Abstract
Objective To evaluate the effectiveness of comprehensive geriatric assessment in hospital for older adults admitted as an emergency.
Search strategy We searched the EPOC Register, Cochrane’s Controlled Trials Register, the Database of Abstracts of Reviews of Effects (DARE), Medline, Embase, CINAHL, AARP Ageline, and handsearched high yield journals.
Selection criteria Randomised controlled trials of comprehensive geriatric assessment (whether by mobile teams or in designated wards) compared with usual care. Comprehensive geriatric assessment is a multidimensional interdisciplinary diagnostic process used to determine the medical, psychological, and functional capabilities of a frail elderly person to develop a coordinated and integrated plan for treatment and long term follow-up.
Data collection and analysis Three independent reviewers assessed eligibility and trial quality and extracted published data. Two additional reviewers moderated.
Results Twenty two trials evaluating 10 315 participants in six countries were identified. For the primary outcome “living at home,” patients who underwent comprehensive geriatric assessment were more likely to be alive and in their own homes at the end of scheduled follow-up (odds ratio 1.16 (95% confidence interval 1.05 to 1.28; P=0.003; number needed to treat 33) at a median follow-up of 12 months versus 1.25 (1.11 to 1.42; P<0.001; number needed to treat 17) at a median follow-up of six months) compared with patients who received general medical care. In addition, patients were less likely to be living in residential care (0.78, 0.69 to 0.88; P<0.001). Subgroup interaction suggested differences between the subgroups “wards” and “teams” in favour of wards. Patients were also less likely to die or experience deterioration (0.76, 0.64 to 0.90; P=0.001) and were more likely to experience improved cognition (standardised mean difference 0.08, 0.01 to 0.15; P=0.02) in the comprehensive geriatric assessment group.
Conclusions Comprehensive geriatric assessment increases patients’ likelihood of being alive and in their own homes after an emergency admission to hospital. This seems to be especially true for trials of wards designated for comprehensive geriatric assessment and is associated with a potential cost reduction compared with general medical care.
Introduction
Older people represent the fastest growing sector of society and account for the largest increase in hospital admissions.1 2 They are at highest risk of acquired disability, cognitive decline, or admission to residential care, either as a consequence of illness or as an unfortunate consequence of treatment.3 4 5 Older people’s needs are more complex with potentially coexistent medical, functional, psychological, and social needs.6 This can lead to an atypical presentation that can often be misunderstood and requires a different approach to care.
One of the cornerstones of modern geriatric care is comprehensive geriatric assessment (CGA). This is defined as a “multidimensional interdisciplinary diagnostic process focused on determining a frail older person’s medical, psychological and functional capability in order to develop a coordinated and integrated plan for treatment and long term follow up.”7 Comprehensive geriatric assessment is therefore both a diagnostic and therapeutic process. It seeks to ensure that problems are identified, quantified, and managed appropriately. The likelihood of multiple overlapping problems necessitates assessment across several domains and therefore involves several disciplines. These assessments across medical, psychiatric, functional, and social domains are required to develop a broad or multifaceted therapeutic plan to enhance recovery and promote independence.
There are two broad models of inpatient comprehensive geriatric assessment.8 The first is delivered in a discrete ward with a coordinated specialist multidisciplinary team. Patients are admitted into this facility and cared for by the specialist team, who provide the assessment and rehabilitation. There are several names for these wards, including acute care for elders (ACE units), geriatric evaluation and management units (GEMU), or rehabilitation wards. For the purposes of this review we have grouped these together as “wards.” In the second model, a mobile or peripatetic team visit appropriate patients wherever they are admitted in a general ward setting. The team will assess the patients and make recommendations to the physicians who care for the patients. These are sometimes referred to as interdisciplinary geriatric consultation services (grouped here under the heading of “teams”).9
Various reviews of comprehensive geriatric assessment already exist in the literature but have shortfalls in their comprehensiveness. One of the earliest reviews9 included analysis of trials of stroke care as well as orthogeriatrics and has now been superseded by individual specialty reviews.10 11 Others have looked at specific subgroups of comprehensive geriatric assessment based on timing of admission,12 patient defined criteria,13 or ward title14 or have simply had inadequate analysis data.8
We determined whether inpatient comprehensive geriatric assessment for frail older adults admitted to hospital as an emergency is more effective than routine or general medical care in hospital.
Methods
Types of participants
Participants were adults aged 65 or older who were admitted to hospital care as an emergency, including all unplanned, unscheduled, or acute presentations.
Intervention
We sought to evaluate only randomised controlled trials comparing comprehensive geriatric assessment with usual care, such as general medical ward care. We considered both discrete geriatric units (“wards”) and inpatient geriatric consultation service (“team”) models. Studies of organised care for specific conditions (such as stroke units, geriatric orthopaedic rehabilitation) were excluded.10 15 Studies that did not evaluate comprehensive geriatric assessment in an inpatient setting were also excluded.
Comparisons
Trials were included only where comparison was with usual care. Comparisons with other forms of comprehensive geriatric assessment were not included. Usual care generally involved admission to a general medical ward setting under the care of a non-specialist.
Pre-planned subgroup analyses included comparisons by wards and teams, admission criteria (such as age alone versus age plus other criteria), the timing of admission to specialist care, analysis by geographical healthcare setting, and specialist outpatient follow-up versus none.
Outcomes
The primary outcome measure—“living at home”—is the inverse of death or admission to residential care combined and describes the odds of someone being alive and in their own home at a point in time. (Patients admitted from and returned to residential care were not classified as living at home.)
Secondary outcome measures included death; residential care; dependence (defined as a dichotomous outcome from scales measuring activities of daily living); death or dependence; death or deterioration (defined as the number of patients who had died or deteriorated in functional ability); activities of daily living (a continuous outcome describing change in score); cognitive status (a continuous outcome describing change in score); readmissions; length of stay; and use of resources.
In April 2010 we searched the EPOC Register (including studies awaiting assessment), the Controlled Trials Register (CCTR), the Database of Abstracts of Reviews of Effects (DARE); the Cochrane Central Register of Controlled Trials (CENTRAL); Medline (from 1966); Embase (from 1980); CINAHL (from 1982); and AARP Ageline (from 1978). Other sources included the hand searching of high yield journals and conference proceedings and the reference lists of any relevant reviews identified. The Medline search strategy is shown in appendix 1 on bmj.com.
Three independent reviewers screened titles and abstracts of papers identified by the literature searches for their potential relevance or assessed the full text for inclusion in the review.
Three reviewers abstracted the data independently, including data on design characteristics, the study population, the intervention, outcome measures used, and length of follow-up. Classification of the intervention was determined by characteristics of the type of assessment used (such as discrete geographical unit versus mobile team) and the components of the interventions.
We assessed all relevant trials to evaluate and record potential sources of bias,16 17 including assessment of randomisation procedure, concealment of treatment allocation, blinding of participants, and documentation or evidence of intention to treat analysis.18
We considered study characteristics such as population, time to enrolment, and length of follow-up in the analysis and grouped studies were according to these characteristics. We have presented results separately for wards and teams for each domain of outcome. Data were combined in analyses and the odds ratios and 95% confidence intervals for each study presented alongside the summary statistic for each subgroup. Studies were included in the analysis only when published data were available for each outcome. For this reason the number of studies reported for each outcome reflect the available data. We used the fixed effects model for analyses. When there was heterogeneity (I2>30%), we used and compared both random effect and fixed effects models.16 When continuous scales were used, we attempted meta-analysis using weighted mean differences.
For the purpose of the outcome of admission to residential care, we combined the presence of the patient in a care home, a hospital ward, or long term residential care.
Results
We searched 28 843 titles and identified 22 relevant randomised controlled trials giving information on 10 315 participants across six countries (fig 1) (full details of all included studies are in appendix 2 on bmj.com).19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53
Fig 1 Identification of studies for inclusion in analysis (CGA=comprehensive geriatric assessment)
Risk of bias in included studies
The studies identified were heterogeneous in quality.18 All used some method of individual patient randomisation, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded. Some trials noted attrition for functional or cognitive outcomes.25 29 For these outcomes, analysis was conducted with and without inclusion of these trial results for comparison (table 1).
Table 1.
Odds ratios (OR) or standardised mean differences (SMD) for secondary outcomes: comprehensive geriatric assessment versus usual care (fixed effects analysis)
| Outcome | Wards | Teams | Overall | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials | No of patients | OR or SMD (95% CI) | P value | Trials | No of patients | OR or SMD (95% CI) | P value | Trials | No of patients | OR or SMD (95% CI) | P value | |||
| Mortality: | ||||||||||||||
| Up to 6 months | 14 | 6048 | 0.93 (0.81 to 1.08) | 0.35 | 5 | 738 | 0.74 (0.47 to 1.17) | 0.19 | 19 | 6786 | 0.91 (0.80 to 1.05) | 0.20 | ||
| End of follow-up | 16 | 6593 | 0.98 (0.87 to 1.11) | 0.75 | 7 | 3370 | 1.00 (0.86 to 1.18) | 0.97 | 23 | 9963 | 0.99 (0.90 to 1.09) | 0.82 | ||
| Living in residential care: | ||||||||||||||
| Up to 6 months | 10 | 4319 | 0.72 (0.61 to 0.85) | <0.001 | 4 | 606 | 1.07 (0.73 to 1.57) | 0.71 | 14 | 4925 | 0.76 (0.66 to 0.89) | <0.001* | ||
| End of follow-up | 14 | 6252 | 0.73 (0.64 to 0.84) | <0.001 | 5 | 885 | 1.16 (0.83 to 1.63) | 0.39 | 19 | 7137 | 0.78 (0.69 to 0.88) | <0.001* | ||
| Dependence | 8 | 4128 | 0.94 (0.81 to 1.10) | 0.44 | 0 | 0 | Not estimable | — | 8 | 4128 | 0.94 (0.81 to 1.10) | 0.44 | ||
| Death or dependence | 4 | 1212 | 0.98 (0.77 to 1.25) | 0.87 | 0 | 0 | Not estimable | — | 4 | 1212 | 0.98 (0.77 to 1.25) | 0.87 | ||
| Death or deterioration | 3 | 2305 | 0.78 (0.65 to 0.93) | 0.006 | 2 | 317 | 0.65 (0.41 to 1.03) | 0.07 | 5 | 2622 | 0.76 (0.64 to 0.90) | 0.001 | ||
| Activities of daily living | 4 | 967 | 0.11† (−0.03 to 0.24) | 0.12 | 2 | 329 | −0.08† (−0.30 to 0.14) | 0.47 | 6 | 1296 | 0.06† (−0.06 to 0.17) | 0.33 | ||
| Cognitive function | 1 | 375 | 0.06† (−0.14 to 0.27) | 0.55 | 4 | 2942 | 0.09† (0.01 to 0.16) | 0.02 | 5 | 3317 | 0.08† (0.01 to 0.15) | 0.02 | ||
| Readmissions | 8 | 3543 | 1.01 (0.87 to 1.17) | 0.94 | 1 | 279 | 1.25 (0.78 to 2.01) | 0.35 | 9 | 3822 | 1.03 (0.89 to 1.18) | 0.72 | ||
*Subgroup interaction.
†Standardised mean difference.
Effects of interventions
The odds of a patient living at home at the end of scheduled follow-up (median 12 months, range six weeks to 12 months) were higher for those patients who had undergone comprehensive geriatric assessment (odds ratio 1.16, 95% confidence interval 1.05 to 1.28; P=0.003; 18 trials, 7062 participants).
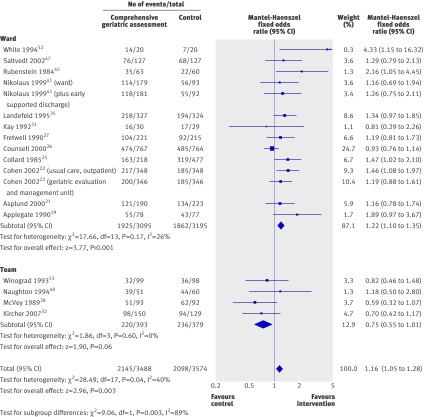
There was significant interaction between subgroups (χ2=9.06, P=0.003, I2=89%), with a significant difference from the effect of comprehensive geriatric assessment wards (1.22, 1.10 to 1.35; P<0.001; 14 trials, 6290 participants), whereas mobile comprehensive geriatric assessment teams were associated with a trend towards a worse outcome (0.75, 0.55 to 1.01; P=0.06; four trials, 772 participants). The overall effect equates to a number needed to treat of 33 to prevent one unnecessary death or admission to residential care, compared with general medical care. This effect is most pronounced for comprehensive geriatric assessment wards where the number needed to treat would be 20 (fig 2).
Fig 2 Odds ratios for living at home at end of follow-up (median 12 months) in elderly patients according to comprehensive geriatric assessment after emergency admission
This effect was more pronounced at analysis up to six months (median six months, range six weeks to six months; 1.25, 1.11 to 1.42; P<0.001; 14 trials, 5117 participants) equating to a number needed to treat of 17. There was interaction between the subgroups: comprehensive geriatric assessment wards were associated with a significantly improved odds of being alive and at home at six months (1.31, 1.15 to 1.49; P<0.001; 11 trials, 4624 participants), equating to a number needed to treat of 13 to avoid one unnecessary death or admission to residential care, compared with general medical care. By contrast, comprehensive geriatric assessment teams were not associated with a benefit (three trials, 493 participants, 0.84, 0.57 to 1.24, P=0.39), though numbers were smaller in this subgroup.
Analysis of data for living in residential care at the end of scheduled follow-up (median 12 months) showed a significant reduction for patients who had undergone comprehensive geriatric assessment (0.78, 0.69 to 0.88; P<0.001; 19 trials, 7137 participants). This equates to a number needed to treat of 25 to avoid one unnecessary admission to residential care, compared with general medical care. There was interaction between the subgroups, showing a difference between the benefits of comprehensive geriatric assessment wards (0.73, 0.64 to 0.84; P<0.001; 14 trials, 6252 participants; number needed to treat 20) and teams (1.16, 0.83 to 1.63; P=0.39; five trials, 485 participants,). This suggests that the overall benefit results from trials of wards.
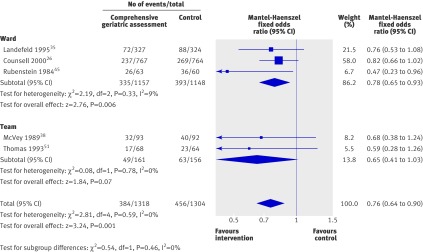
Analysis of data for the outcome of death or deterioration (a combined outcome of death or functional decline) showed a significant reduction in death or deterioration (0.76, 0.64 to 0.90; P=0.001; five trials, 2622 participants). This equates to a number needed to treat of 17 to avoid one unnecessary death or deterioration compared with general medical care (fig 3). There was no interaction between subgroups.
Fig 3 Odds ratios for death or deterioration at the end of follow-up (median 12 months) in elderly patients according to comprehensive geriatric assessment after emergency admission at baseline
Analysis of cognitive function showed an overall benefit on cognitive measures (five trials, 3317 participants, standardised mean difference 0.08, 0.01 to 0.15, P=0.02) for patients who underwent comprehensive geriatric assessment. There was no subgroup interaction, though data were available from only one comprehensive geriatric assessment ward (375 participants).
Analysis of mortality at the end of scheduled follow-up showed no significant difference between intervention and control groups (odds ratio 0.99, 0.90 to 1.09; P=0.82; 23 trial arms, 9963 participants). There was also no difference at up to six months’ follow-up (0.91, 0.80 to 1.05; P=0.20; 19 trials, 6787 participants).
Eight trials (4128 participants) reported data on dependence. All of the trials tested comprehensive geriatric assessment wards. No usable dependence data were recorded for teams. There was no significant difference between intervention and control groups (odds ratio 0.94, 0.81 to 1.10, P=0.44) (table 1). Though attrition was marked in one trial, exclusion of this trial from analysis did not significantly alter the outcome (0.89, 0.76 to 1.06; P=0.19; seven trials, 3669 participants). Similarly there was no significant difference for the outcome of death or dependence (0.98, 0.77 to 1.25; P=0.87; three trials, 1212 participants) or activities of daily living (standardised mean difference 0.06, −0.06 to 0.17; P=0.33; five trials, 1296 participants). There was no significant difference between the groups for the outcome of readmission to hospital (odds ratio 1.03, 0.89 to 1.18; P=0.72; nine trials, 3822 participants).
Data on length of stay were analysed for 12 trials (4034 participants). As there was significant heterogeneity (P<0.001, I2=86%), we did not carry out a meta-analysis.
Trials reported the costs of comprehensive geriatric assessment differently and with differing outcome measures. For this reason, we did not attempt meta-analysis. Table 2 shows the costs quoted by the papers that reported resource use with a brief explanation of how they were reported. Most costs reported are those incurred by the trial hospital and only rarely have the costs of nursing home care been taken into consideration. Most of the differences in cost are attributed to differences in length of stay or differences in the type and number of investigations requested between the groups. Many of the hospital costs (although not exclusively) seem to show a reduction in costs associated with comprehensive geriatric assessment.21 22 25 26 27 35 40 Some trials reported greater costs in the treatment group for hospitals.19 30 If nursing home costs are taken into consideration, the potential benefit of comprehensive geriatric assessment might be greater. The few trials that reported these cost showed reduced costs in the comprehensive geriatric assessment group.19 22 41 45
Table 2.
Costs associated with comprehensive geriatric assessment (intervention) versus usual care (control)
| Costs | Comments | ||
|---|---|---|---|
| Intervention | Control | ||
| Cohen 2002,22 US: | |||
| Geriatric unit-usual care outpatient v usual care inpatient-usual care outpatient | $36 592 (SD 1844) | $38 624 (SD 2037) | Cost-cost analysis separated into institutional costs and costings estimated for nursing home admissions based on standardised HMO rates |
| Geriatric unit-geriatric outpatient v usual care inpatient-geriatric outpatient | $35 935 (SD 1829) | $35 951 (SD 1827) | |
| Collard 1985,25 US: | |||
| Choate | $4015.17 (SE 0.03) | $4545.13 (SE 0.03) | Cost-cost analysis (hospital costs only) |
| Symmes | $3591.42 (SE 0.03) | $4155.54 (SE 0.02) | |
| Fretwell 1990,27 US | $3148 (SD 7210) | $4163 (SD 18 406) | Cost-cost analysis (hospital costs only) |
| Applegate 1990,19 US: | |||
| Geriatric unit (rehab diagnosis) v usual care (rehab diagnosis) | $32 978 (SD 35 130) | $18 409 (SD 16 555) | Health and social care costs up to 1 year after randomisation |
| Geriatric unit (medical/surgical diagnosis) v usual care (medical/surgical diagnosis) | $25 846 (SD 29 628) | $15 248 (SD 13 152) | |
| Asplund 2000,21 Sweden (Swedish kroner) | 10 800 (IQR 9300-12 300) | 12 800 (IQR 11 500-14 100) | Cost-cost analysis (hospital costs only) |
| Counsell 2000,26 US | $5640 | $5754 | Included in experimental group costs are costs of renovation of geriatric unit |
| Hogan 1987,30 Canada | $C98.36 | $C77.68 | Monthly costings for physician services only |
| Landefeld 1995,35 US | $6608 | $7240 | Cost-cost analysis (hospital costs only) |
| Nikolaus 1999,41 Germany (deutschmark): | |||
| Geriatric unit-early supported discharge | 3 365 000 ($1 922 400) | 4 145 000 ($2 368 300) | Costs for hospital care and nursing homes (estimated as costs per 100 people per year) |
| Geriatric unit only | 3 983 000 ($2 276 600) | ||
| Rubenstein 1984,45 US | $22 597 | $27 826 | Costs per year survived including hospital and nursing home costs |
| Naughton 1994,40 US | $4525 (SD 5087) | $6474 (SD 7000) | Cost-cost analysis (hospital costs only) |
| White 1994,52 US | $23 906 | $45 189 | Cost-cost analysis (hospital costs only) |
SD=standard deviation, IQR=interquartile range, SE=standard error.
Discussion
In this review of specialist organised geriatric care (normally referred to as comprehensive geriatric assessment or CGA) we found a clear and significant improvement in the odds of a patient being alive and in their own home if they receive coordinated specialist services rather than conventional care in a hospital setting.
Comparison with other studies
Most of the other reviews covering this issue have considered comprehensive geriatric assessment only in subgroups of inpatients 12 13 14 or have such assessment with care for specific conditions,9 13 in some cases making assumptions about what criteria differentiate one subgroup from another.13 These have generally not reported costs or included comprehensive geriatric assessment teams. These reviews have concluded that there are benefits for these subgroups but because of decisions regarding inclusion criteria are limited in their recommendations and might be making premature conclusions about which aspects of comprehensive geriatric assessment are effective and why.
Strengths and limitations
The quality and breadth of meta-analysis was limited by the availability and quality of data in individual studies. This was variable because of differences in reporting standards and formats, thus limiting the results for some outcome categories (such as activities of daily living). Reporting was generally high quality for the primary outcome (the inverse of death and admission to residential care combined).
As with other meta-analyses, trials that are combined might differ in important ways from each other (for example, acute and post-acute care). In this form of analysis, it is not possible to compare different forms of comprehensive geriatric assessment with each other nor to evaluate the benefit of combining different models. There remains a need for further trials that compare differing forms of comprehensive geriatric assessment. Wards that admit direct from the emergency department (sometimes called ACE units) show improved outcomes in patients compared with general medical settings12; so do trials admitting patients later in their hospital stay.13 14 Direct comparison between acute geriatric wards and post-acute wards is not possible from this analysis. Furthermore, the trials of acute geriatric wards and post-acute geriatric wards in this analysis differ in their admission criteria.
Trials evaluating direct admission from the emergency department all have admission criteria related to age, whereas trials evaluating post-acute care all have criteria related to needs (the exception being Shamian et al50). This makes comparison difficult. It could be that the optimal model of comprehensive geriatric assessment for hospitals includes both acute and post-acute models. Nevertheless, we did combine trials where key aspects of comprehensive geriatric assessment are carried out and have shown a distinct difference from general medical care.
Meta-analysis such as this can provide only limited guidance on which types of patients should undergo comprehensive geriatric assessment. In attempting to answer this question we dichotomised the data according to trials that admit patients primarily on the basis of age alone (for example, all those aged over 75) or those that included patients on the basis of need (defined as age plus several specific criteria). These needs related criteria are generally descriptive, including classic geriatric presentations (falls, delirium, immobility, etc) and some consideration of at risk criteria (such as functional impairment or risk of admission to nursing home). Both geriatric wards that admit patients on an age related basis and those that admit on a needs related basis seem to result in improved outcomes. Teams did not significantly benefit care of patients.
Analysis by healthcare setting and by the inclusion of specialist follow-up did not show a clear relation with outcomes, as had been previously suggested.7 9
The decision to create subgroups by wards and teams as two forms of comprehensive geriatric assessment is based on similarities in both the staffing of teams and wards and the processes of care (see appendix 2 on bmj.com). In clinical practice the two are often considered to be equivalent, and, in some cases, comprehensive geriatric assessment teams are seen as a more expedient form of assessment as it does not require fixed beds. The only initially apparent differences between the interventions relate to the physical geography of the admission of patients.
While it is possible to conclude that comprehensive geriatric assessment is beneficial, the benefits seem to arise solely from trials of geriatric wards and are not seen for geriatric teams. This effect is similar to the differences observed between stroke wards and stroke teams10 54 and might have multiple explanations. Firstly, a dedicated ward with more time focused on the care of older people allows greater learning within the ward staff, fostering the development of greater skills and expertise. In addition, working in close proximity as a group allows more efficient and effective multidisciplinary working and team building. Secondly, mobile teams often find it difficult to modify the behaviour of other health professionals directly involved in the patient’s care. As a consequence, recommendations for treatment and therapy are not always carried through,36 and having control over this process seems to lead to a better outcome.
There is evidence that specialisation within the ward team (for example, medical, physiotherapy and occupational therapy) is critical to the successful multidisciplinary team outcome, and this might be especially true in relation to gerontological nursing.55 As with stroke wards, the role of nursing in delivering direct care 24 hours a day might be critical to the success of care and can include both the avoidance of complications and the creation of an enabling therapeutic environment. In addition, communication with family members and patients might be key to goal setting or discharge planning.
Other factors that seem to benefit discrete units include the development of protocols of care for the management of key conditions, which can be implemented and acted on with a higher degree of consistency. A modified ward environment designed to be more suitable for frailer older adults is also important.
Several trials analysed costs. Most reported equitable or cost effective care from comprehensive geriatric assessment in a hospital setting. Further economic evaluation is worthwhile considering the demographic changes and potential societal costs from healthcare for an ageing population.
Conclusions
Significantly more older patients are likely to survive admission to hospital and return home if they undergo comprehensive geriatric assessment while they are inpatients. Fewer will die or experience deterioration and more will have improved cognitive functioning. These effects of acute geriatric medicine programmes are consistently shown in trials of geriatric wards but are not replicated in trials of geriatric consultation teams on general wards. These benefits might be cost effective.
Policy implications
All frail elderly patients admitted to hospital as an emergency should have access to comprehensive geriatric assessment beds. Compliance with best practice should be audited across healthcare providers, and the provision of geriatric services needs reviewed. Further evaluation (through additional research) of the unanswered questions as to who should receive specialist services and which forms of comprehensive geriatric assessment are most appropriate to which inpatient setting (acute or post-acute) are a priority.
What is already known on this topic
Older people represent a considerable proportion of hospital admissions and are at greatest risk of acquired disability, cognitive impairment, or admission to residential care
Some subgroups of in-hospital comprehensive geriatric assessment have been shown to be effective
What this study adds
Comprehensive geriatric assessment in hospital is effective and results in an increased likelihood of a patient returning home and avoiding admission to residential care or death and deterioration
The key features of successful comprehensive geriatric assessment seem to be treatment in discrete units, with expertise in the care of older people and control over the delivery of direct care
These benefits seem to be cost effective for hospitals and might be cost avoiding for society
Contributors: GE, MAW, and DR were responsible for trial searching, selection, and data extraction. PL and D’ON moderated. GE was responsible for analysis and writing up and is guarantor. All authors were involved in reviewing documents.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: Technical appendix, statistical code, and dataset available from the corresponding author. Consent was not obtained but the presented data are anonymised and risk of identification is low.
Cite this as: BMJ 2011;343:d6553
Web Extra. Extra material supplied by the author
Appendix 1: Search strategy for Medline
Appendix 2: Full details of all included trials
References
- 1.OECD. Ageing societies and the looming pension crisis. Paris: OECD, 2004.
- 2.National Statistics (UK). Population. Office for National Statistics, 2011. www.statistics.gov.uk/cci/nugget.asp?ID=949.
- 3.Kortebein P, Ferrando A, Lombedia J, Wolf R, Evans W. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007;297:1772-3. [DOI] [PubMed] [Google Scholar]
- 4.Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic DM, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc 2003;51:451-8. [DOI] [PubMed] [Google Scholar]
- 5.Mudge AM, O’Rourke P, Denaro CP. Timing and risk factors for functional changes associated with medical hospitalization in older patients. J Gerontol A Biol Sci Med Sci 2010;65:866-72. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K, Hubbard R. Frailty and the geriatrician. Age Ageing 2004;33:429-30. [DOI] [PubMed] [Google Scholar]
- 7.Rubenstein LZ, Stuck AE, Siu AL, Wieland D. Impact of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J Am Geriatr Soc 1991;39:8-16S. [DOI] [PubMed] [Google Scholar]
- 8.Ellis G, Langhorne P. Comprehensive geriatric assessment for older hospital patients. Br Med Bull 2005;71:45-59. [DOI] [PubMed] [Google Scholar]
- 9.Stuck AE, Siu AL, Wieland D, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet 1993;342:1032-6. [DOI] [PubMed] [Google Scholar]
- 10.Stoke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2009;4:CD000197. [DOI] [PubMed] [Google Scholar]
- 11.Cameron ID, Handoll HHG, Finnegan TP, Madhok R, Langhorne P. Co-ordinated multidisciplinary approaches for inpatient rehabilitation of older patients with proximal femoral fractures. Cochrane Database Syst Rev 2009;4:CD000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baztan JJ, Suarez-Garcia FM, Lopez-Arrieta J, Rodriguez-Manas L, Rodriguez-Artalejo F. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta-analysis. BMJ 2009;338:b50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachmann S, Finger C, Huss A, Egger M, Stuck AE, Clough-Gorr KM. Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ 2010;340:c1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Craen K, Braes T, Wellens N, Denhaerynck K, Flamaing J, Moons P, et al. The effectiveness of inpatient geriatric evaluation and management units: a systematic review and meta-analysis. J Am Geriatr Soc 2010;58:83-92. [DOI] [PubMed] [Google Scholar]
- 15.Handoll HHG, Cameron ID, Mak JCS, Finnegan TP. Multidisciplinary rehabilitation for older people with hip fractures. Cochrane Database Syst Rev 2009;4:CD007125. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions Cochrane Collaboration, 2011.
- 17.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427-37. [DOI] [PubMed] [Google Scholar]
- 18.Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2011;7:CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Applegate WB, Miller ST, Graney MJ, Elam JT, Burns R, Akins DE. A randomized, controlled trial of a geriatric assessment unit in a community rehabilitation hospital. N Engl J Med 1990;322:1572-8. [DOI] [PubMed] [Google Scholar]
- 20.Miller ST, Applegate WB, Elam JT, Graney MJ. Influence of diagnostic classification on outcomes and charges in geriatric assessment and rehabilitation. J Am Geriatr Soc 1994;42:11-5. [DOI] [PubMed] [Google Scholar]
- 21.Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, et al. Geriatric-based versus general wards for older acute medical patients: a randomised comparison of outcomes and use of resources. J Am Geriatr Soc 2000;48:1381-8. [DOI] [PubMed] [Google Scholar]
- 22.Cohen HJ, Feussner JR, Weinberger M, Carnes M, Hamdy RC, Hseih F, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med 2002;346:905-12. [DOI] [PubMed] [Google Scholar]
- 23.Phibbs CS, Holty JEC, Goldstein MK, Garber AM, Wang Y, Feussner JR, et al. The effect of geriatrics evaluation and management on nursing home use and health care costs. Results from a randomised trial. Med Care 2006;44:91-5. [DOI] [PubMed] [Google Scholar]
- 24.Bachman SS, Collard AF, Greenberg JN, Fountain E, Huebner TW, Kimball B, et al. An innovative approach to geriatric acute care delivery: the Choate-Symmes experience. Hosp Health Serv Admin 1987;November:509-20. [PubMed]
- 25.Collard AF, Bachman SS, Beatrice DF. Acute care delivery for the geriatric patient: an innovative approach. Q Rev Bull 1985;June:180-5. [PubMed]
- 26.Counsell SR, Holder CM, Liebenauer LL, Palmer RM, Fortinsky RH, Kresevic DM, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalised older patients: a randomised controlled trial of acute care for elders (ACE) in a community hospital. J Am Geriatr Soc 2000;48:1572-81. [DOI] [PubMed] [Google Scholar]
- 27.Fretwell MD, Raymond PM, McGarvey ST, Owens N, Traines M, Silliman RA, et al. A controlled trial of a consultative/unit-based geriatric assessment program in acute care. The Senior Care Study. J Am Geriatr Soc 1990;38:1073-81. [DOI] [PubMed] [Google Scholar]
- 28.Silliman RA, McGarvey ST, Raymond PM, Fretwell MD. Senior care study: does inpatient interdisciplinary geriatric assessment help the family caregivers of acutely ill older patients? J Am Geriatr Soc 1990;38:461-6. [DOI] [PubMed] [Google Scholar]
- 29.Harris RD, Henschke PJ, Popplewell PY, Radford AJ, Bond MJ, Turnbull RJ, et al. A randomised study of outcomes in a defined group of acutely ill elderly patients managed in a geriatric assessment unit or a general medical unit. Aust NZ J Med 1991;21:230-4. [DOI] [PubMed] [Google Scholar]
- 30.Hogan DB, Fox RA, Badley BWD, Mann OE. Effect of a geriatric consultation service on management of patients in an acute care hospital. CMAJ 1987;136:713-7. [PMC free article] [PubMed] [Google Scholar]
- 31.Kay G, MacTavish M, Moffat C, Lau G. Development and evaluation of a geriatric assessment unit in a community Hospital. Fall 1992;16:2-9. [PubMed] [Google Scholar]
- 32.Kircher TJ, Wormstall H, Muller PH, Schwarzler F, Buchkremer G, Wild K, et al. A randomised trial of a geriatric evaluation and management consultation services in frail hospitalised patients. Age Ageing 2007;36:36-42. [DOI] [PubMed] [Google Scholar]
- 33.Covinsky KE, King JT, Quinn LM, Siddique R, Palmer R, Kresevic DM, et al. Do acute care for elders units increase hospital costs? A cost analysis using the hospital perspective. J Am Geriatr Soc 1997;45:729-34. [DOI] [PubMed] [Google Scholar]
- 34.Covinsky KE, Palmer R, Kresevic DM, Kahana E, Counsell C, Fortinsky RH, et al. Improving functional outcomes in older patients: lessons from an acute care for elders unit. JT Comm J Qual Improv 1998;24:63-76. [DOI] [PubMed] [Google Scholar]
- 35.Landefeld CS, Palmer RM, Krescevic DM, Fortinsky RH, Kowal J. A randomised trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med 1995;332:1338-44. [DOI] [PubMed] [Google Scholar]
- 36.Allen CA, Becker PM, McVey LJ, Saltz CC, Feussner JR, Cohen HJ. A randomized, controlled clinical trial of a geriatric consultation team. JAMA 1986;255:2617-21. [PubMed] [Google Scholar]
- 37.Becker PM, McVey LJ, Saltz CC, Feussner JR, Cohen HJ. Hospital-acquired complications in a randomised controlled clinical trial of a geriatric consultation team. JAMA 1987;17:2313-7. [PubMed] [Google Scholar]
- 38.McVey LJ, Becker PM, Saltz CC, Feussner JR, Cohen HJ. Effect of a geriatric consultation team on functional status of elderly hospitalized patients. Ann Intern Med 1989;110:79-84. [DOI] [PubMed] [Google Scholar]
- 39.Saltz CC, McVey LJ, Becker PM, Feussner JR, Cohen HJ. Impact of a geriatric consultation team on discharge placement and repeat hospitalization. Gerontologist 1988;28:344-50. [DOI] [PubMed] [Google Scholar]
- 40.Naughton BJ, Moran MB, Feinglass J, Falconer J, Williams ME. Reducing hospital costs for the geriatric patient admitted from the emergency department: a randomized trial. J Am Geriatr Soc 1994;41:1045-9. [DOI] [PubMed] [Google Scholar]
- 41.Nikolaus T, Specht-Leible N, Bach M, Oster P, Schuerf G. A randomised trial of comprehensive geriatric assessment and home intervention in the care of hospitalised patients. Age Ageing 1999;28:543-50. [DOI] [PubMed] [Google Scholar]
- 42.Powell C, Montgomery P. The age study: the admission of geriatric patients through emergency. Age Ageing 1990;19(supp):21-22. [Google Scholar]
- 43.Reuben DB, Borok GM, Wolde-Tsadik G, Ershoff DH, Fishman LK, Ambrosini VL, et al. A randomised trial of comprehensive geriatric assessment in the care of hospitalised patients. N Engl J Med 1995;332:1345-50. [DOI] [PubMed] [Google Scholar]
- 44.Rubenstein LZ, Josephson KR, Harker JO, Miller DK, Wieland DG. The Sepulveda GEU Study revisited: long-term outcomes, use of services, and costs. Ageing (Milano) 1995;7:212-7. [DOI] [PubMed] [Google Scholar]
- 45.Rubenstein LZ, Josephson KR, Wieland DG, English PA, Sayre JA, Kane RL. Effectiveness of a geriatric evaluation unit. N Engl J Med 1984;311:1664-70. [DOI] [PubMed] [Google Scholar]
- 46.Saltvedt I, Jordhoy M, Opdahl Mo ES, Fayers P, Kaasa S, Sletvold O. Randomised trial of in-hospital geriatric intervention: impact on function and morale. Gerontology 2006;52:223-30. [DOI] [PubMed] [Google Scholar]
- 47.Saltvedt I, Opdahl Mo ES, Fayers P, Kaasa S, Sletvold O. Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomised trial. J Am Geriatr Soc 2002;50:792-8. [DOI] [PubMed] [Google Scholar]
- 48.Saltvedt I, Spigset O, Ruths S, Fayers P, Kaasa S, Sletvold O. Patterns of drug prescription in a geriatric evaluation and management unit as compared with the general medical wards: a randomised study. Eur J Clin Pharmacol 2005;61:921-8. [DOI] [PubMed] [Google Scholar]
- 49.Saltvedt I, Saltnes T, Mo ES, Fayers P, Kaasa S, Sletvold O. Acute geriatric intervention increases the number of patients able to live at home. A prospective randomised study. Aging Clin Exp Res 2004;16:300-6. [DOI] [PubMed] [Google Scholar]
- 50.Shamian J, Clarfield AM, Maclean J. A randomized trial of intra-hospital relocation of geriatric patients in a tertiary-care teaching hospital. J Am Geriatr Soc 1984;32:794-800. [DOI] [PubMed] [Google Scholar]
- 51.Thomas DR, Brahan R, Haywood BP. Inpatient community-based geriatric assessment reduces subsequent mortality. J Am Geriatr Soc 1993;41:101-4. [DOI] [PubMed] [Google Scholar]
- 52.White SJ, Powers JS, Knight JR, Harrell D, Varnell L, Vaughn C, et al. Effectiveness of an inpatient geriatric service in a university hospital. J Tenn Med Assoc 1994;87:425-8. [PubMed] [Google Scholar]
- 53.Winograd CH, Gerety MB, Lai NA. A negative trial of inpatient geriatric consultation. Arch Intern Med 1993;153:2017-23. [PubMed] [Google Scholar]
- 54.Langhorne P, Dey P, Woodman M, Kalra L, Wood-Dauhinee S, Patel N, et al. Is stroke unit care portable? A systemaic review of the clinical trials. Age Ageing 2005;34:324-30. [DOI] [PubMed] [Google Scholar]
- 55.Pound P, Sabin C, Ebrahim S. Observing the process of care: a stroke unit, elderly care unit and general medical ward compared. Age Ageing 1999;28:433-40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Search strategy for Medline
Appendix 2: Full details of all included trials