Abstract
Endomyocardial biopsy (EMB) is widely used for surveillance of cardiac allograft rejection and for the diagnosis of unexplained ventricular dysfunction. Typically, EMB is performed through the jugular or femoral veins and is associated with a serious acute complication rate of less than 1% using current flexible bioptomes. Although it is accepted that EMB should be used to monitor for rejection after transplant, use of EMB for the diagnosis of various myocardial diseases is controversial. Diagnosis of myocardial disease in the nontransplant recipient is often successful via noninvasive investigations including laboratory evaluation; echocardiography, nuclear studies, and magnetic resonance imaging can yield specific diagnoses in the absence of invasive EMB. Therefore, use of the technique is patient specific and depends on the potential prognostic and treatment information gained by establishing a pathologic diagnosis beyond noninvasive testing.
Acc = American college of cardiology; AHA = American heart Association; EMB = endomyocardial biopsy
Endomyocardial biopsy (EMB) is an established invasive clinical tool used for surveillance of cardiac allograft rejection and, to a lesser extent, in the evaluation of dilated and restrictive cardiomyopathies. Although a safe procedure, EMB is clearly associated with both a risk of procedural complications and long-term sequelae. This article reviews the current status of EMB, the technique, indications, diagnostic yield, and future directions.
TECHNIQUE
Since the introduction of the flexible Stanford-Caves Schultz bioptome1 and the King’s bioptome,2 the preferred access site for EMB has been the right internal jugular vein. Access through the right femoral vein and femoral artery for access to the right and left ventricle, respectively, is also feasible.3 Sheath size depends in part on type of bioptome used. Commonly used bioptomes are shown in Figure 1.
FIGURE 1.
Commonly used bioptomes. A, Single-use 50-cm Novatome (Sholten Surgical Instruments, Inc, Lodi, CA) with a 2.3-mm tip that requires a 9-F sheath. B, Argon endomyocardial biopsy forceps (Argon Medical Devices, Inc, Athens, TX) with a 1.8-mm tip that requires a 6-F sheath or a 2.3-mm tip that requires a 7-F sheath. C, Bipal 7 bioptome, 50 cm and 104 cm (Cordis Corp, Miami Lakes, FL) with a 2.3-mm tip that requires a 7-F sheath. D, 8-F Transseptal Mullens (Medtronic, Inc, Minneapolis, MN) sheath when using the longer Bipal 7 bioptome through right femoral vein access to improve tip control and placement.
Because the right ventricular free wall is thin, obtaining biopsy specimens from this area is dangerous; thus, biopsy samples should be taken from the interventricular septum.4 Endomyocardial biopsy may be guided by fluoroscopy, 2-dimensional echocardiography, or both. Studies that have reported on the utility and safety of fluoroscopic guidance with or without echocardiographic guidance while performing EMB have reported discordant data.5-7 In a study by Blomstrom-Lundqvist et al7 of more than 200 biopsies in 74 patients by either echocardiographic or fluoroscopic guidance, myocardial perforation occurred during 1 fluoroscopically guided procedure (1.7%), whereas Han et al8 reported a right ventricular perforation rate of 3.3% using echocardiography to guide transfemoral biopsies in 90 patients. However, reported case numbers are too small to draw firm conclusions. Both fluoroscopy and 2-dimensional echocardiography have limitations. Fluoroscopy provides planar imaging, but soft tissues are not visualized; directionality of the bioptome cannot be determined in only one projection. Most fluoroscopic suites have single-plane imaging systems, making control of tip directionality in the anteroposterior plane problematic unless care is taken to move the gantry before each biopsy. Echocardiography is a tomographic technique and may miss the tip of the deflectable bioptome; thus, care must be taken to ensure adequate tip visualization. The resolution of current 2-dimensional echocardiography is insufficient to visualize the chordae tendineae, which can be damaged during EMB. Technical advances, including 3-dimensional imaging, may allow better visualization of cardiac structures and biopsy instrumentation.9,10 Furthermore, the risk of perforation is different between patients who have had prior cardiac procedures, especially cardiac transplant, and patients in whom the pericardial space has never been violated. Clinical experience suggests that the risk of clinically relevant cardiac perforation in transplant recipients is small in part because the pericardial space has been nearly obliterated and any right ventricular perforation is contained by the pericardium. Therefore, transthoracic echocardiographic guidance is suggested for patients undergoing a first-time biopsy or within 3 months of cardiac transplant by which time the pericardium should be adhered to the myocardium, thereby decreasing the risk of tamponade should perforation occur. Cardiac allograft recipients may undergo EMB numerous times, which leads to scarring of the interventricular septum and increases the difficulty in obtaining adequate tissue samples for histologic analysis.
Article Highlights
Endomyocardial biopsy (EMB) is associated with a serious acute complication rate of less than 1% using current flexible bioptomes
Among cardiac transplant recipients, EMB is used routinely for immediate rejection surveillance
In general, EMB is still preferred for long-term allograft rejection surveillance and has not been replaced by gene expression profiling
EMB should be performed in young patients in whom myocarditis is strongly suspected or in older patients with suspected infiltrative cardiomyopathy who may be appropriate candidates for aggressive treatment
In general, EMB is not routinely recommended to diagnose other cardiac disorders, unless dictated by research protocols
COMPLICATIONS
All patients who undergo EMB are at risk of complications. The rate of complication during EMB is reported as less than 6% in most case series.8,11-19 Reported complications include access site hematoma, transient right bundle branch block, transient arrhythmias, tricuspid regurgitation, and occult pulmonary embolism.20,21 Life-threatening complications occur far less frequently. Right ventricular perforation was reported in less than 1% of patients in recent reports.12-19 Although rare, procedure-related deaths have been reported20,22; however, recent case series reported no mortality in patients undergoing EMB.8,11-19
In general, only patients who undergo repeated EMB during a prolonged period (ie, posttransplant surveillance) are at risk of long-term complications of the procedure. Serious late sequelae from EMB can include coronary artery-to-right ventricular fistula and severe tricuspid valve regurgitation.18,23 Tricuspid valve regurgitation occurs relatively frequently, especially among cardiac transplant recipients who undergo frequent biopsies, with reported rates of symptomatic regurgitation up to 23%.18,24-27 Tucker et al26 reported similar long-term rates of late tricuspid regurgitation for both femoral and internal jugular access. Unfortunately, even asymptomatic patients experience increased late mortality when EMB-induced tricuspid regurgitation occurs.28 As previously mentioned, neither 2-dimensional transthoracic echocardiography nor fluoroscopic guidance allows adequate visualization of the chordae tendineae attachments to the tricuspid valve. Prevention against damage to the tricuspid valve and supporting structures may be enhanced by using longer sheaths that allow the bioptome to pass through the tricuspid valve with minimal contact of the valve apparatus, by prophylactic tricuspid valve annuloplasty on the donor heart at the time of transplant.29
ANALYSIS OF BIOPSY SPECIMENS
Although technical skill and patient safety are the immediate concerns during any procedure, it is the responsibility of the operator to ensure careful handling and correct preparation of the tissue. The specimen should be moved off the biopsy catheter with a needle and placed on gauze soaked in isotonic saline. Subsequent storage is dictated by the clinical question to be answered: 10% neutral-buffered formalin is needed to diagnose transplant rejection, unexplained cardiomyopathy, myocarditis, infiltrative cardiomyopathy, or tumors. Zeus fixative (used primarily for immunofluorescence studies to evaluate antibody-mediated rejection) can be used to assess allograft rejection. Trump’s fixative or 4% glutaraldehyde can be used when assessing an unexplained cardiomyopathy or anthracycline-induced cardiotoxicity, and specimens should be snap-frozen when assessing possible myocarditis. Crush artifact may be caused by the bioptome, possibly by cutting a large specimen with a blade, or by handling with forceps. Crush artifact can make pathologic diagnosis difficult or impossible (Figure 2).20,30 A minimum of 5 right ventricular samples should be obtained. Although this number has been associated with a 98% sensitivity for detection of transplant rejection,30,31 it has been shown to yield only a 45% sensitivity for the diagnosis of myocarditis.31 Subsequent specimen preparation depends on the clinical question to be answered and testing that will be done. Standard histological preparation for light microscopy, which can be used in the diagnosis of transplant rejection, myocarditis, or amyloidosis, requires paraffin wax embedding, sectioning, and staining.32 Specimens prepared in Trump’s fixative or 4% glutaraldehyde can be viewed with transmission electron microscopy, which may be helpful in assessing drug toxicity, metabolic or storage disorders, or light chain deposition disease.6,33
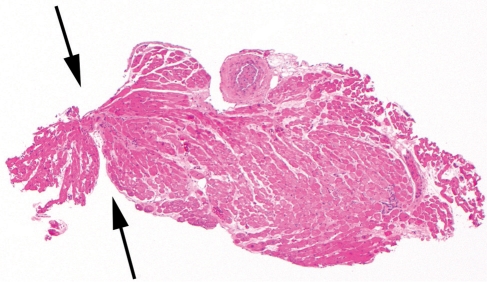
FIGURE 2.
Crush artifact, showing pinching of the sample at the time of procurement (arrows) (hematoxylin-eosin, original magnification, x40).
Viruses are an increasingly recognized cause of myocarditis.34 Polymerase chain reaction for viral DNA amplification may change the role of EMB in the future—from one of simple diagnosis to one of guiding focused treatment.35 Although paraffin-embedded tissue may be used for many polymerase chain reaction–based viral genome assays, optimum sensitivity is achieved by using tissue that has been snap-frozen in liquid nitrogen and stored at –80°C at the time of biopsy. This is particularly true for RNA virus detection. Additionally, frozen tissue is often necessary for immunohistochemical detection of dystrophin in the setting of muscular dystrophies.
INDICATIONS FOR EMB
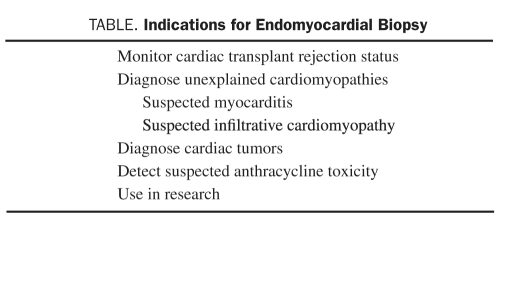
With the possible exception of monitoring for allograft rejection immediately after cardiac transplant, there is no standard or definite indication for EMB. For each possible indication, the utility of the technique is patient specific, dependent on the information gained, and an assessment of potential risks. Information from EMB may be of prognostic value or be useful in guiding treatment by establishing a diagnosis from direct tissue sampling. There is considerable overlap between diseases that can be diagnosed via EMB, diseases that can be diagnosed by noninvasive testing, and those that have effective treatments. Endomyocardial biopsy is absolutely necessary only for the diagnosis of a small number of diseases or conditions, including anthracycline-induced cardiomyopathy, cardiac allograft rejection, sarcoidosis, giant cell myocarditis, and hypereosinophilic syndrome, of which only allograft rejection, sarcoidosis, and hypereosinophilic syndrome have proven therapies. Many diseases diagnosed by EMB are often suspected before the procedure is performed.36,37 Unfortunately, a specific tissue diagnosis is achieved only in a minority of cases because histologic findings are frequently nonspecific. Indications for performing EMB that are consistent with current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure are listed in the Table.
TABLE.
indications for endomyocardial Biopsy
Monitoring Cardiac Transplant Rejection Status
The most common and clearly established indication for EMB is monitoring transplant rejection status, including both cell-mediated and antibody-mediated types.30,37 Unfortunately, because monitoring of tissue rejection requires numerous consecutive biopsies, especially within the first several months of cardiac transplant, the risk of developing tricuspid regurgitation and coronary-to-right ventricle fistulas is increased in such patients.14,17,19,23,38
Noninvasive means of monitoring tissue rejection, such as gene expression profiling, are under way and may have utility as primary screening methods in cardiac transplant recipients at low risk of rejection.39,40 None of the currently available techniques can be used to actually diagnose rejection, but they can be used as a pretest assessment of the low risk and lower probability of rejection, the implication being that those at low risk may not require long-term consecutive EMB. The usefulness of gene expression profiling has been brought into question by some, despite demonstration of so-called noninferiority, because of the relatively narrow cohort studied and the remaining questions regarding the general usefulness of long-term surveillance.41 Worldwide, a few centers do not perform EMB after the first year following cardiac transplant because they prefer noninvasive rejection surveillance; however, most institutions in the United States continue to use EMB as the primary method of rejection surveillance due to the limited data on clinical outcomes with the noninvasive methods.39,42,43
The diagnosis of antibody-mediated (humoral) rejection remains an area of some controversy and generally requires demonstration of histopathologic changes on EMB, clinical allograft dysfunction, and the presence of donor-specific alloantibodies; however, standard diagnostic criteria have not been agreed on. Typical histopathologic changes may include not only morphologic abnormalities but also immunoperoxidase and immunofluorescence studies for complement split products, with C4d being the most frequently examined in North America.44 The usefulness of looking for additional markers and defining diagnostic criteria for antibody-mediated (humoral) rejection are current topics of great interest.
Unexplained Cardiomyopathies
Suspected Myocarditis. Endomyocardial biopsy has been shown to be the only method for diagnosing myocarditis in more than 30% of unexplained cardiomyopathy cases.36 Indeed, in patients with suspected myocarditis, determining the exact etiology has both important prognostic and treatment implications. Both giant cell myocarditis and necrotizing eosinophilic myocarditis carry a grave prognosis, but they can be responsive to corticosteroid therapy.45,46 Lymphocytic myocarditis and hypersensitivity myocarditis have better prognoses.45 Hypersensitivity myocarditis is not only responsive to corticosteroid therapy but also is often a manifestation of a reaction to medications, the use of which can be discontinued or modified.47 Thus, differentiating these specific types of myocarditis with EMB can lead to appropriate therapy, focused discussions regarding prognosis, and, in some cases, expedited listing for cardiac transplant or ventricular assist device placement.
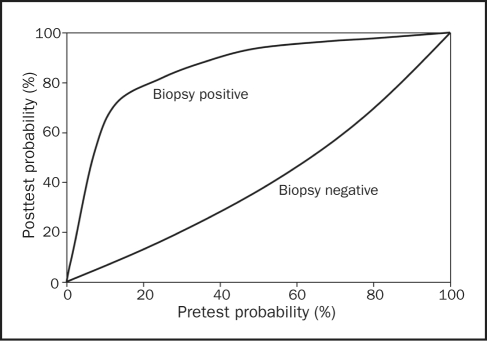
The recent ACC/AHA practice guidelines identify specific patient scenarios in which myocarditis may be suspected and EMB considered, including heart failure with hemodynamic compromise of less than 2 weeks’ duration (class I recommendation) or less than 3 months’ duration if associated with heart block or new ventricular arrhythmias (class I recommendation). Also, recent-onset heart failure associated with potential allergic reaction is included in the report as a scenario in which biopsy can be considered (class IIa recommendation).6 Despite the recent guidelines and recognized patient presentations that suggest underlying myocarditis, the decision to perform EMB to provide a pathologic diagnosis of myocarditis can be difficult. Among a population of patients in whom the clinician more likely suspects myocarditis on the basis of a careful history, physical examination, and laboratory test results, the utility of EMB may be assessed using the Bayesian theory as with any other diagnostic test.48 Mills and Lauer48 have constructed a Bayesian model for EMB in myocarditis using a previously reported false-positive biopsy interpretation rate of 3%49 and a false-negative biopsy interpretation rate of 55%31 (Figure 3).
FIGURE 3.
Bayesian model for utility of endomyocardial biopsy in myocarditis. As with any diagnostic procedure, utility is maximized when the pretest probability of disease is intermediate. Ultimately, clinical and noninvasive parameters may supplant routine biopsy as the initial test and serve to categorize patients into low, medium, and high likelihood of rejection strata. Those with an intermediate likelihood of rejection stand to benefit most from a diagnostic biopsy. From Am Heart J,48 with permission from elsevier.
We suggest that patients in whom acute fulminant myocarditis is suspected undergo confirmatory biopsy. Pathologic diagnosis will be reassuring to the clinical care team when rapid decisions regarding transplant and ventricularassist support are needed.
Suspected Infiltrative Diseases. Cardiac sarcoidosis, amyloidosis, and Fabry disease can all be diagnosed by EMB. Cardiac sarcoidosis is suspected in patients with systemic sarcoidosis or in those who present with a dilated left ventricle in systolic failure with either ventricular arrhythmias or heart block.6,50 In a study by Mehta et al,51 approximately 40% of patients with known systemic sarcoidosis and palpations had syncope or presyncope with cardiac involvement diagnosed subsequently. Because cardiac sarcoidosis is responsive to corticosteroid therapy and associated with a poor prognosis, diagnosis by EMB can be considered an important part of patient care.52-54
Unlike sarcoidosis, which is associated with systolic dysfunction, many other infiltrative diseases cause restrictive cardiomyopathies with thickened ventricles that may mimic the appearance of hypertrophic cardiomyopathy. Although hypertrophic cardiomyopathy does not require EMB for diagnosis, EMB may be appropriate if cardiac amyloidosis or Fabry disease is strongly suspected.6,55,56 Identification of the specific infiltrative disease has implications for both prognosis and treatment. For instance, differentiating primary (AL) amyloidosis from senile or familial forms of amyloidosis is prognostically important. Patients with primary amyloidosis have a significantly higher mortality rate57 but are potentially responsive to chemotherapeutic agents or stem-cell transplant.58 Fabry disease may be responsive to enzyme replacement therapy.59,60 Furthermore, both fibrosis of the myocardium and eosinophilic cardiomyopathy (Löffler endocarditis) are potentially responsive to corticosteroid therapy.61,62
Unfortunately, the need for EMB in the aforementioned conditions is not well defined, and in some cases it is unnecessary. Only one-quarter of patients with a clinical diagnosis of cardiac sarcoidosis were found to have noncaseating granulomas on EMB.55 Diagnosis of cardiac sarcoidosis using magnetic resonance imaging can be achieved with high specificity, whereas positron emission tomography may provide higher sensitivity.52,63 Therefore, patients strongly suspected of having cardiac sarcoidosis should be treated despite normal findings on biopsy, raising questions about the clinical utility of EMB.64 Similarly, cardiac amyloidosis and myocardial fibrosis are often suspected on the basis of clinical examination and other testing, such as echocardiography, fat aspirate, nuclear studies, or magnetic resonance imaging, possibly rendering pathologic diagnosis by EMB redundant or unnecessary.65
Diagnosing Cardiac Tumors
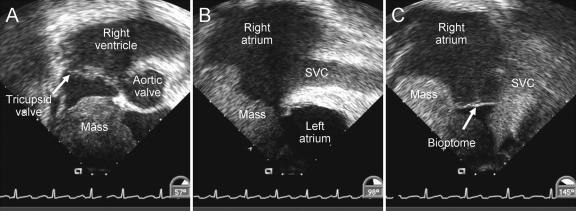
Case reports illustrate that cardiac tumors (both neoplastic and nonneoplastic), with the possible exception of typical myxomas, can be biopsied and diagnosed with EMB with imaging (usually transesophageal) guidance (Figure 4).66,67 Current ACC/AHA guidelines state that EMB is reasonable for the diagnosis of cardiac tumors (class IIa recommendation) if 4 specific criteria are met: (1) diagnosis cannot be made in any other way, (2) the diagnosis with EMB will alter therapy, (3) the success of biopsy is believed to be reasonably high, and (4) the biopsy will be performed by an experienced operator.6 Usual biopsy forceps may not yield adequate tissue samples for histologic analysis because of the inability to penetrate an overlying capsule or organized hematoma. Coring needles may be used to safely obtain a deep tissue sample. When EMB is performed to diagnose an intracardiac mass, frozen sections should be examined to ensure adequate tissue samples for analysis before the procedure is stopped.
FIGURE 4.
Transesophageal echocardiographic images of right atrial mass in a 56-year-old man with small cell lung cancer. The mass was biopsied using a 9-F bioptome through the superior vena cava (SVC). Subsequent pathologic analysis revealed metastatic small cell carcinoma.
Anthracycline-Induced Cardiomyopathy
Biopsy specimens from patients with anthracycline-induced cardiotoxicity demonstrate loss of myofilaments and vacuolar degeneration on both light microscopy and electron microscopy.68,69 Early studies and reviews suggested that routine monitoring with EMB in patients treated with anthracycline chemotherapeutic agents was appropriate.11 However, recent guidelines conclude that EMB is best suited for situations in which the cause of cardiomyopathy is unclear, for determining whether higher doses of an anthracycline can be given, or for research purposes.6
Unexplained Tachyarrhythmias
Case reports show that myocarditis has been diagnosed by EMB in as many as 50% of patients with malignant ventricular arrhythmias and 12% of patients with various supraventricular tachycardias.6,70,71 Complicating matters is the fact that, in many patients with unexplained arrhythmias, there are often pathologic abnormalities of unclear clinical importance that may not clarify either diagnosis or treatment. Thus, EMB in patients with malignant arrhythmias (originating in the ventricle or atrium) without clear association to ventricular function or other cardiac abnormalities remains a class IIB recommendation, with the specification that the procedure should be used only in “exceptional cases” in which the benefits of biopsy out-weigh the procedural risks.6
Research And Future Directions
Identification of new molecular and genomic or proteomic studies among patients with cardiomyopathy compared with healthy controls are currently under way. In this continued research, EMB is an important resource.4 For clinical diagnosis and prognostication, moving tissue analysis beyond standard light microscopic examination will be important to improve diagnostic yields, to enhance clinical utility, and to gain new pathophysiologic insights into cardiac dysfunction.
GENERAL CONCLUSIONS
Endomyocardial biopsy is an important clinical tool with an established role in surveillance for posttransplant myocardial rejection, and, to a lesser extent, in the evaluation of dilated and restrictive cardiomyopathies, both acute and chronic. Although a safe procedure, EMB is clearly associated with both a risk of procedural complications and serious long-term sequelae such as tricuspid regurgitation when performed repeatedly. Analyzing tissue specimens with more sophisticated methods, rather than routine staining used in light microscopy, may improve the diagnostic yield and clinical and research utility of EMB. Establishing specific myocardial diagnoses can be aided with noninvasive imaging techniques, and ultimately newer imaging techniques incorporating molecular imaging may eliminate the need for routine EMB. Ideally, these procedures should be performed at centers with advanced heart failure and transplant programs that have the technical capabilities and the expertise to appropriately analyze the specimens.
Clinical Recommendations for the Use of EMB
Endomyocardial biopsy can be used in 2 broad groups of patients: cardiac transplant recipients and nontransplant patients. Among transplant recipients, we recommend EMB for both immediate and long-term cardiac surveillance because noninvasive methods require further data before adoption into routine practice. Among patients with suspected myocardial disease who have not undergone cardiac transplant, we recommend biopsy for young patients in whom there is a strong suspicion of myocarditis or in older patients with suspected infiltrative cardiomyopathy who may be appropriate candidates for aggressive treatment. We do not routinely recommend EMB for other cardiac disorders, unless dictated by research protocols.
Acknowledgments
We thank Sudhir S. Kushwaha, MD, and William D. Edwards, MD, for critical review of the submitted manuscript.
REFERENCES
- 1. Caves PK, Stinson EB, Graham AF, Billingham ME, Grehl TM, Shumway NE. Percutaneous transvenous endomyocardial biopsy. JAMA. 1973; 225:288-291 [PubMed] [Google Scholar]
- 2. Richardson PJ. King’s endomyocardial bioptome. Lancet. 1974;1:660-661 [DOI] [PubMed] [Google Scholar]
- 3. Anderson JL, Marshall HW. The femoral venous approach to endomyocardial biopsy: comparison with internal jugular and transarterial approaches. Am J Cardiol. 1984;53:833-837 [DOI] [PubMed] [Google Scholar]
- 4. Ardehali H, Kasper EK, Baughman KL. Diagnostic approach to the patient with cardiomyopathy: whom to biopsy. Am Heart J. 2005;149:7-12 [DOI] [PubMed] [Google Scholar]
- 5. Miller LW, Labovitz AJ, McBride LA, Pennington DG, Kanter K. Echocardiography-guided endomyocardial biopsy: a 5-year experience. Circulation. 1988;78(5 pt 2):III99-III102 [PubMed] [Google Scholar]
- 6. Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific state ment from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216-2233 [DOI] [PubMed] [Google Scholar]
- 7. Blomstrom-Lundqvist C, Noor AM, Eskilsson J, Persson S. Safety of transvenous right ventricular endomyocardial biopsy guided by two-dimensional echocardiography. Clin Cardiol. 1993;16:487-492 [DOI] [PubMed] [Google Scholar]
- 8. Han J, Park Y, Lee H, et al. Complications of 2-D echocardiography guided transfemoral right ventricular endomyocardial biopsy. J Korean Med Sci. 2006;21:989-994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amitai ME, Schnittger I, Popp RL, Chow J, Brown P, Liang DH. Comparison of three-dimensional echocardiography to two-dimensional echocardiography and fluoroscopy for monitoring of endomyocardial biopsy. Am J Cardiol. 2007;99:864-866 [DOI] [PubMed] [Google Scholar]
- 10. Platts D, Brown M, Javorsky G, West C, Kelly N, Burstow D. Comparison of fluoroscopic versus real-time three-dimensional transthoracic echocardiographic guidance of endomyocardial biopsies. Eur J Echocardiogr. 2010;11(7): 637-643 [DOI] [PubMed] [Google Scholar]
- 11. Fowles RE, Mason JW. Endomyocardial biopsy. Ann Intern Med. 1982;97:885-894 [DOI] [PubMed] [Google Scholar]
- 12. Hiramitsu S, Hiroe M, Uemura A, Kimura K, Hishida H, Morimoto S. National survey of the use of endomyocardial biopsy in Japan. Jpn Circ J. 1998;62:909-912 [DOI] [PubMed] [Google Scholar]
- 13. Nippoldt TB, Edwards WD, Holmes DR, Jr, Reeder GS, Hartzler GO, Smith HC. Right ventricular endomyocardial biopsy: clinicopathologic correlates in 100 consecutive patients. Mayo Clin Proc. 1982;57:407-418 [PubMed] [Google Scholar]
- 14. Drury JH, Labovitz AJ, Miller LW. Echocardiographic guidance for endomyocardial biopsy. Echocardiography. 1997;14:469-474 [DOI] [PubMed] [Google Scholar]
- 15. Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: a seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol. 1992;19:43-47 [DOI] [PubMed] [Google Scholar]
- 16. Baraldi-Junkins C, Levin HR, Kasper EK, Rayburn BK, Herskowitz A, Baughman KL. Complications of endomyocardial biopsy in heart transplant patients. J Heart Lung Transplant. 1993;12:63-67 [PubMed] [Google Scholar]
- 17. Ragni T, Martinelli L, Goggi C, et al. Echo-controlled endomyocardial biopsy. J Heart Transplant. 1990;9:538-542 [PubMed] [Google Scholar]
- 18. Wong RC, Abrahams Z, Hanna M, et al. Tricuspid regurgitation after cardiac transplantation: an old problem revisited. J Heart Lung Transplant. 2008;27:247-252 [DOI] [PubMed] [Google Scholar]
- 19. Sloan KP, Bruce CJ, Oh JK, Rihal CS. Complications of echocardiography-guided endomyocardial biopsy. J Am Soc Echocardiogr. 2009;22(3):324.e1-324.e4 [DOI] [PubMed] [Google Scholar]
- 20. Baim DS. Endomyocardial biopsy. In: Baim DS, Grossman W, eds. Grossman’s Cardiac Catheterization, Angiography, and Intervention. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:198-205 [Google Scholar]
- 21. Kreher SK, Ulstad VK, Dick CD, DeGroff R, Olivari MT, Homans DC. Frequent occurrence of occult pulmonary embolism from venous sheaths during endomyocardial biopsy. J Am Coll Cardiol. 1992;19:581-585 [DOI] [PubMed] [Google Scholar]
- 22. Sekiguchi M, Take M. World survey of catheter biopsy of the heart. In: Sekiguchi M, Olsen EG, eds. Cardiomyopathy: Clinical, Logical and Theortical Aspects. Baltimore, MD: Univeristy Park Press; 1980:217-225 [Google Scholar]
- 23. Sandhu JS, Uretsky BF, Zerbe TR, et al. Coronary artery fistula in the heart transplant patient: a potential complication of endomyocardial biopsy. Circulation. 1989;79:350-356 [DOI] [PubMed] [Google Scholar]
- 24. Chan MC, Giannetti N, Kato T, et al. Severe tricuspid regurgitation after heart transplantation. J Heart Lung Transplant. 2001;20:709-717 [DOI] [PubMed] [Google Scholar]
- 25. Huddleston CB, Rosenbloom M, Goldstein JA, Pasque MK. Biopsy-induced tricuspid regurgitation after cardiac transplantation. Ann Thorac Surg. 1994;57:832-836 [DOI] [PubMed] [Google Scholar]
- 26. Tucker PA, II, Jin BS, Gaos CM, Radovancevic B, Frazier OH, Wilansky S. Flail tricuspid leaflet after multiple biopsies following orthotopic heart transplantation: echocardiographic and hemodynamic correlation. J Heart Lung Transplant. 1994;13:466-472 [PubMed] [Google Scholar]
- 27. Nguyen V, Cantarovich M, Cecere R, Giannetti N. Tricuspid regurgitation after cardiac transplantation: how many biopsies are too many? J Heart Lung Transplant. 2005;24:S227-S231 [DOI] [PubMed] [Google Scholar]
- 28. Burgess MI, Aziz T, Yonan N. Clinical relevance of subclinical tricuspid regurgitation after orthotopic cardiac transplantation [letter]. J Am Soc Echocardiogr. 1999;12(2):164 [DOI] [PubMed] [Google Scholar]
- 29. Jeevanandam V, Russell H, Mather P, Furukawa S, Anderson A, Raman J. Donor tricuspid annuloplasty during orthotopic heart transplantation: long-term results of a prospective controlled study. Ann Thorac Surg. 2006;82:2089-2095 [DOI] [PubMed] [Google Scholar]
- 30. Cunningham KS, Veinot JP, Butany J. An approach to endomyocardial biopsy interpretation. J Clin Pathol. 2006;59:121-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64:1235-1245 [DOI] [PubMed] [Google Scholar]
- 32. Zerbe TR, Arena V. Diagnostic reliability of endomyocardial biopsy for assessment of cardiac allograft rejection. Hum Pathol. 1988;19:1307-1314 [DOI] [PubMed] [Google Scholar]
- 33. Toor AA, Ramdane BA, Joseph J, et al. Cardiac nonamyloidotic immunoglobulin deposition disease. Mod Pathol. 2006;19:233-237 [DOI] [PubMed] [Google Scholar]
- 34. Schultz JC, Hilliard AA, Cooper LT, Jr, Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clin Proc. 2009;84:1001-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frustaci A, Chimenti C, Calabrese F, Pieroni M, Thiene G, Maseri A. Immunosuppressive therapy for active lymphocytic myocarditis: virological and immunologic profile of responders versus nonresponders. Circulation. 2003;107:857-863 [DOI] [PubMed] [Google Scholar]
- 36. Ardehali H, Qasim A, Cappola T, et al. Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. Am Heart J. 2004;147:919-923 [DOI] [PubMed] [Google Scholar]
- 37. Mason JW, O’Connell JB. Clinical merit of endomyocardial biopsy. Circulation. 1989;79:971-979 [DOI] [PubMed] [Google Scholar]
- 38. Bhat G, Burwig S, Walsh R. Morbidity of endomyocardial biopsy in cardiac transplant recipients. Am Heart J. 1993;125:1180-1181 [DOI] [PubMed] [Google Scholar]
- 39. Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362:1890-1900 [DOI] [PubMed] [Google Scholar]
- 40. Horwitz PA, Tsai EJ, Putt ME, et al. Detection of cardiac allograft rejection and response to immunosuppressive therapy with peripheral blood gene expression. Circulation. 2004;110:3815-3821 [DOI] [PubMed] [Google Scholar]
- 41. Mehra MR, Parameshwar J. Gene expression profiling and cardiac allograft rejection monitoring: is IMAGE just a mirage? J Heart Lung Transplant. 2010;29:599-602 [DOI] [PubMed] [Google Scholar]
- 42. White JA, Guiraudon C, Pflugfelder PW, Kostuk WJ. Routine surveillance myocardial biopsies are unnecessary beyond one year after heart transplantation. J Heart Lung Transplant. 1995;14:1052-1056 [PubMed] [Google Scholar]
- 43. Stehlik J, Starling RC, Movsesian MA, et al. Utility of long-term surveillance endomyocardial biopsy: a multi-institutional analysis. J Heart Lung Transplant. 2006;25:1402-1409 [DOI] [PubMed] [Google Scholar]
- 44. Kucirka LM, Maleszewski JJ, Segev DL, Halushka MK. Survey of North American pathologist practices regarding antibody-mediated rejection in cardiac transplant biopsies. Cardiovasc Pathol. 2011;20(3):132-138 [DOI] [PubMed] [Google Scholar]
- 45. Cooper LT, Jr, Berry GJ, Shabetai R; Multicenter Giant Cell Myocarditis Study Group Investigators Idiopathic giant-cell myocarditis: natural history and treatment. N Engl J Med. 1997;336:1860-1866 [DOI] [PubMed] [Google Scholar]
- 46. deMello DE, Liapis H, Jureidini S, Nouri S, Kephart GM, Gleich GJ. Cardiac localization of eosinophil-granule major basic protein in acute necrotizing myocarditis. N Engl J Med. 1990;323:1542-1545 [DOI] [PubMed] [Google Scholar]
- 47. Taliercio CP, Olney BA, Lie JT. Myocarditis related to drug hypersensitivity. Mayo Clin Proc. 1985;60:463-468 [DOI] [PubMed] [Google Scholar]
- 48. Mills RM, Lauer MS. Endomyocardial biopsy: a procedure in search of an indication. Am Heart J. 2004;147:759-760 [DOI] [PubMed] [Google Scholar]
- 49. Chow LH, Radio SJ, Sears TD, McManus BM. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14:915-920 [DOI] [PubMed] [Google Scholar]
- 50. Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82:537-540 [DOI] [PubMed] [Google Scholar]
- 51. Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133:1426-1435 [DOI] [PubMed] [Google Scholar]
- 52. Kim JS, Judson MA, Donnino R, et al. Cardiac sarcoidosis. Am Heart J. 2009;157:9-21 [DOI] [PubMed] [Google Scholar]
- 53. Okura Y, Dec GW, Hare JM, et al. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003;41:322-329 [DOI] [PubMed] [Google Scholar]
- 54. Ardehali H, Howard DL, Hariri A, et al. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J. 2005;150:459-463 [DOI] [PubMed] [Google Scholar]
- 55. Falk RH, Skinner M. The systemic amyloidoses: an overview. Adv Intern Med. 2000;45:107-137 [PubMed] [Google Scholar]
- 56. Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898-909 [DOI] [PubMed] [Google Scholar]
- 57. Dubrey SW, Cha K, Skinner M, LaValley M, Falk RH. Familial and primary (AL) cardiac amyloidosis: echocardiographically similar diseases with distinctly different clinical outcomes. Heart. 1997;78:74-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Skinner M, Sanchorawala V, Seldin DC, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85-93 [DOI] [PubMed] [Google Scholar]
- 59. Frustaci A, Chimenti C, Ricci R, et al. Improvement in cardiac function in the cardiac variant of Fabry’s disease with galactose-infusion therapy. N Engl J Med. 2001;345:25-32 [DOI] [PubMed] [Google Scholar]
- 60. Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha-galactosidase A: replacement therapy in Fabry’s disease. N Engl J Med. 2001;345:9-16 [DOI] [PubMed] [Google Scholar]
- 61. Fawzy ME, Ziady G, Halim M, Guindy R, Mercer EN, Feteih N. Endomyocardial fibrosis: report of eight cases. J Am Coll Cardiol. 1985;5: 983-988 [DOI] [PubMed] [Google Scholar]
- 62. Kushwaha SS, Fallon JT, Fuster V. Restrictive cardiomyopathy. N Engl J Med. 1997;336:267-276 [DOI] [PubMed] [Google Scholar]
- 63. Tadamura E, Yamamuro M, Kubo S, et al. Multimodality imaging of cardiac sarcoidosis before and after steroid therapy. Circulation. 2006;113: e771-e773 [DOI] [PubMed] [Google Scholar]
- 64. Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138:299-302 [DOI] [PubMed] [Google Scholar]
- 65. Maceira AM, Joshi J, Prasad SK, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186-193 [DOI] [PubMed] [Google Scholar]
- 66. Auriti A, Pandozi C, Altamura V, Cianfrocca C, Manente L, Santini M. Transthoracic echocardiography-guided biopsy of a right ventricular mass. J Cardiovasc Med (Hagerstown). 2007;8:274-276 [DOI] [PubMed] [Google Scholar]
- 67. Unger P, Kentos A, Cogan E, Renard M, Crasset V, Stoupel E. Primary cardiac lymphoma: diagnosis by transvenous biopsy under transesophageal echocardiographic guidance. J Am Soc Echocardiogr. 1998;11:89-91 [DOI] [PubMed] [Google Scholar]
- 68. Meinardi MT, van der Graaf WT, van Veldhuisen DJ, Gietema JA, de Vries EG, Sleijfer DT. Detection of anthracycline-induced cardiotoxicity. Cancer Treat Rev. 1999;25:237-247 [DOI] [PubMed] [Google Scholar]
- 69. Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47-58 [DOI] [PubMed] [Google Scholar]
- 70. Vignola PA, Aonuma K, Swaye PS, et al. Lymphocytic myocarditis presenting as unexplained ventricular arrhythmias: diagnosis with endomyocardial biopsy and response to immunosuppression. J Am Coll Cardiol. 1984;4:812-819 [DOI] [PubMed] [Google Scholar]
- 71. Kobayashi Y, Yazawa T, Baba T, et al. Clinical, electrophysiological, and histopathological observations in supraventricular tachycardia. Pacing Clin Electrophysiol. 1988;11:1154-1167 [DOI] [PubMed] [Google Scholar]