Abstract
We describe an algorithm to calculate an index that characterizes spatial differences in broadband near-infrared [(NIR), 650–1000 nm] absorption spectra of tumor-containing breast tissue. Patient-specific tumor spatial heterogeneities are visualized through a heterogeneity spectrum function (HS). HS is a biomarker that can be attributed to different molecular distributions within the tumor. To classify lesion heterogeneities, we built a heterogeneity index (HI) derived from the HS by weighing the HS in specific NIR absorption bands. It is shown that neoadjuvant chemotherapy (NAC) response is potentially related to the tumor heterogeneity. Therefore, we correlate the heterogeneity index obtained prior to treatment with the final response to NAC. From a pilot study of 15 cancer patients treated with NAC, pathological complete responders (pCR) were separated from non-pCR according to their HI (–44 ± 12 and 43 ± 17, p = 3 × 10−8, respectively). We conclude that the HS function is a biomarker that can be used to visualize spatial heterogeneities in lesions, and the baseline HI prior to therapy correlates with chemotherapy pathological response.
Keywords: neoadjuvant chemotherapy; heterogeneity; breast, cancer; spectra; near-infrared; biomarkers
Breast cancer accounts for >20% of all newly diagnosed female cancers.1 New treatment regimens and early detection have reduced mortality rates. Patients diagnosed with locally advanced breast cancer are considered to be at increased risk of disseminated disease. As a result, many of these patients now receive neoadjuvant chemotherapy (NAC), even if the tumor is primarily operable. Although there is no evidence to suggest that there is a survival benefit of neoadjuvant chemotherapy when compared to adjuvant treatment, some studies have shown an overall survival benefit for those patients who achieve a pathologic complete response (pCR) after neoadjuvant chemotherapy compared to patients without a complete response (non-pCR).2 Breast cancer is a heterogeneous disease that comes in several clinical and histological forms. Its clinical progression is difficult to predict using current prognostic factors, and treatment is therefore not as effective as it should be.3, 4, 5, 6, 7 The prediction of the efficacy of chemotherapy would potentially select good candidates who would respond well while excluding poor candidates who would not benefit from treatment. This would prevent many patients from experiencing unnecessary side effects. Several studies have been performed using conventional radiologic imaging techniques to investigate predictive factors for various chemotherapeutic modalities in breast cancer, but many of them have been found to be unsatisfactory.8, 9, 10
A previous study11 was able to predict chemotherapy response based on specific histological features of breast cancer. In this work, we investigate the possibility of noninvasively predicting chemotherapy response prior to treatment based on biomarkers obtained from tumor spatial heterogeneities of spectral features measured using diffuse optical spectroscopy (DOS).
DOS is a noninvasive, bedside technique that is commonly used to provide biochemical information on hemoglobin, bulk lipids, and water concentration by measuring near-infrared [(NIR), 650–1000 nm] tissue absorption and scattering.12 DOS does not require exogenous contrast and rapidly (e.g., tens of seconds) provides quantitative, functional information about tumor biochemical composition, making it desirable from a patient perspective.
Multiple pilot studies have shown the utility of DOS in predicting early tumor response to neoadjuvant treatment.13, 14 These studies correlated changes in tumor optical properties with tumor final pathological response; thus, multiple time points were required to predict therapeutic response. There are inherent limitations in this approach because therapies are often multistage and the optimal timing of the post-treatment measurements is unknown. Furthermore, hormonal status and age introduce high interpatient variability in absorption spectra and might contribute additional functional changes independent of tumor response.15
Using a self-referencing differential spectroscopy (SRDS) method, which accounts for interpatient variability, metabolic differences were previously observed between malignant and normal tissues. These differences are assumed to be the result of subtle changes in molecular disposition (i.e., the location, concentration, and environment of molecular species of NIR absorbers in tissues).16 The SRDS method exploits the presence or absence of a unique endogenous spectral absorption fingerprint, called the specific tumor component (STC), which has been shown to separate malignant disease from benign tumors.17 The STCs are patient-specific signatures derived from scatter-corrected absorption spectra. In brief, the STC for each location over the tumor is calculated using the following documented formula:
| (1) |
where D(λ,x,y) is the difference spectrum calculated subtracting the average absorption in the tumor-free reference region from the absorption of the spatial tumor point being considered. Δi(x,y) is the difference of the fractional contribution of each tissue main absorber (lipid, water, oxy-hemoglobin, deoxy-hemoglobin) present in the spectra of the reference and the tumor point. are the known tissue absorber spectra. The STC is then the residue from the fit of the difference spectra to the tissue main absorbers.16 We observed that tumor spectra can vary spatially within the same lesion and hypothesize that STCs may contain specific information on tumor heterogeneity. NAC response is potentially related to tumor cellular and structural heterogeneity.11 These heterogeneous domains could be clustered by histological type or more homogeneously distributed throughout the tumor. Because heterogeneous clustering likely reduces therapeutic efficacy, our goal was to determine whether pretherapeutic spatial variations of STCs could be correlated with NAC response.
We first introduce an algorithm for calculating the tumor spatial heterogeneity spectrum (HS). Then using the HS, a heterogeneity index (HI) is developed to quantify the amount of heterogeneity. Furthermore, the HI is correlated with NAC pathologic response in 15 patients. This suggests that noninvasive scatter-corrected absorption spectra could be used to predict neoadjuvant chemotherapy response in patients prior to therapy.
Absorption spectra were obtained at 1-cm-spaced locations on grids over both tumor-containing tissue and normal tissue using a broadband DOS instrument.18 The amount of points in a grid varied (from 6 to 15), depending on tumor dimension that was previously localized by palpation and/or ultrasound. The lesion-free region in the contralateral breast served as the patient-specific reference for the calculation of the STCs.16
Tissue absorption and scattering spectra were calculated using custom software in MATLAB™. The resulting absorption spectra were analyzed by in-house Elantest software (Laboratory for Fluorescence Dynamics—University of California, Irvine) to calculate the STC spectra. Custom software for the calculation of the HS and HI was developed using MATLAB.
First, we considered the STC spectra in the locations within the lesion for each patient.16 We hypothesized that the variations of the lesion STC spectra are due to tumor heterogeneity.
In order to mathematically characterize tumor spatial heterogeneity for each patient using the normalized STCs spectra, we defined the HS as follows:
| (2) |
where λ are the different wavelengths used during the measurements, i is the location within the lesion, Ni is the number of spatial points sampled within the lesion and Nλ is the number of wavelengths.
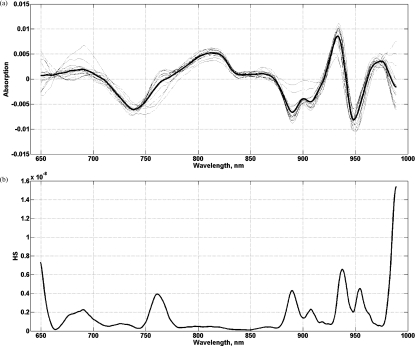
The HS, computed per subject, represents a biomarker obtained from variations of the STC spectra at each wavelength (Fig. 1). The HS provides information on spatial absorption variations, likely resulting from regional differences in lipid, hemoglobin, and water disposition. Specific wavelength domains contain information on the lesion heterogeneity in that particular range. For instance, it has been shown that the water absorption peak around 975 nm undergoes redshifting and broadening in vivo due to macromolecular binding.19 The heterogeneity in the absorption spectra may provide additional insight regarding tissue pathophysiology. Further investigation of biochemical/histological origins of the heterogeneity spectra will be the subject of future work.
Figure 1.
(a) STC spectra within the lesion, 2 × 3 cm grid containing 12 points and (b) HS of the lesion computed from normalized (a). HS represents variations of the STCs spectra at each wavelength.
Hypothesizing that spatial STCs variations are associated with heterogeneous lesions, the amplitude of the HS would be correlated with treatment effectiveness. In other words, a highly variable HS would suggest that the subject will respond poorly to NAC. To verify this assumption, we investigated 15 patients, ranging in age from 31 to 71 years, seven pre- and eight postmenopausal, with pathologically confirmed diagnosis of invasive ductal carcinoma (IDC) (ranging from 1.3 to 9.8 cm) (Table 1). Only IDC tumors were included in the study for consistency of the data set and the ability to compare absorption signatures from different patients. We performed pretreatment absorption and scattering measurements. Final treatment pathological response was determined from standard pathology. Pathological complete response was defined as the absence of invasive cancer, and non-pCR was defined as residual invasive tumor of any size, regardless of the presence of ductal carcinoma in situ. Among the 15 patients, six were pCR and nine were non-pCR (Table 1). Data were acquired in compliance with an institutionally approved human subjects research protocol [University of California, Irvine (UCI) 02–2306].
Table 1.
Patients diagnosed with IDC and undergoing NAC considered in the study. AC = doxorubicin + cyclophosphamide, CarbPac = carboplatin + paclitaxel, Her = trastuzumab, Bev = bevacizumab.
| Patient No. | Age | Menopausal status | Lesion size (cm) | Tumor depth (cm) | NAC Response | NAC Treatment |
|---|---|---|---|---|---|---|
| 1 | 57 | POST | 2.7 | NA | pCR | AC-CarbPac+Her |
| 2 | 45 | PRE | 1.6 | 0.72 | pCR | AC-CarbPac+Her |
| 3 | 38 | PRE | 1.3 | NA | pCR | AC-CarbPac |
| 4 | 56 | POST | 2.7 | 0.75 | pCR | AC-CarbPac+Her |
| 5 | 45 | POST | 1.6 | NA | pCR | CarbPac+Bev |
| 6 | 38 | PRE | 3 | NA | pCR | CarbPac+Her |
| 7 | 45 | PRE | 1.3 | NA | non-pCR | AC-CarbPac |
| 8 | 51 | POST | 6 | 0.85 | non-pCR | AC-CarbPac+Bev |
| 9 | 33 | PRE | 2.3 | 0.99 | non-pCR | AC-CarbPac+Bev+Her |
| 10 | 61 | POST | 3.3 | NA | non-pCR | AC-CarbPac |
| 11 | 58 | POST | 1.4 | NA | non-pCR | AC |
| 12 | 55 | POST | 1.8 | NA | non-pCR | AC-CarbPac+Her |
| 13 | 43 | PRE | 4 | 0.67 | non-pCR | AC-CarbPac |
| 14 | 71 | POST | 3.4 | 0.36 | non-pCR | CarbPac+Her |
| 15 | 31 | PRE | 9.8 | 1.5 | non-pCR | CarbPac+Bev |
A weighted wavelength analysis method was used to exploit the HS to discriminate between pCR and non-pCR. The algorithm separates two types of spectra by using the distance of a given spectrum from the average spectrum of each type. To account for spectral differences across the full wavelength region, the distance is calculated at each wavelength point at which data were obtained (i.e., every half nanometer in the 650–1000 nm range). Furthermore, to maximize the differences, wavelength regions are weighted. Weighting factors for HS of non-pCR (ωNR) and HS of pCR (ωpCR) are determined by an iterative process that calculates what combination of values for each wavelength region would best separate the coordinates of the pCR (DpCR) from the non-pCR (DNR) patients. For every patient (or HS), the “similarity” between the HS and the average HS spectrum of a pCR (HSpCR) and a non-pCR (HSNR) lesion was calculated and translated into the HI, which quantifies the amount of heterogeneities in the HS. The HI ranges from −100 to 100, where negative and positive values would describe a homogeneous and heterogeneous lesion, respectively,
| (3) |
where is the average distance of the considered HS from the average HS of all the pCR and is the average distance of the considered HS from the average HS of all the non-pCR (the factor of 2 is used to convert it from ±50) and are calculated as follows:
| (4) |
With nNR and npCR being the normalization factors in order to have the shape of the spectra as the main source of difference, not the amplitude, k being the number of wavelength points and Δp being the wavelength points in the weighed region.
The 15 patients (six pCR and nine non-pCR) were used to optimize the weights for building the HI. The average HI values obtained for pCR and non-pCR tumors were –44 ± 12 and 43 ± 17 (p = 3 × 10−8), respectively. As observed in Fig. 2, the HI values for all patients clearly separate the two groups. The HI was positive for non-pCR tumors and negative for pCR tumors. A negative HI would describe a homogeneous lesion. The results confirm the hypothesis that spatially varying lesions, with a positive HI, result in a partially or non-effective therapy.
Figure 2.
Distribution of the heterogeneity index. The pCR patients are clearly separated from the non-pCR patients.
The results were not affected by the number of points chosen for the calculation of the HS. Further analyses were performed using a fixed number of spatial points for all the patients (using six as a maximum due to the size of the smallest tumor) and led to the same separation. The addition of spatial points in the HS for tumors bigger than six spatial locations did not alter the HS shape; therefore, we could conclude that tumor size did not affect HI. Information on tumor depth was not available for all the patients, but it would be of interest to investigate any dependence between tumor depth and HI.
The data set was tested using a round-robin analysis to determine the dependence of the classification of the patients’ HS on the particular group. Each patient was systematically removed from the set, and for each of the reduced sets, the weighting factors were optimized. The HI of the omitted patient was calculated according to the new weights as a test patient. We found that the HI changed slightly for each patient. However, the classification remained unchanged from the round-robin analysis.
We were able to obtain two additional subjects, both non-pCR, whose measurements have been used to further test the classification algorithm. The lesions were correctly classified with an HI of 33 for one subject and 40 for the other subject.
In conclusion, we have introduced a method for visualizing and analyzing the spatial heterogeneity of noninvasive, scatter-corrected tumor absorption spectra based on SRDS. We present an application of the method that, for the first time, relates tumor spatial heterogeneity to the pathological response of neoadjuvant chemotherapy. Preliminary results in 17 patients reveal that a spatial heterogeneity index (HI), calculated from the HS, can separate pCR from non-pCR patients. These results show that the SRDS technique is a promising approach for potentially predicting NAC outcome prior to therapy.
Acknowledgments
This research was supported by the National Institutes of Health under Grants No. P41-RR01192 (Laser Microbeam and Medical Program: LAMMP) and No. PHS 5 P41- RR03155 (Laboratory for Fluorescence Dynamics), No. U54-CA136400 (Network for Translational Research), No. R01-CA142989, No. P30-CA62203 (UC Irvine Cancer Center Support Grant), and the Beckman Foundation. Finally, the authors also thank Montana Compton, Amanda F. Durkin, Laura Estrada, Darren Roblyer, Waya W. Tanamai, and Shigeto Ueda for their assistance, as well as the patients who generously volunteered their time for the study.
References
- Fangberget A., Nilsen L. B., Hole K. H., Holmen M. M., Engebraaten O., Naume B., Smith H.-J., Olsen D. R., and Seierstad T., “Neoadjuvant chemotherapy in breast cancer-response evaluation and prediction of response to treatment using dynamic contrast-enhanced and diffusion-weighted MR imaging,” Eur. Radiol. 21(6), 1188–1199 (2011). 10.1007/s00330-010-2020-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman H., Gunasekara A., Rycroft M., Zubovits J., Dent R., Spayne J., Yaffe M. J., and Czarnota1 G. J., “Functional imaging using diffuse optical spectroscopy of neoadjuvant chemotherapy response in women with locally advanced breast cancer,” Clin. Cancer Res. 16, 2605–2614, (April 2010). 10.1158/1078-0432.CCR-09-1510 [DOI] [PubMed] [Google Scholar]
- Fisher B., Bryant J., Wolmark N., Mamounas E., Brown A., Fisher E. R., Wickerham D. L., Begovic M., DeCillis A., Robidoux A., Margolese R. G., A. B.CruzJr., Hoehn J. L., Lees A. W., Dimitrov N. V., and Bear H. D., “Effect of preoperative chemotherapy on the outcome of women with operable breast cancer,” J. Clin. Oncol. 16, 2672–2685 (1998). 10.1016/S0959-8049(98)80142-1 [DOI] [PubMed] [Google Scholar]
- Wolmark N., Wang J., Mamounas E., Bryant J., and Fisher B., “Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18,” J. Natl. Inst. Monogr. 30, 96–102 (2001). 10.1053/sonc.2001.26151 [DOI] [PubMed] [Google Scholar]
- van der Hage J. A., van de Velde C. J., Julien J. P., Tubiana-Hulin M., Vandervelden C., and Duchateau L., “Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer Trial,” J. Clin. Oncol. 19, 4224–4237 (2001). 10.1016/S0959-8049(01)00294-5 [DOI] [PubMed] [Google Scholar]
- Symmans W. F., Peintinger F., Hatzis C., Rajan R., Kuerer H., Valero V., Assad L., Poniecka A., Hennessy B., Gree M., Buzdar A. U., Singletary S. E., Hortobagyi G. N., and Pusztai L., “Measurements of residual breast cancer burden to predict survival after neoadjuvant chemotherapy,” J. Clin. Oncol. 25, 4414–4422 (2007). 10.1200/JCO.2007.10.6823 [DOI] [PubMed] [Google Scholar]
- Rastogi P., Anderson S. J., Bear H. D., Geyer C. E., Kahlenberg M. S., Robidoux A., Margolese R. G., Hoehn J. L., Vogel V. G., Dakhil S. R., Tamkus D., King K. M., Pajon E. R., Wright M. J., Robert J., Paik S., Mamounas E. P., and Wolmark N., “Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project protocols B-18 and B-27,” J Clin. Oncol. 26, 778–785 (2008). 10.1200/JCO.2007.15.0235 [DOI] [PubMed] [Google Scholar]
- Londer V., Bazzocchi M., Del Frate C., Puglisi F., Di Loreto C., Francescutti G., and Zuiani C., “Locally advanced breast cancer comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy,” Eur. Radiol. 14, 1371–1379 (2004). [DOI] [PubMed] [Google Scholar]
- Yeh E., Slanetz P., Kopans D. B., Rafferty E., Georgian-Smith D., Moy L., Halpern E., Moore R., Kuter I., and Taghian A., “Prospective comparison of mammography, sonography and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer,” Am. J. Roentgenol. 184, 868–877 (2005). [DOI] [PubMed] [Google Scholar]
- Grimsby G. M., Gra R., Amylou D., Carpenter S., Stucky C., Aspey H., Giurescu M. E., and Pockaj B., “Is there concordance of invasive breast cancer pathologic tumor size with magnetic resonance imaging?” Am. J. Surg. 198, 500–504 (2009). 10.1016/j.amjsurg.2009.07.012 [DOI] [PubMed] [Google Scholar]
- Horii R., Akiyama F., Ito Y., Matsuura M., Miki Y., and Iwase T., “Histological features of breast cancer, highly sensitive to chemotherapy,” Breast Cancer 14, 393–400 (2007). 10.2325/jbcs.14.393 [DOI] [PubMed] [Google Scholar]
- Cerussi A., Shah N., Hsiang D., Durkin A., Butler J., and Tromberg B. J., “In vivo absorption, scattering, and physiologic properties of 58 malignant breast tumors determined by broadband diffuse optical spectroscopy,” J. Biomed. Opt. 11, 044005 (2006). 10.1117/1.2337546 [DOI] [PubMed] [Google Scholar]
- Cerussi A., Hsiang D., Shah N., Mehta R., Durkin A., Butler J., and Tromberg B. J “Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy,” Proc. Nat. Acad. Sci. USA 104, 4014–4019 (2007). 10.1073/pnas.0611058104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromberg B. J., Cerussi A., Shah N., Compton M., Fedyk A., Hsiang D., Butler J., and Mehta R., “Diffuse optics in breast cancer: detecting tumors in pre-menopausal women and monitoring neoadjuvant chemotherapy,” Breast Cancer Res. 7, 279–285 (2005). 10.1186/bcr1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N., Cerussi A., Eker C., Espinoza J., Butler J., Fishkin J., Hornung R., and Tromberg B. J., “Noninvasive functional optical spectroscopy of human breast tissue,” Proc. Nat. Acad. Sci. U S A 98(8), 4420–4425 (2001). 10.1073/pnas.071511098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukreti S., Cerussi A., Tromberg B. J., and Gratton E., “Intrinsic tumor biomarkers revealed by novel double-differential spectroscopic analysis of near-infrared spectra,” J. Biomed. Opt. 12. 020509 (2007). 10.1117/1.2709701 [DOI] [PubMed] [Google Scholar]
- Kukreti S., Cerussi A. E., Tanamai W., Hsiang D., Tromberg B. J., and Gratton E., “Characterization of metabolic differences between benign and malignant tumors: high-spectral-resolution diffuse optical spectroscopy,” Radiology 254(1), 277–284 (2010). 10.1148/radiol.09082134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerussi A. E., Tanamai V. W., Mehta R. S., Hsiang D., Butler J., and Tromberg B. J., “Frequent optical imaging during breast cancer neoadjuvant chemotherapy reveals dynamic tumor physiology in an individual patient,” Acad. Radiol. 17, 1031–1039 (2010). 10.1016/j.acra.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. H., Cerussi A. E., Klifa C., Baek H. M., Birgul O., Gulsen G., Merritt S. I., Hsiang D., and Tromberg B. J., “In vivo water state measurements in breast cancer using broadband diffuse optical spectroscopy,” Phys. Med. Biol. 53, 6713–6727 (2008). 10.1088/0031-9155/53/23/005 [DOI] [PMC free article] [PubMed] [Google Scholar]