine on the treatment of experimental Staphylococcus aureus endocarditis. Int J Antimicrob Agents. 1998;9:227-229 [DOI] [PubMed] [Google Scholar]