Abstract
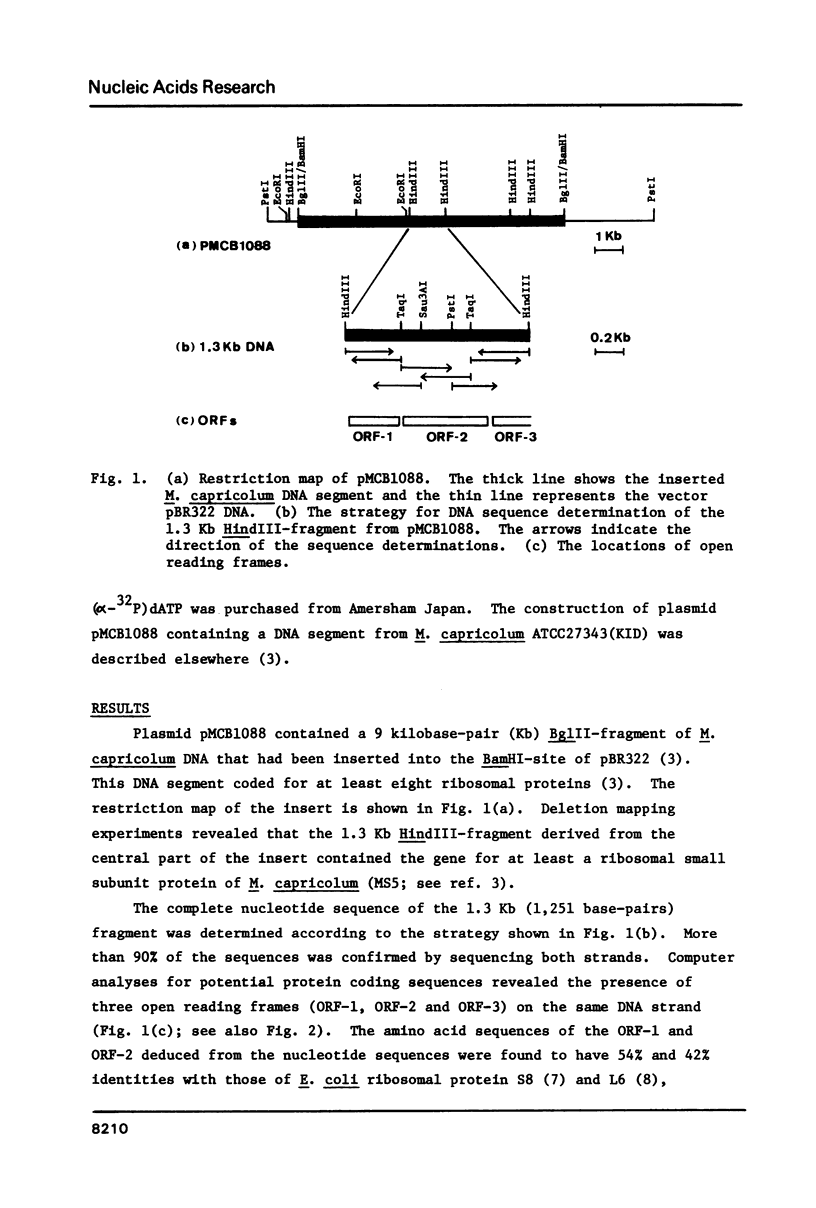
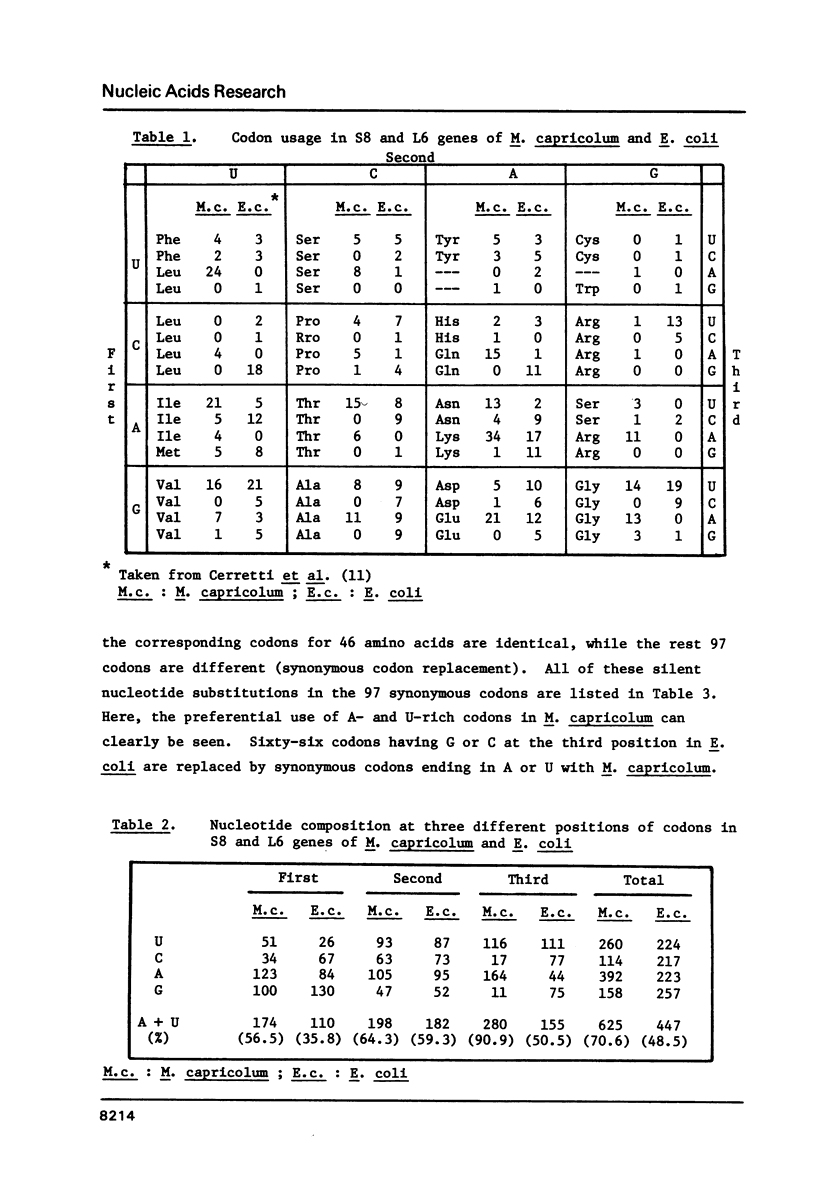
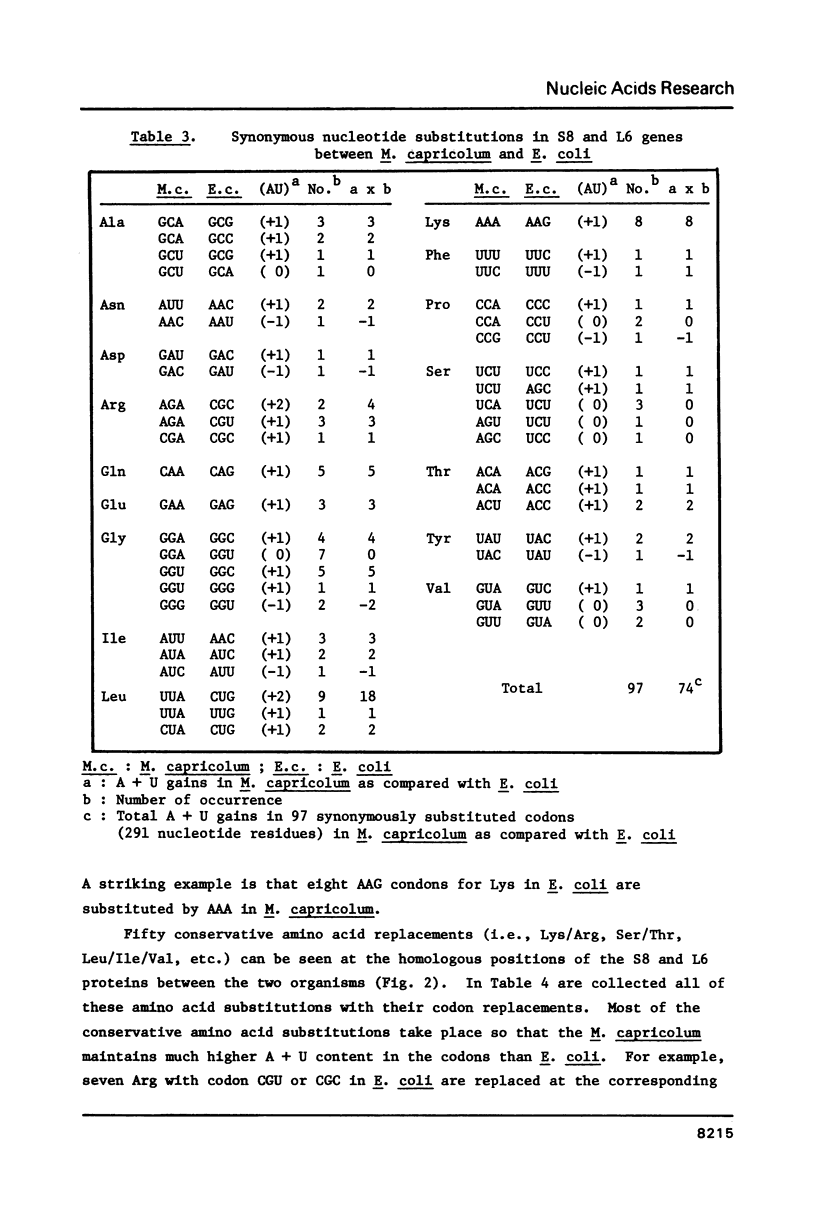
The nucleotide sequence of the 1.3 kilobase-pair DNA segment, which contains the genes for ribosomal proteins S8 and L6, and a part of L18 of Mycoplasma capricolum, has been determined and compared with the corresponding sequence in Escherichia coli (Cerretti et al., Nucl. Acids Res. 11, 2599, 1983). Identities of the predicted amino acid sequences of S8 and L6 between the two organisms are 54% and 42%, respectively. The A + T content of the M. capricolum genes is 71%, which is much higher than that of E. coli (49%). Comparisons of codon usage between the two organisms have revealed that M. capricolum preferentially uses A- and U-rich codons. More than 90% of the codon third positions and 57% of the first positions in M. capricolum is either A or U, whereas E. coli uses A or U for the third and the first positions at a frequency of 51% and 36%, respectively. The biased choice of the A- and U-rich codons in this organism has been also observed in the codon replacements for conservative amino acid substitutions between M. capricolum and E. coli. These facts suggest that the codon usage of M. capricolum is strongly influenced by the high A + T content of the genome.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Wittmann-Liebold B. The amino acid sequence of the ribosomal protein S8 of Escherichia coli. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1509–1525. doi: 10.1515/bchm2.1978.359.2.1509. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Arfsten U., Chen-Schmeisser U. The primary structure of protein L6 from the aminoacyl-tRNA binding site of the Escherichia coli ribosome. Hoppe Seylers Z Physiol Chem. 1977 Apr;358(4):531–535. [PubMed] [Google Scholar]
- Higo K., Otaka E., Osawa S. Purification and characterization of 30S ribosomal proteins from Bacillus subtilis: correlation to Escherichia coli 30S proteins. Mol Gen Genet. 1982;185(2):239–244. doi: 10.1007/BF00330792. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J. Cell biology of the mycoplasmas. Bacteriol Rev. 1972 Sep;36(3):263–290. doi: 10.1128/br.36.3.263-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Neimark H. C. Division of mycoplasmas into subgroups. J Gen Microbiol. 1970 Oct;63(2):249–263. doi: 10.1099/00221287-63-2-249. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. L., Morowitz H. J. Partial purification of native rRNA and tRNA cistrons from mycoplasma sp. (Kid). Proc Natl Acad Sci U S A. 1969 Aug;63(4):1282–1289. doi: 10.1073/pnas.63.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]



