Abstract
Background
Traditional methods of diagnosing mucosal leishmaniasis (ML), such as biopsy with histopathology, are insensitive and require collection of an invasive diagnostic specimen.
Methods
We compared standard invasive procedures including biopsy histopathology, biopsy PCR, and leishmanin skin test (LST) to a novel, non-invasive, cytology-brush based PCR for the diagnosis of ML in Lima, Peru. Consensus reference standard was 2/4 tests positive, and outcome measures were sensitivity and specificity. Leishmania species identification was performed by PCR-based assays of positive specimens.
Results
Twenty-eight patients were enrolled, 23 of whom fulfilled criteria for a diagnosis of ML. Sensitivity and specificity of biopsy with histopathology were 21.7% [95% CI 4.9–38.5%] and 100%; 69.6% [95% CI 50.8–88.4%] and 100% for LST; 95.7% [95% CI 87.4–100%] and 100% for biopsy PCR; and 95.7% [95% CI 87.4–100%] and 90% [95% CI 71.4–100%] for cytology brush PCR using both Cervisoft® and Histobrush® cervical cytology brushes. Represented species identified by PCR-RFLP included: L. (V). braziliensis (n = 4), and L. (V). peruviana (n = 3).
Conclusions
Use of commercial grade cytology brush PCR for diagnosis of ML is sensitive, rapid, well tolerated, and carries none of the risks of invasive diagnostic procedures such as biopsy. Further optimization is required for adequate species identification. Further evaluation of this method in field and other settings is warranted.
Introduction
Mucosal leishmaniasis (ML) is a severe and stigmatizing chronic sequela of infection with predominantly New World species of Leishmania including L. (Viannia) braziliensis [1]–[5]. Along with Brazil and Bolivia, Peru contributes more than 90% of ML cases worldwide [1]. Differentiating ML from other endemic etiologies such as tuberculosis, non-tuberculous mycobacterial infections, rhinoscleroma, paracoccidioidomycosis, and malignancy is difficult on clinical grounds as manifestations such as nasal injection, pruritus, and infiltration, epistaxis, dysphonia, and palatal infiltration may be common to all. Coupled with the highly toxic nature of standard antimonial therapy, the broad differential diagnosis of mucosal lesions in Peruvian patients necessitates the use of accurate diagnostic modalities.
Traditional methods of diagnosing ML, such as biopsy with histopathology, are insensitive and require collection of an invasive diagnostic specimen [2], [6]. Invasive specimen collection is difficult to perform in remote under-resourced settings, and without technical expertise [7]–[9]. While PCR is a highly sensitive technique and is quickly becoming a favored ‘gold standard’ for the diagnosis of leishmaniasis [6], [10]–[14], this platform has mostly been used on invasive diagnostic specimens in ML such as biopsies [2], [9], [14]. Accurate diagnosis in the absence of a well-performing gold standard is an ongoing challenge in leishmaniasis [15]. There is therefore a need for sensitive, accurate non-invasive diagnostic testing in ML.
We herein compared several ‘traditional’ methods for diagnosing ML including biopsy with histopathology, biopsy PCR, and leishmanin skin test (LST) to the novel, non-invasive method of cytology brush PCR using 2 different commercial grade cervical cytology brushes. In addition, we performed species identification using PCR-based assays of clinical specimens, which is important in countries like Peru where several members of the Leishmania (Viannia) subgenus can cause mucosal disease.
Methods
Ethics Statement
This study was approved by the Institutional Review Boards of Hospital Nacional Cayetano Heredia (HNCH) and the University of Toronto. All patients provided written informed consent for the study procedures prior to enrolment.
Study Site
The study was conducted at the Leishmania Clinic of the Instituto de Medicina Tropical “Alexander Von Humboldt” and HNCH, in Lima, Peru, between January and December 2010. The Institute houses a large outpatient clinic for the diagnosis and management of American tegumentary leishmaniasis, with an average of 30–40 new cases diagnosed per month [7], [16].
Study Population
Consecutive patients presenting to the Leishmania Clinic for the evaluation of mucosal (nasal, buccal, oral, pharyngeal) and/or skin lesions were approached to participate in this study, and screened for eligibility criteria. All patients were interviewed and examined by a clinic physician. Direct anterior rhinoscopy and oropharyngoscopy were performed on all patients (including those referred for cutaneous ulcers), to determine if mucosal abnormalities such as erythema, infiltration, or ulceration were present. We included patients who were referred to the Leishmania Clinic for suspected ML or CL; had one or more mucosal lesions with a clinical indication for mucosal biopsy; and were able to give informed consent for the diagnostic procedures. We excluded patients undergoing active treatment for ML or CL, and those with any contraindication to mucosal biopsy.
Sampling
Cytology brushes
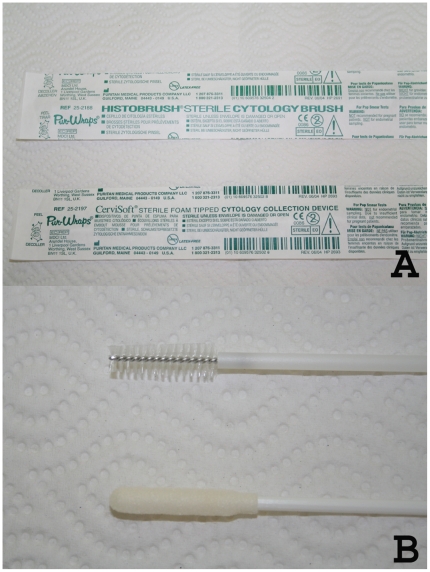
After removing any overlying scab or crust with moistened gauze, and cleansing the mucosal lesion with isopropyl alcohol, sterile and duplicate CerviSoft® (Puritan Medical Products, Maine) and Histobrush® (Puritan Medical Products, Maine) cervical cytology brushes (Figure 1) were rolled clockwise on the lesion 5 times each in sequence. Each cytology brush has a cylindrical handle of several inches in length, and a foam or bristled tip of 1-inch in length for collection of clinical specimens (Figure 1). Cytology brush tips were then cut off with sterile scissors directly into 1.5-mL Eppendorf tubes containing 700 µL 100% ethanol and stored at −20 C for qualitative PCR testing. Control nasal septum and buccal cytology brush specimens were collected and processed as above from 5 healthy volunteers with normal mucosa living in a non-endemic area.
Figure 1. Cervical cytology brushes.
A, sterile wrapped CerviSoft® and Histobrush® cervical cytology brushes; B, CerviSoft® and Histobrush® cytology brush tips.
Mucosal biopsy
After collection of the cytology brush specimens and cleansing the lesion again with isopropyl alcohol, mucosal lesions were anesthetized with 20 mg/mL lidocaine spray. Two small biopsy specimens were then obtained from lesions using sterile nasal or ethmoid biopsy forceps. The tissue was then stored in 1.5-mL Eppendorf tubes containing 700 µL 100% ethanol at −20°C for qualitative PCR testing, or placed in 10% formalin for histopathology with hematoxylin and eosin, Ziehl-Neelsen, and Giemsa staining. Sterile gauze was applied with pressure to the mucosal lesion until hemostasis was achieved.
Leishmanin Skin Test
Leishmanin skin tests were applied and read as described [7], [16]. A positive result was indicated by ≥5 mm of erythema and induration as previously described [7], [16], [17].
Isolation of DNA from Cytology Brushes and Biopsy Specimens
Prior to DNA extraction, samples were centrifuged at 3000 g for 5 min and ethanol was discarded. Biopsied tissues were disaggregated with a sterile scalpel. Disaggregated tissue and cytology brushes were processed for DNA isolation using the High Pure PCR Template Preparation Kit® (Roche, Mannheim, Germany) according to manufacturer's instructions.
Kinetoplastid DNA (kDNA) Polymerase Chain Reaction
Leishmania kDNA PCR was performed using the HotStar Taq Plus DNA Polymerase kit (QIAGEN, Hilden, Germany) and conditions were as described [7]. Two pairs of primers were used as previously described [7], [18]. Amplicons were visualized on 3% agarose gels (Promega, Madrid, Spain) and stained with ethidium bromide.
Species Identification by PCR and Restriction Fragment Length Polymorphism (PCR-RFLP) of Genomic Targets
Four PCR assays targeting different sequences specific to Leishmania sub-genus Viannia species including L. (V.) braziliensis, L. (V.) peruviana, and L. (V.) guyanensis, the principal causative species in Peru, were used for the species identification following initial kDNA PCR. PCR assays were performed using the HotStar Taq Plus DNA Polymerase kit (QIAGEN, Hilden, Germany) as previously described [7].
The first assay, targeting the mannose phosphate isomerase gene (MPI), consisted of two separate reactions employing allele-specific reverse primers, which distinguish L. (V.) peruviana from L. (V.) braziliensis and L. (V.) guyanensis, as previously described [7], [19]. MPI PCR conditions were as described [7], [19]. The second assay, targeting the cysteine proteinase B (Cpb) gene, employed primers which distinguish between L. (V.) braziliensis and non-L. (V.) braziliensis species as previously described [7], [20], [21]. Cpb PCR conditions were as described [7], [20], [21]. The third assay, targeting heat shock protein 70 (hsp70), employed primers which distinguish between L. (V.) guyanensis and non-L. (V.) guyanensis species as previously described [7], [21], [22]. Hsp70 PCR conditions were as described [21], [22].
A fourth and final PCR assay was used to confirm species on those samples that yielded weak bands or lack of amplification products in previous assays. The PCR target was a 870 bp fragment of Leishmania glycoprotein of 63 kDa (gp63), with the following primer sequences: MUS (fwd) 5′- GTGGGTGTCATCAACATCCC – 3′ and MUSA3 (rev) 5′- CTGCTGCCGTACACCTGGAC – 3′ [23]. Gp63 PCR conditions were as follows: 95°C for 5 min, followed by 45 cycles of denaturation at 94°C for 30 s; primer annealing at 63°C for 60 s; extension at 72°C for 60 s, and a final extension step at 72°C for 6 min (iCycler iQ, Bio-Rad). All PCR products were visualized on 1.5% agarose gels (Promega, Madrid, Spain) and stained with ethidium bromide.
Restriction fragment length polymorphism analysis of Cpb, Hsp70, and gp63 PCR products (PCR-RFLP)
Following cpb, hsp70 and gp63 PCR amplification as above, products were separately digested overnight at 65°C for the cpb assay, or 37°C for the hsp70 and gp63 assay, in a total volume of 20 µL, with 5 U of each restriction enzyme. The following enzymes were used in each reaction: TaqI (cpb) and HaeIII (hsp70) (Fermentas, Burlington, Canada). For gp63, products were digested in duplicate. One reaction was digested with HincII and the second with SalI restriction enzyme (Fermentas, Burlington, Canada). Restriction fragments were then analyzed separately using 2.5% agarose gels for cpb and gp63 or 4% agarose gels for hsp70 (Promega, Madrid, Spain), and stained with ethidium bromide. When weak amplification product was observed after PCR, restriction fragments were separated using 12% polyacrylamide gel electrophoresis using the MiniProtean III system (Bio-Rad, Hercules, CA, USA), and stained with silver stain (Promega, Madrid, Spain). Mpi PCR distinguishes L. (V.) peruviana, while Cpb PCR-RFLP distinguishes L. (V.) braziliensis, and hsp70 PCR-RFLP differentiates L. (V.) guyanensis from L. (V.) lainsoni.
Composite Reference Standard
We defined a lesion as ML when any 2 of 4 tests were positive, where tests refer to biopsy with histopathology; biopsy PCR; LST; or cytology brush PCR. These 4 tests served as the composite reference standard against which each individual diagnostic test was compared. Assessors of LST, histopathology, and PCR were blind to the results of the other assays.
Sample Size Calculation
Based on existing literature [2], [14], [24]–[26], we estimated the overall sensitivity of gold standard biopsy with histopathology to be 40%, and the sensitivity of biopsy PCR to be 90–95%. In order to achieve a sensitivity of cytology brush PCR better than the gold standard histopathology and comparable to biopsy PCR, assuming an α = 0.05 and a power of 80%, 28 patients were required per group. For sensitivity analysis, the aforementioned composite reference standard was applied, and the unit of analysis was the patient.
Statistical Analysis
Descriptive statistics (mean, SD, median, range) were calculated for continuous variables, and differences were compared using 2-tailed t-testing. Categorical variables were quantitated by proportions, and differences between the groups were compared using Yate's corrected Chi-square analysis. Differences in sensitivities and specificities were compared using the z-test. Statistical analyses were performed using SigmaStat 2.03 software (SPSS Inc., Chicago, IL). Level of significance was set at p<0.05.
Results
Twenty-eight patients were enrolled in the study: 23 males and 5 females. Of 28 patients enrolled, 23 were referred for suspicious mucosal lesions only, and 5 were referred for evaluation of cutaneous lesions who were then noted to have mucosal involvement on examination. Clinical and demographic characteristics of the cohort are summarized in table 1. Median age was 48 years (range 16–87 years), and median duration of exposure in the risk area was 7.5 years (range 2 days–75 years). Median duration of illness was 25.5 months (range 1 month–20 years). Of 28 patients enrolled, 15 (54%) had a past history of CL, and 4 (14%) had old scars suspicious for past CL, but no previous definitive diagnosis (Table 1). Of 23 patients diagnosed with ML, 4 had intercurrent cutaneous leishmaniasis.
Table 1. Clinical and Demographic Characteristics of 28 Patients with Suspected Mucosal Leishmaniasis.
| Age, Sex | Lesion Location | Intercurrent CL* | LST | Histopathology | PCR Biopsy | PCR Brush** |
| 60, M§ | NS | No | Pos | Lymphoeosinophilic infiltrate | Pos | Pos |
| 46, M¶ | NS | No | Neg | Granulomatous inflammation | Pos | Pos |
| 62, F | NS | No | Neg | Lymphoplasmo-histiocytosis | Pos | Pos |
| 68, M | NS | Yes | Neg | Lymphoeosinophilic infiltrate | Pos | Pos† |
| 66, M§ | NS | No | Pos | Parasitic granuloma with amastigotes | Pos | Pos |
| 57, M | Canthus | Yes | Pos | Granulomatous inflammation with amastigotes present | Pos | Pos |
| 36, M | NS | Yes | Pos | Normal cartilage | Pos | Pos |
| 49, F¶ | NS | No | Neg | Polymorphonuclear lymphocytic infiltrate | Pos | Pos |
| 75, F | NS | Yes | Pos | Lymphocytic infiltrate | Pos | Pos |
| 16, M | NS | No | Pos | Parasitic granuloma with amastigotes | Pos | Pos |
| 38, M§ | Buccal | No | Neg | PAS-positive bodies suspicious for Paracoccidioides braziliensis infection | Pos | Pos |
| 31, M§ | Hard Palate | No | Neg | Granulomatous inflammation | Pos | Pos |
| 87, M§ | Buccal | No | Neg | Epidermoid carcinoma | Neg | Neg |
| 52, M§ | NS | No (stasis ulcers) | Pos | Granulomatous inflammation | Pos | Pos |
| 63, M¶ | NS | No | Pos | Granulomatous inflammation | Pos | Pos |
| 32, M§ | NS | No | Pos | Granulomatous inflammation | Pos | Neg |
| 19, M | NS | No | Neg | Perivascular eosinophilic infiltrate | Neg | Neg |
| 41, M | Hard Palate | No | Pos | Granulomatous inflammation | Pos | Pos |
| 40, M§ | NS | No | Neg | Granulomatous inflammation | Neg | Pos |
| 20, F§ | NS | No | Pos | Granulomatous inflammation | Pos | Pos |
| 50, M§ | NS | No | Pos | Granulomatous inflammation | Neg | Pos |
| 47, M§ | NS | No | Pos | Parasitic granuloma with amastigotes | Pos | Pos |
| 62, F¶ | NS | No | Neg | Acute and chronic inflammatory infiltrate | Neg | Neg |
| 43, M§ | NS | No | Pos | Parasitic granuloma with amastigotes | Pos | Pos |
| 62, M | NS | No | Neg | Granulomatous inflammation | Neg | Neg |
| 35, M§ | NS | No | Neg | Granulomatous inflammation | Pos | Pos |
| 55, M§ | NS | No | Pos | Granulomatous inflammation | Pos | Pos |
| 23, M§ | NS | No | Pos | Epithelial hyperplasia and necrosis | Pos | Pos |
*Intercurrent CL confirmed by smear or culture of cutaneous lesion specimens.
**All 5 control volunteers had negative cytology brush specimens.
CerviSoft® and Histobrush® PCR demonstrated 97% concordance; in 1 patient, CerviSoft® PCR was positive and Histobrush® PCR was negative.
Patient with known previous CL on history.
Patient with scars suspicious to be healed CL but no definitive history of diagnosis.
Abbreviations: CL, cutaneous leishmaniasis; F, female; LST, leishmanin skin test; M, male; Neg, negative; NS, nasal septum; Pos, positive.
Performance characteristics of each tested assay are summarized in table 2. Biopsy PCR was 95.7% sensitive [95% CI 87.4–100%], and 100% specific, with pooled cytology brush PCR demonstrating 95.7% [95% CI 87.4–100%] sensitivity and 90% [95% CI 71.4–100%] specificity. Sensitivity and specificity of Cervisoft® brush PCR were 95.7% and 90%, while Histobrush® PCR demonstrated sensitivity of 91.3% [95% CI 79.8–100%], and specificity of 90% [95% CI 71.4–100%]. Compared to cytology brush PCR, traditional biopsy with histopathology and LST had poorer performance characteristics with sensitivities of 21.7% [95% CI 4.9–38.5%] (p<0.001) and 69.6% [95% CI 50.8–88.4%] (p = 0.016), respectively [Table 2]. Expanding the definition of biopsy with histopathological diagnosis to include granulomatous inflammation, rather than strictly to the presence of visible amastigotes, increased the sensitivity of biopsy with histopathology to 71.4% [95% CI 52.9–89.9%], which was still inferior to cytology brush PCR or biopsy PCR (p = 0.024).
Table 2. Analysis of 5 Diagnostic Tests used in the Evaluation of 28 Patients with Suspected Mucosal Leishmaniasis.
| Assay | Number Positive | Number Negative | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| kDNA PCR of Biopsy Specimen | 22 | 6 | 95.7 | 100.0 | 100.0 | 83.3 |
| kDNA PCR of CerviSoft® brushes | 23 | 10* | 95.7 | 90.0 | 95.7 | 90.0 |
| kDNA PCR of Histobrush® brushes | 22 | 11* | 91.3 | 90.0 | 95.5 | 81.8 |
| LST | 16 | 12 | 69.6 | 100.0 | 100.0 | 41.7 |
| Biopsy with Histopathology | 5 | 23 | 21.7 | 100.0 | 100.0 | 21.7 |
*includes nasal and buccal specimens from 5 healthy control volunteers.
Abbreviations: LST, leishmanin skin test; NPV, negative predictive value; PPV, positive predictive value.
Subjective tolerability of the CerviSoft® brush was superior to that of the Histobrush®, as it was reported to cause less discomfort and was softer on the mucosa, particularly in the nose. All healthy control volunteers had negative CerviSoft® and Histobrush® cytology brush PCR.
Of 23 patients with ML, PCR-based assays led to species identification in 7. Represented causative species included: L. (V.) braziliensis, 4 patients; and L. (V.) peruviana, 3 patients. There were no cases of L. (V.) guyanensis or L. (V.) lainsoni identified (Table 3).
Table 3. Species identification from 2 sets of specimens out of 16 PCR-positive lesions subsequently tested with PCR-based assays targeting the mannose phosphate isomerase, cysteine proteinase B, heat shock protein 70, and gp63 genes.
| Leishmania Species | Number (% of those tested) |
| L. (V.) braziliensis | 4 (25%) |
| L. (V.) guyanensis | 0 |
| L. (V.) peruviana | 3 (19%) |
| Not identifiable | 9 (56%) |
| Not tested* | 12 |
*Only specimens with sufficient amplifiable DNA from the kDNA PCR of cytology brushes were selected for direct clinical specimen PCR.
Discussion
We have demonstrated that Leishmania (Viannia) kDNA can be detected in non-invasive cytology brush specimens for the diagnosis of ML. PCR of non-invasive cytology brush specimens had a comparable performance to PCR of biopsy specimens, and was superior to either conventional biopsy with histopathology or LST. Compared to biopsy, non-invasive cytology brushes are easier to obtain, require no technical expertise or anesthesia, cause no discomfort to the patient, do not carry risks of bleeding or infection, and obviate the need for sharps, sharps biosafety disposal, or concerns regarding needle stick injuries in the health care worker. Unlike biopsies, cytology brush specimens can be easily collected in the field and transported back to a reference center for testing. Thus, our data represent an advance in the approach to diagnostic testing in ML that will benefit the patient and health care worker alike.
Although PCR of biopsy and brush specimens were highly sensitive and specific, there may have been 1 patient with known previously treated CL (1990) and ML (1992), and new isolated involvement of the buccal mucosa with no other focus of infection, who was a biological false positive. This patient had PAS-positive bodies on histopathology suspicious for Paracoccidioides braziliensis, but also had a buccal mucosa biopsy and cytology brushes that were positive for L. (Viannia) kDNA by PCR. It is therefore possible that this patient had detectable amounts of persistent parasite DNA in the mucosa and new infection with Paracoccidioides braziliensis. Alternatively, the patient may have had active infection with both paracoccidioidomycosis and leishmaniasis. In any case, this patient highlights the need for non-invasive multiplex assays that can differentiate between common causes of mucosal lesions in the tropics. Although the patient fulfilled reference criteria for a diagnosis of ML, the uncertainty surrounding the diagnosis given the histopathology also suggests that further refinements to the cytology brush method, possibly including a quantitative component, are warranted.
Leishmania kDNA has been detected in the normal mucosa of Latin American patients with CL [9], which raises the possibility that detectable kDNA in the mucosa does not necessarily reflect ML. While it is possible that patients in our series merely had detectable kDNA in the mucosa, and not necessarily true ML, that they all had clinical evidence of ML (ie, mucosal lesions) and fulfilled a consensus reference standard for diagnosis argues against this possibility. However, in one patient with confirmed CL (by smear and culture of skin lesion aspirate) and erythematous nasal mucosa, a diagnosis of ML was made based on positive nasal biopsy PCR, cytology brush PCR, and LST, despite normal nasal septal histopathology. It is possible that this patient simply had detectable kDNA in the mucosa by multiple means, thus fulfilling reference standard criteria for diagnosis, but no true ML. Given the paucity of data surrounding treatment outcomes on patients like this who have CL and detectable kDNA in the mucosa, we erred on the side of caution and implemented a 28-day ML treatment regimen rather than a 21-day CL regimen. Prospective testing of CL patients with normal mucosa by nasal and buccal cytology brush PCR in our center may be indicated to better inform management of this unique situation. Regardless of how the mucosa is labeled histopathologically, treatment of detectable kDNA in the mucosa of CL patients with a ML regimen is likely warranted until further data, which inform our understanding of mucosal dissemination, are accrued.
Species identification is important in countries like Peru where several members of the L. Viannia subgenus are co-endemic and portend different prognoses and response to therapy [4]. L. (V). braziliensis is historically the most well represented causative species in ML [2], [4], [5]. We have demonstrated that related species including L. (V). peruviana are implicated in mucosal disease as well. Case-report level data implicating non-braziliensis sub-species in ML is also mounting [2], [5], [27], [28]. Further optimization of direct-cytology brush species identification is required. As genomic targets were used for species identification PCR and RFLP assays (rather than the kinetoplast target used for the diagnostic PCR), enhanced collection of higher concentrations of amastigotes or parasite DNA from the lesion by additional revolutions of the brush may improve the yield.
In summary, we have demonstrated that cytology brush PCR using CerviSoft® and Histobrush® cervical cytology brushes is adequate for the diagnosis of ML though identification of causative species requires further optimization. We have further demonstrated its superior performance to the gold standard biopsy with histopathology. Cytology brush PCR offers numerous practical advantages over biopsy PCR including simplicity, tolerability, and cost efficiency due to the lack of need for highly trained personnel to collect the specimen, anesthesia, sterile biopsy instruments, and sharps biohazard disposal and precautions. At just 30–50 cents (US) per cytology brush, this novel diagnostic specimen is practical and comparatively affordable. It can be transported easily to a reference center for diagnostic testing and is likely appropriate for field situations. Future field studies are indicated, as are studies that aim to differentiate between common causes of mucosal lesions in the tropics using this simple non-invasive specimen.
Acknowledgments
The authors would like to thank Dr. Eduardo Gotuzzo, Sra. Ana Luz Quispe, and Sra. Carmen Medina of the Instituto de Medicina Tropical “Alexander von Humboldt” (UPCH) for logistical support. The authors would like to thank Sra. Milena Alba of the Instituto de Medicina Tropical “Alexander von Humboldt” (UPCH) for technical support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by the Royal Society of Tropical Medicine and Hygiene through a Garnham Fellowship (www.rstmh.org). AKB was supported by a Detweiler Traveling Fellowship through the Royal College of Physicians and Surgeons of Canada during the study period (www.rcpsc.medical.org). Personnel and facility support for the Arevalo molecular laboratory (NV, JA) were provided by the Institutional Collaboration Framework Agreement 3 from the Belgian Development Cooperation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Leishmaniasis: burden of disease. Available at (accessed May 10, 2010): http://www.who.int/leishmaniasis/burden/en/
- 2.David CV, Craft N. Cutaneous and mucocutaneous leishmaniasis. Derm Ther. 2009;22:491–502. doi: 10.1111/j.1529-8019.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 3.Lucas CM, Franke ED, Cachay MI, Tejada A, Cruz ME, et al. Geographic distribution and clinical description of leishmaniasis cases in Peru. Am J Trop Med Hyg. 1998;59:312–317. doi: 10.4269/ajtmh.1998.59.312. [DOI] [PubMed] [Google Scholar]
- 4.Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, et al. Influence of leishmania (viannia) species on the response to antimonial treatment in patients with american tegumentary leishmaniasis. J Infect Dis. 2007;195:1846–1851. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- 5.Santrich C, Segura I, Arias AL, Saravia NG. Mucosal disease caused by Leishmania braziliensis guyanensis. Am J Trop Med Hyg. 1990;42:51–55. doi: 10.4269/ajtmh.1990.42.51. [DOI] [PubMed] [Google Scholar]
- 6.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 7.Boggild AK, Valencia BM, Espinosa DE, Veland N, Pilar Ramos A, et al. Detection and species identification of Leishmania DNA from filter paper lesion impressions in patients with American cutaneous leishmaniasis. Clin Infect Dis. 2010;50:e1–6. doi: 10.1086/648730. [DOI] [PubMed] [Google Scholar]
- 8.Mimori T, Matsumoto T, Calvopina MH, Gomez EA, Saya H, et al. Usefulness of sampling with a cotton swab for PCR-diagnosis of cutaneous leishmaniasis in the New World. Acta Trop. 2002;81:197–202. doi: 10.1016/s0001-706x(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa RA, Lozano LE, Romero IC, Cardona MT, Prager M, et al. Detection of Leishmania in unaffected mucosal tissues of patients with cutaneous leishmaniasis caused by Leishmania (Viannia) species. J Infect Dis. 2009;200:638–646. doi: 10.1086/600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 12.Tavares CA, Fernandes AP, Melo MN. Molecular diagnosis of leishmaniasis. Expert Rev Mol Diagn. 2003;3:657–667. doi: 10.1586/14737159.3.5.657. [DOI] [PubMed] [Google Scholar]
- 13.Reithinger R, Dujardin JC. Molecular diagnosis of leishmaniasis: current status and future applications. J Clin Micro. 2007;45:21–25. doi: 10.1128/JCM.02029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira JGS, Novais FO, de Oliveira CI, da Cruz Junior AC, Campos LF, et al. Polymerase chain reaction (PCR) is highly sensitive for diagnosis of mucosal leishmaniasis. Acta Trop. 2005;94:55–59. doi: 10.1016/j.actatropica.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Cortes A, Ojeda A, Francino O, Lopez-Fuertes L, Timon M, Alberola J. Leishmania infection: laboratory diagnosing in the absence of a “gold standard”. Am J Trop Med Hyg. 2010;82:251–256. doi: 10.4269/ajtmh.2010.09-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boggild AK, Miranda-Verastegui C, Espinosa D, Adaui V, Arevalo J, et al. Evaluation of a Microculture Method for the Isolation of Leishmania parasites from Cutaneous Lesions in Peru. J Clin Microbiol. 2007;45:3680–3684. doi: 10.1128/JCM.01286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokal JE. Measurement of delayed skin-test responses. N Eng J Med. 1975;293:501–502. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 18.Lopez M, Orrego C, Cangalaya M, Inga R, Arevalo J. Diagnosis of Leishmania via the polymerase chain reaction: A simplified procedure for field work. Am J Trop Med Hyg. 1993;49:348–356. doi: 10.4269/ajtmh.1993.49.348. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W-W, Miranda-Verastegui C, Arevalo J, Ndao M, Ward B, et al. Development of a genetic assay to distinguish between Leishmania viannia species on the basis of isoenzyme differences. Clin Infect Dis. 2006;42:801–809. doi: 10.1086/500326. [DOI] [PubMed] [Google Scholar]
- 20.Garcia AL, Kindt A, Quispe-Tintaya KW, Bermudez H, Llanos A, et al. American tegumentary leishmaniasis: antigen-gene polymorphism, taxonomy and clinical pleomorphism. Infect Genet Evol. 2005;5:109–116. doi: 10.1016/j.meegid.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Perez JE, Veland N, Espinosa D, Torres K, Ogusuku E, et al. Isolation and molecular identification of Leishmania (Viannia) peruviana from naturally infected Lutzomyia peruensis (Diptera: Psychodidae) in the Peruvian Andes. Mem Inst Oswaldo Cruz. 2007;102:655–658. doi: 10.1590/s0074-02762007005000077. [DOI] [PubMed] [Google Scholar]
- 22.Garcia L, Kindt A, Bermudez H, Llanos-Cuentas A, De Doncker S, et al. Culture-independent species typing of neotropical Leishmania for clinical validation of a PCR-based assay targeting heat shock protein 70 genes. J Clin Micro. 2004;42:2294–2297. doi: 10.1128/JCM.42.5.2294-2297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Victoir K, de Doncker S, Cabrera L, Alvarez E, Arevalo J, et al. Direct identification of Leishmania species in biopsies from patients with American tegumentary leishmaniasis. Trans R Soc Trop Med Hyg. 2003;97:80–87. doi: 10.1016/s0035-9203(03)90031-9. [DOI] [PubMed] [Google Scholar]
- 24.Weigle KA, de Davalos M, Heredia P, Molineros R, Saravia NG, et al. Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: A comparison of seven methods. Am J Trop Med Hyg. 1987;36:489–496. doi: 10.4269/ajtmh.1987.36.489. [DOI] [PubMed] [Google Scholar]
- 25.Scope A, Trau H, Anders G, Barzilai A, Confino Y, et al. Experience with New World cutaneous leishmaniasis in travelers. J Am Acad Dermatol. 2003;49:672–678. doi: 10.1067/s0190-9622(03)01576-7. [DOI] [PubMed] [Google Scholar]
- 26.Pirmez C, da Silva Trajano V, Neto M, da-Cruz AM, Gonçalves-da-Costa SC, et al. Use of PCR in diagnosis of human American tegumentary leishmaniasis in Rio de Janeiro, Brazil. J Clin Micro. 1999;37:1819–1823. doi: 10.1128/jcm.37.6.1819-1823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas CM, Franke ED, Cachay MI, Tejada A, Cruz ME, et al. Geographic distribution and clinical description of leishmaniasis cases in Peru. Am J Trop Med Hyg. 1998;59:312–317. doi: 10.4269/ajtmh.1998.59.312. [DOI] [PubMed] [Google Scholar]
- 28.Osorio LE, Castillo CM, Ochoa MT. Mucosal leishmaniasis due to Leishmania (Viannia) panamensis in Colombia: clinical characteristics. Am J Trop Med Hyg. 1998;59:49–52. doi: 10.4269/ajtmh.1998.59.49. [DOI] [PubMed] [Google Scholar]