Abstract
OBJECTIVE: To investigate the association between 347 single-nucleotide polymorphisms within candidate genes of the tumor necrosis factor, interleukin 1 and interleukin 6 families with neutrophil count.
PATIENTS AND METHODS: Four hundred cases with heart failure after myocardial infarction (MI) were matched by age, sex, and date of incident MI to 694 controls (MI without post-MI heart failure). Both genotypes and neutrophil count at admission for incident MI were available in 314 cases and 515 controls.
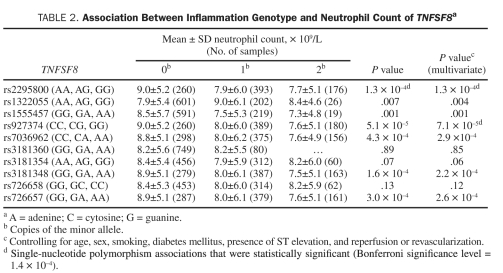
RESULTS: We found significant associations between the TNFSF8 poly morphisms rs927374 (P=5.1 x 10–5) and rs2295800 (P=1.3 x 10–4) and neutrophil count; these single-nucleotide polymorphisms are in high linkage disequilibrium (r2=0.97). Associations persisted after controlling for clinical characteristics and were unchanged after adjusting for case-control status. For rs927374, the neutrophil count of GG homozygotes (7.6±5.1) was 16% lower than that of CC homozygotes (9.0±5.2).
CONCLUSION: The TNFSF8 polymorphisms rs927374 and rs2295800 were associated with neutrophil count. This finding suggests that post-MI inflammatory response is genetically modulated.
GWAS = genome-wide association study; HF = heart failure; IL = interleukin; MAF = minor allele frequency; MI = myocardial infarction; SNP = single-nucleotide polymorphism; TNF = tumor necrosis factor
Myocardial infarction (MI) triggers an inflammatory response.1,2 Early in the post-MI inflammatory phase, proinflammatory cytokines with chemoattractant properties are activated and contribute to neutrophil recruitment to the infarcted area.1,2 In addition, proinflammatory cytokines3 promote demargination of intravascular neutrophils, acceleration of the release of neutrophils by the bone marrow, and activation of neutrophils.4-6 Indeed, the early post-MI period is frequently marked by activation of neutrophils7,8 and elevation of the peripheral neutrophil count. We have previously demonstrated that neutrophil count on incident MI presentation is strongly and independently associated with adverse outcomes.9 Secondary analysis of clinical trials of ST-elevation MI also showed associations between peripheral neutrophil count and post-MI adverse outcomes.10
The proinflammatory cytokines include the tumor necrosis factor (TNF), interleukin (IL) 1 and IL-6 families,2,11,12 which are not constitutively expressed in the heart.13,14 However, these cytokines are consistently and rapidly expressed in response to myocardial injury, including MI.15-19 The short-term limited expression of cytokines provides the heart with an adaptive response to injury. This protective response may occur at the cost of unwanted deleterious effects, including left ventricular dysfunction,20-23 which may manifest clinically as heart failure (HF) syndrome. In HF, increased levels of both TNF and IL-6 are associated with worse survival.6,24 After MI, levels of IL-6 are associated with an increase in left ventricular end-diastolic volume and remodeling.25
Production of proinflammatory cytokines is genetically modulated with heritability estimates ranging from 53% to 86%.26,27 It is unknown whether genetic variants of the proinflammatory cytokine families are associated with post-MI inflammation as assessed by the inflammatory marker of neutrophil count. Therefore, we investigated the association between 347 single-nucleotide polymorphisms (SNPs) within candidate genes of the proinflammatory cytokine families, including TNF (18 genes), IL-1 (10 genes), and IL-6 (12 genes), and peripheral neutrophil count in a nested case-control study from the Olmsted County, Minnesota, cohort of incident MI.28 This study tests the hypothesis that variants within candidate genes of these proinflammatory cytokine families are associated with peripheral neutrophil count in patients with MI.
PATIENTS AND METHODS
Study Design
The parent study, which enabled the current investigation, consists of an observational cohort of patients with incident MI within the geographically defined population of Olmsted County, where Mayo Clinic and Olmsted Medical Center provide medical care for all county residents. These facilities use a unified record linkage system that accumulates comprehensive clinical records. The Rochester Epidemiology Project enables these records to be easily retrieved.29 Details of assembly of the DNA repository that was used for the identification and recruitment of participants in this study have been previously published.28
The current study design was a case-control study of white patients nested in the incident MI cohort. The case group consisted of 400 patients with MI and post-MI HF (HF within 90 days after MI), and the control group consisted of 694 MI patients who did not experience post-MI HF (within 90 days after MI). We attempted to match cases and controls 1:2 by age (±5 years), sex, and date of diagnosis of the acute MI (±5 years). If 1:2 matching was not possible, we applied 1:1 matching. The Mayo Clinic Institutional Review Board approved this study (1194-05), and all participants signed a written informed consent form.
The Framingham Heart Study criteria were used to diagnose HF.30 Masked abstractors unaware of the case-control status reviewed the records of all study participants and validated the diagnosis of post-MI HF. Ascertaining HF based on the Framingham Heart Study criteria in our experience yielded an excellent intraobserver agreement (κ=0.87; 95% confidence interval, 0.78-0.96).31 Clinical diagnoses were used to ascertain systemic hypertension, diabetes mellitus, hyperlipidemia, familial coronary artery disease, and smoking status (defined as current or prior smoking history). Body mass index was calculated using the admission height and weight. Clinical data obtained also included Killip class. Reperfusion therapy was defined as the use of thrombolytic therapy, percutaneous coronary intervention, or coronary artery bypass surgery.
Left ventricular ejection fraction was measured during hospitalization for the acute MI by previously validated methods, including M-mode or bidimensional echocardiography using the Quinones formula from the parasternal views,32 by the quantitative bidimensional biplane volumetric Simpson method,33 and by the bidimensional estimate method from multiple echocardiographic views.34 Ejection fraction values were averaged when multiple measurements were performed. Wall motion in each of 16 segments was scored 1 through 5.35 The wall motion score index was determined as the sum of the segmental scores divided by the number of visualized segments.35 Diastolic function was assessed by a previously described approach,36 which integrates Doppler measurements of the mitral inflow and Doppler tissue imaging of the mitral annulus using the medial annulus velocity.37
Peripheral Neutrophil Count
Clinically indicated blood samples were drawn at presentation for the incident MI. The peripheral blood leukocyte count was estimated with an automated hematology analyzer. This instrument uses approximately 100,000 cells per differential to produce a differential count, including absolute neutrophil count,38 which was used for this study.
SNP Selection
The candidate genes included the proinflammatory cytokine families TNF (18 genes), IL-1 (10 genes), and IL-6 (12 genes), as described in the Kyoto Encyclopedia of Genes and Genomes (Table S1 [a link to which is provided at the end of this article]). Two approaches were used to select SNPs for genotyping, one based on haplotypes and the other on linkage disequilibrium. We based our haplotype tag SNP selection on the technique of Stram et al,39 using an r2 of 0.90, tagging haplotypes with a frequency of 2% or higher. We applied the linkage disequilibrium select method developed by Carlson et al40 (http://droog.gs.washington.edu/ldSelect.html) on HapMap central ethnicity, Utah origin data. This population consists of Utah residents with Northern and Western European ancestry from the Centre d’etude du Polymorphisme Humain collection. We used an r2 of 0.90 and a minor allele frequency (MAF) of 0.05. The SNPs with design scores greater than 0.60 (Illumina Inc, San Diego, CA) were preferentially selected.
Genotyping and Quality Control
Genotyping was performed in the Mayo Genotyping Shared Resource in 2 series using the Illumina Golden-Gate assay.41 All pairwise replicate sample comparisons showed more than 99.5% genotype call concordance rate. Because all X-linked genes of this study lie outside the pseudoautosomal regions, males only have homozygous genotype for the relevant SNPs. We deleted 5 reported male samples because of varying degrees of heterozygosity in the X-linked SNPs. Evaluation of paired identity by state in the remaining 1034 nonduplicated incident MI samples revealed one related pair of samples, indicating possible sample mix-up. This pair together with 12 other samples having genotype call rates of less than 98% was also excluded from the study. Besides these 23 SNPs, we eliminated an additional 33 SNPs for the following reasons: 30 with an MAF less than 5%, 2 with a call rate less than 98%, and 1 with a difference in MAF of more than 10% between the 2 series. Three SNPs deviated significantly from the Hardy-Weinberg equilibrium (χ2 test, P<.0001) but were retained on graphic review of their genotype clusters. Note that only female samples were included in the calculation of MAF and Hardy-Weinberg equilibrium for the X-link SNPs.
Statistical Analyses
The clinical characteristics of patient groups were compared using the χ2 test for categorical variables and the Wilcoxon rank sum test for continuous variables. Individual SNP associations with neutrophil count were assessed with linear regression modeling adjusted for the clinical covariates of age, sex, smoking, diabetes mellitus, presence of ST elevation, and reperfusion or revascularization. Analysis was also completed with no clinical covariates included in the linear regression model. The distribution of neutrophil count was highly positively skewed; hence, a natural log transformation was applied. Autosomal SNPs were coded as nominal variables with homozygotes for the major allele in the reference group, whereas minor homozygotes and heterozygotes were used as the alternative categories. For the X-linked SNPs, the alternative categories were replaced by male hemizygotes, female heterozygotes, and female homozygotes. When homozygotes for the minor allele of an SNP had a frequency of less than 5, we combined them with the heterozygotes. We performed a case-control stratification analysis to ensure that any significant association was not a consequence of the study design. To correct for multiple comparisons, we used a Bonferroni correction based on the number of SNPs tested (0.05/347=1.4 × 10–4). The confidence interval–based haplotype block definition in Haploview version 4.2 (Broad Institute, Cambridge, MA)42 was used to generate a linkage disequilibrium plot of the genomic region of interest.
RESULTS
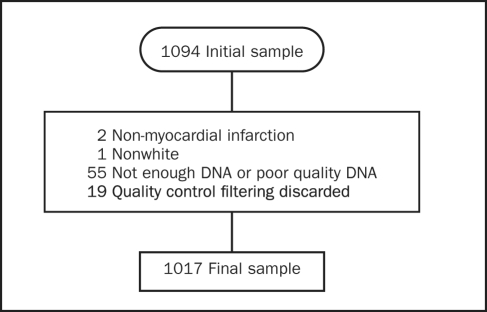
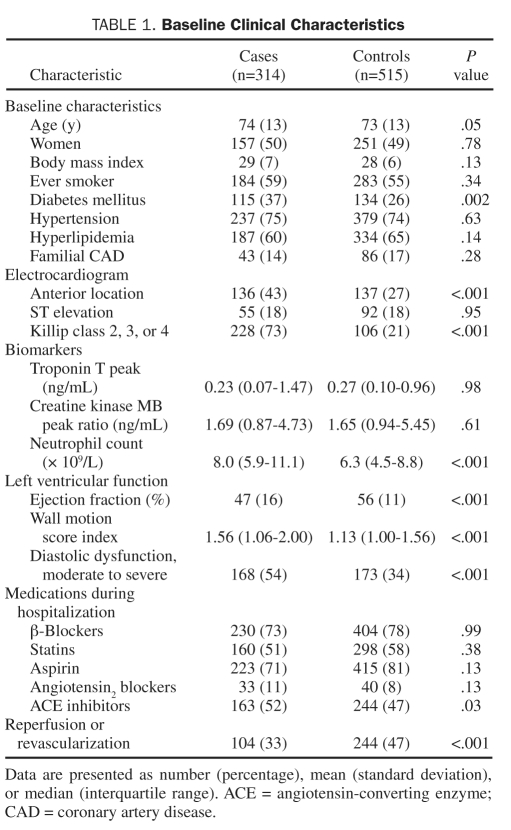
After application of the exclusion criteria previously detailed (Figure 1), 1017 samples had adequate genotyping; of those, neutrophil count was available in 314 cases and 515 controls. Baseline clinical characteristics are summarized in Table 1. Cases had higher neutrophil counts and worse left ventricular function as indicated by higher Killip class, worse ejection fraction, and higher wall motion score index.
FIGURE 1.
Reasons for exclusion of samples.
TABLE 1.
Baseline Clinical Characteristics
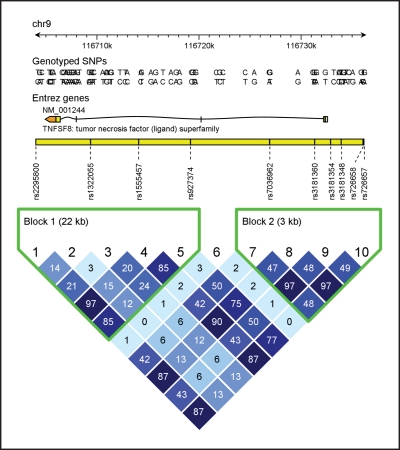
The associations between the TNFSF8 polymorphisms rs927374 (P=5.1 x10–5) and rs2295800 (P=1.3 × 10–4) and peripheral neutrophil count (Table 2) were deemed significant with the Bonferroni correction. These associations remained significant after controlling for age, sex, smoking, diabetes mellitus, presence of ST elevation, and reperfusion or revascularization. Ancillary analysis, including casecontrol status as covariate and stratifying by case-control status, indicated that the associations were unaffected by case-control status (data not shown). The association of all TNFSF8 SNPs with neutrophil count is shown in Table 2. Other SNPs were marginally associated with neutrophil count. This finding could be explained by the strong linkage disequilibrium in the region (Figure 2). In particular, the r2 value was 0.97 between TNFSF8 rs927374 and rs2295800 (Figure 2). The results of the other SNP associations with neutrophil count are summarized in Table S2 (see Supporting Online Material.
TABLE 2.
Association Between Inflammation Genotype and Neutrophil Count of TNFSF8a
FIGURE 2.
Linkage disequilibrium plot for variants in the TNFSF8 gene. A = adenine; C = cytosine; chr9 = chromosome 9; G = guanine; SNP = single-nucleotide polymorphism; T = thymine.
DISCUSSION
In this case-control study nested in the Olmsted County cohort of incident MI, we found significant associations between the TNFSF8 rs927374 and rs2295800 polymorphisms and peripheral neutrophil count in MI patients independent of case-control status. These 2 SNPs are in high linkage disequilibrium (r2=0.97), with rs927374 residing in an intron and rs2295800 outside the gene. For rs927374, the neutrophil count of the GG homozygotes was 16% lower than that of the CC homozygotes.
In a previous analysis of a multicenter trial of acute coronary syndromes, there was no evidence of an association between selected genotypes of the IL-6 and IL-1 family of genes with white blood cell count after MI; however, variants in the TNF family of genes were not studied.43 In contrast to the previous study, we expanded the list of candidate genes, included TNF family and the IL-6 and IL-1 families, evaluated their association with neutrophil count after MI, and identified an association between polymorphisms of the TNFSF8 gene with neutrophils in MI.
The SNP rs927374 is located in an intron of TNFSF8, and rs2295800 resides outside the gene. The TNFSF8 gene encodes the protein CD30L, which functions as the ligand for TNFRSF8 (CD30), a cell surface antigen. CD30L is a type 2 glycoprotein expressed on activated T cells and macrophages, as well as on B cells, mast cells, neutrophils, eosinophils, and various cancer cells.44-46 CD30L is constitutively expressed on neutrophils.47 Cross-linking of CD30L in neutrophils induced production of IL-8. That experiment also induced a strong, rapid oxidative burst,47 which suggested that CD30L may contribute to activation of neutrophils. Activated neutrophils generate oxidizing agents through the myeloperoxidase pathway. Previously, in patients with acute MI and unstable angina, there was reduction in the neutrophil myeloperoxidase, which suggested a significant release of myeloperoxidase from neutrophils and was a marker of neutrophil activation.7,8 However, the mechanism through which TNFSF8 variations might influence peripheral neutrophil count is unclear.
The association between genetic variations of TNFSF8 and neutrophil count in MI is interesting given that CD30L has been shown to be elevated in patients with cutaneous inflammatory diseases (psoriasis and atopic dermatitis),48 thyroid autoimmune diseases,49 and rheumatoid arthritis.50 Patients with rheumatoid arthritis are more likely to have MI51 and HF52 when compared with controls without rheumatoid arthritis. In addition, a prior autopsy study showed increased coronary inflammation in patients with rheumatoid arthritis.53 In a prospective cohort study, patients with rheumatoid arthritis who developed subsequent HF had a higher erythrocyte sedimentation rate, a marker of inflammation, than patients with rheumatoid arthritis without HF at follow-up.53 Collectively, those prior studies suggest that inflammation is a common mechanism for coronary artery disease, HF, and rheumatoid arthritis. Thus, there is a need to further investigate TNFSF8 variants with susceptibility to MI, HF, and systemic inflammatory diseases, such as rheumatoid arthritis.
A genome-wide association study (GWAS) in healthy Europeans identified a variant in CSF3 associated with total white blood cell count,54 but the genetic association with neutrophil count was not evaluated. Another GWAS demonstrated that variants in CSF3 were associated with neutrophil count in 5771 Japanese patients with 14 different diseases55 recruited to a Japanese biorepository. Conversely, in the current study, variants in the CSF3 gene were not associated with peripheral neutrophil count in incident MI. This difference in results could relate to differences in study design. The neutrophil count reported herein was likely regulated by a genetically modulated expression of proinflammatory cytokines released by the early post-MI inflammation, whereas participants of the GWAS were relatively healthy adults.54,55 Indeed, the European study recruited healthy volunteers,54 whereas the Japanese study included 6% of patients with a history of prior MI.55 When comparing our results with the Japanese study, the differences in ethnicity could also have contributed to the inconsistent findings.
Our study has several strengths. It is a population-based, case-control study with detailed phenotype characterization that follows strict and previously validated epidemiological criteria.31,56,57 Clinically indicated blood samples were drawn at admission for the incident MI and were used to determine peripheral neutrophil count, which is an inflammatory marker. In addition, we report high-quality genotyping data. The primary limitations of our study are a limited sample size, lack of expression level of TNFSF8, and the unavailability of admission neutrophil count for all study participants. The positive findings in our report require replication in larger studies with greater power and ethnic diversity. Future studies should also address the expression level of TNFSF8.
CONCLUSION
In this case-control study nested in the Olmsted County cohort of incident MI, we found significant associations between TNFSF8 rs927374 and rs2295800 with peripheral neutrophil count in patients with MI. Moreover, our data were consistent with an association between neutrophil count and genotype. This finding suggests that post-MI inflammatory response is genetically modulated. These findings require replication in larger multiethnic studies.
Supplementary Material
Acknowledgments
We thank the Mayo Biospecimens Accessioning and Processing Shared Resource for DNA extraction, the Mayo Genomics Shared Resource for genotyping (supported by CA15083), Ellen Koepsell, RN, for study management, Kay Traverse, RN, and Susan Stotz, RN, for their assistance with data collection, Jill Killian, BS, for assistance with statistical analysis, and Kristie Shorter for secretarial support.
Footnotes
Supporting Online Material
www.mayoclinicproceedings.com/content/86/11/1075/suppl/DC1 Tables S1-S2
This study was supported by grants from a Clinician Investigator Fellowship Award from Mayo Clinic; grants from the Public Health Service; and the National Institutes of Health (R03 AG031347-01, R01 HL 59205, R01 HL 72435) and was made possible by the Rochester Epidemiology Project (AG034676, National Institute on Aging).
REFERENCES
- 1. Collins T. Acute and chronic inflammation. In: Kumar V, Abbas AK, eds. Robbins Pathologic Basis of Disease. Philadelphia, PA: WB Saunders Company; 1999. [Google Scholar]
- 2. Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem. 2006;13:1877-1893 [DOI] [PubMed] [Google Scholar]
- 3. Tousoulis D, Antoniades C, Koumallos N, Stefanadis C. Pro-inflammatory cytokines in acute coronary syndromes: from bench to bedside. Cytokine Growth Factor Rev. 2006;17:225-233 [DOI] [PubMed] [Google Scholar]
- 4. Suwa T, Hogg JC, English D, Van Eeden SF. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol. 2000;279:H2954-H2960 [DOI] [PubMed] [Google Scholar]
- 5. Ulich TR, del Castillo J, Busser K, Guo KZ, Yin SM. Acute in vivo effects of IL-3 alone and in combination with IL-6 on the blood cells of the circulation and bone marrow. Am J Pathol. 1989;135:663-670 [PMC free article] [PubMed] [Google Scholar]
- 6. Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988-998 [DOI] [PubMed] [Google Scholar]
- 7. Biasucci LM, D’Onofrio G, Liuzzo G, et al. Intracellular neutrophil myeloperoxidase is reduced in unstable angina and acute myocardial infarction, but its reduction is not related to ischemia. J Am Coll Cardiol. 1996;27:611-616 [DOI] [PubMed] [Google Scholar]
- 8. Buffon A, Biasucci LM, Liuzzo G, D’Onofrio G, Crea F, Maseri A. Widespread coronary inflammation in unstable angina. N Engl J Med. 2002; 347:5-12 [DOI] [PubMed] [Google Scholar]
- 9. Arruda-Olson AM, Reeder GS, Bell MR, Weston SA, Roger VL. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes. 2009;2(6):656-662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Donoghue M, Morrow DA, Cannon CP, et al. Association between baseline neutrophil count, clopidogrel therapy, and clinical and angiographic outcomes in patients with ST-elevation myocardial infarction receiving fibrinolytic therapy. Eur Heart J. 2008;29:984-991 [DOI] [PubMed] [Google Scholar]
- 11. Wilson EM, Diwan A, Spinale FG, Mann DL. Duality of innate stress responses in cardiac injury, repair, and remodeling. J Mol Cell Cardiol. 2004; 37:801-811 [DOI] [PubMed] [Google Scholar]
- 12. Hellermann JP, Reeder GS, Jacobsen SJ, Weston SA, Killian JM, Roger VL. Longitudinal trends in the severity of acute myocardial infarction: a population study in Olmsted County, Minnesota. Am J Epidemiol. 2002; 156:246-253 [DOI] [PubMed] [Google Scholar]
- 13. Kapadia S, Lee J, Torre-Amione G, Birdsall HH, Ma TS, Mann DL. Tumor necrosis factor-alpha gene and protein expression in adult feline myocardium after endotoxin administration. J Clin Invest. 1995;96:1042-1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kapadia SR, Oral H, Lee J, Nakano M, Taffet GE, Mann DL. Hemodynamic regulation of tumor necrosis factor-alpha gene and protein expression in adult feline myocardium. Circ Res. 1997;81:187-195 [DOI] [PubMed] [Google Scholar]
- 15. Maury CP, Teppo AM. Circulating tumour necrosis factor-alpha (cachectin) in myocardial infarction. J Intern Med. 1989;225:333-336 [DOI] [PubMed] [Google Scholar]
- 16. Basaran Y, Basaran MM, Babacan KF, et al. Serum tumor necrosis factor levels in acute myocardial infarction and unstable angina pectoris. Angiology. 1993;44:332-337 [DOI] [PubMed] [Google Scholar]
- 17. Ikeda U, Ohkawa F, Seino Y, et al. Serum interleukin 6 levels become elevated in acute myocardial infarction. J Mol Cell Cardiol. 1992;24:579-584 [DOI] [PubMed] [Google Scholar]
- 18. Miyao Y, Yasue H, Ogawa H, et al. Elevated plasma interleukin-6 levels in patients with acute myocardial infarction. Am Heart J. 1993;126:1299-1304 [DOI] [PubMed] [Google Scholar]
- 19. Cruickshank AM, Oldroyd KG, Cobbe SM. Serum interleukin-6 in suspected myocardial infarction [letter]. Lancet. 1994;343:974 [DOI] [PubMed] [Google Scholar]
- 20. Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol. 2003;65:81-101 [DOI] [PubMed] [Google Scholar]
- 21. Suffredini AF, Fromm RE, Parker MM, et al. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321:280-287 [DOI] [PubMed] [Google Scholar]
- 22. Pagani FD, Baker LS, Hsi C, Knox M, Fink MP, Visner MS. Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factoralpha in conscious dogs. J Clin Invest. 1992;90:389-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hegewisch S, Weh HJ, Hossfeld DK. TNF-induced cardiomyopathy. Lancet. 1990;335:294-295 [DOI] [PubMed] [Google Scholar]
- 24. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone Trial (VEST). Circulation. 2001;103:2055-2059 [DOI] [PubMed] [Google Scholar]
- 25. Ohtsuka T, Hamada M, Inoue K, et al. Relation of circulating interleukin-6 to left ventricular remodeling in patients with reperfused anterior myocardial infarction. Clin Cardiol. 2004;27:417-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Craen AJ, Posthuma D, Remarque EJ, van den Biggelaar AH, Westendorp RG, Boomsma DI. Heritability estimates of innate immunity: an extended twin study. Genes Immun. 2005;6:167-170 [DOI] [PubMed] [Google Scholar]
- 27. Wörns MA, Victor A, Galle PR, Hohler T. Genetic and environmental contributions to plasma C-reactive protein and interleukin-6 levels: a study in twins. Genes Immun. 2006;7:600-605 [DOI] [PubMed] [Google Scholar]
- 28. Arruda-Olson AM, Weston SA, Fridley BL, Killian JM, Koepsell EE, Roger VL. Participation bias and its impact on the assembly of a genetic specimen repository for a myocardial infarction cohort. Mayo Clin Proc. 2007;82:1185-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266-274 [DOI] [PubMed] [Google Scholar]
- 30. Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107-115 [DOI] [PubMed] [Google Scholar]
- 31. Hellermann JP, Goraya TY, Jacobsen SJ, et al. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol. 2003;157:1101-1107 [DOI] [PubMed] [Google Scholar]
- 32. Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744-753 [DOI] [PubMed] [Google Scholar]
- 33. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440-1463 [DOI] [PubMed] [Google Scholar]
- 34. Amico AF, Lichtenberg GS, Reisner SA, Stone CK, Schwartz RG, Meltzer RS. Superiority of visual versus computerized echocardiographic estimation of radionuclide left ventricular ejection fraction. Am Heart J. 1989;118:1259-1265 [DOI] [PubMed] [Google Scholar]
- 35. Schiller NB, Shah PM, Crawford M, et al. ; American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2: 358-367 [DOI] [PubMed] [Google Scholar]
- 36. Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194-202 [DOI] [PubMed] [Google Scholar]
- 37. Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788-1794 [DOI] [PubMed] [Google Scholar]
- 38. Pierre RV, Payne BA, Lee WK, Hyma BA, Melchert LM, Scheidt RM. Comparison of four leukocyte differential methods with the National Committee for Clinical Laboratory Standards (NCCLS) reference method. Am J Clin Pathol. 1987;87:201-209 [DOI] [PubMed] [Google Scholar]
- 39. Stram DO, Haiman CA, Hirschhorn JN, et al. Choosing haplotype-tagging SNPS based on unphased genotype data using a preliminary sample of unrelated subjects with an example from the Multiethnic Cohort Study. Hum Hered. 2003;55:27-36 [DOI] [PubMed] [Google Scholar]
- 40. Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shen R, Fan JB, Campbell D, et al. High-throughput SNP genotyping on universal bead arrays. Mutat Res. 2005;573:70-82 [DOI] [PubMed] [Google Scholar]
- 42. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263-265 [DOI] [PubMed] [Google Scholar]
- 43. Byrne CE, Fitzgerald A, Cannon CP, Fitzgerald DJ, Shields DC. Elevated white cell count in acute coronary syndromes: relationship to variants in inflammatory and thrombotic genes. BMC Med Genet. 2004;5:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith CA, Gruss HJ, Davis T, et al. CD30 antigen, a marker for Hodgkin’s lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73:1349-1360 [DOI] [PubMed] [Google Scholar]
- 45. Gruss HJ, Pinto A, Gloghini A, et al. CD30 ligand expression in nonmalignant and Hodgkin’s disease-involved lymphoid tissues. Am J Pathol. 1996;149:469-481 [PMC free article] [PubMed] [Google Scholar]
- 46. Pinto A, Aldinucci D, Gloghini A, et al. Human eosinophils express functional CD30 ligand and stimulate proliferation of a Hodgkin’s disease cell line. Blood. 1996;88:3299-3305 [PubMed] [Google Scholar]
- 47. Wiley SR, Goodwin RG, Smith CA. Reverse signaling via CD30 ligand. J Immunol. 1996;157:3635-3639 [PubMed] [Google Scholar]
- 48. Fischer M, Harvima IT, Carvalho RF, et al. Mast cell CD30 ligand is up-regulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion. J Clin Invest. 2006;116:2748-2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ruggeri RM, Barresi G, Sciacchitano S, Trimarchi F, Benvenga S, Trovato M. Immunoexpression of the CD30 ligand/CD30 and IL-6/IL-6R signals in thyroid autoimmune diseases. Histol Histopathol. 2006;21:249-256 [DOI] [PubMed] [Google Scholar]
- 50. Carvalho RF, Ulfgren AK, Engstrom M, Klint E, Nilsson G. CD153 in rheumatoid arthritis: detection of a soluble form in serum and synovial fluid, and expression by mast cells in the rheumatic synovium. J Rheumatol. 2009;36:501-507 [DOI] [PubMed] [Google Scholar]
- 51. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402-411 [DOI] [PubMed] [Google Scholar]
- 52. Nicola PJ, Crowson CS, Maradit-Kremers H, et al. Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis Rheum. 2006;54:60-67 [DOI] [PubMed] [Google Scholar]
- 53. Aubry MC, Maradit-Kremers H, Reinalda MS, Crowson CS, Edwards WD, Gabriel SE. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol. 2007; 34:937-942 [PubMed] [Google Scholar]
- 54. Soranzo N, Spector TD, Mangino M, et al. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat Genet. 2009;41:1182-1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Okada Y, Kamatani Y, Takahashi A, et al. Common variations in PSMD3-CSF3 and PLCB4 are associated with neutrophil count. Hum Mol Genet. 2010; 19(10):2079-2085 [DOI] [PubMed] [Google Scholar]
- 56. Roger VL, Killian JM, Weston SA, et al. Redefinition of myocardial infarction: prospective evaluation in the community. Circulation. 2006;114: 790-797 [DOI] [PubMed] [Google Scholar]
- 57. Roger VL, Jacobsen SJ, Weston SA, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136(5):341-348 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.