Abstract
Introduction:
The purpose of this study was to investigate the use of lung age to motivate a quit attempt among smokers presenting to a hospital pulmonary function testing (PFT) laboratory.
Methods:
Participants were randomized to receive a lung age-based motivational strategy (intervention group) versus standard care (control group). At 1 month, all participants were interviewed by telephone to determine whether they made a quit attempt.
Results:
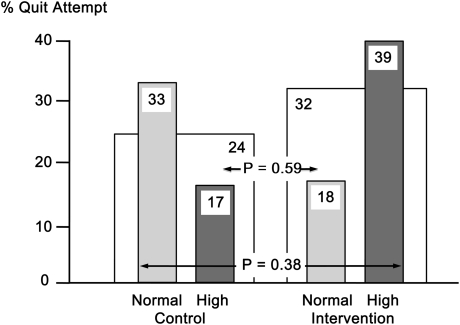
A total of 67 participants were enrolled, and 51 completed the study. Baseline mean data included age = 52 years, 70% women, 40 pack-years of smoking, FEV1 = 69% predicted, and lung age = 83 years. The quit attempt rates were not different between the intervention and control groups (32% vs. 24%, respectively, p = .59). There was a near significant interaction between lung age and intervention strategy (p = .089), with quit attempt rates among those with normal lung age of 18% in the intervention group versus 33% in the control group and among those with high (worse) lung age of 39% in the intervention group versus 17% in the control group; p = .38.
Conclusions:
Using lung age to motivate smokers presenting to the PFT laboratory to quit may succeed in patients with high lung age but may undermine motivation in smokers with normal lung age. Further work is needed to refine the approach to smokers with normal lung age.
Introduction
One strategy to motivate smokers to quit is to assess whether they have abnormal lung function, which is a strong independent risk factor for chronic obstructive pulmonary disease (COPD), lung cancer, cardiovascular disease, stroke, and all-cause mortality (Mannino, Aguayo, Petty, & Redd, 2003; Mannino, Gagnon, Petty, & Lydick, 2000; Wasswa-Kintu, Gan, Man, Pare, & Sin, 2005; Young, Hopkins, & Eaton, 2007). However, this approach has had mixed success in motivating smokers to quit (Bize, Burnand, Mueller, Rege, & Cornuz, 2009; Bohadana, Nilsson, & Martinet, 2005; Boushey, Enright, & Samet, 2005; Enright & Crapo, 2000; Ferguson, Enright, Buist, & Higgins, 2000; Kotz, Huibers, West, Wesseling, & van Schayck, 2009; Mannino, 2006; McClure, Ludman, Grothaus, Pabiniak, & Richards, 2009; Wilt, Niewoehner, & Kim, 2005; Young, Hopkins, Smith, & Hogarth, 2010), which we believe is mainly due to problems with communicating lung function results to smokers (Enright & Kaminsky, 2003; Petty & Enright, 2003). One way to discuss abnormal lung function results with patients is to use the lung age concept (Morris & Temple, 1985), which relates a person’s current lung function to the age at which his/her lung function would be considered normal. Thus, an elevated lung age signifies poor lung function as if the lungs have aged beyond the patient’s chronological age. Recently, Parkes, Greenhalgh, Griffin, and Dent (2008) demonstrated that using lung age to communicate lung function to smokers in the primary care setting enhances smoking abstinence at one year. Importantly, these authors were not able to demonstrate any negative impact of normal lung age; that is, that finding that lung age was normal would result in less motivation to quit. However, this has been a concern (Kotz, Wesseling, Aveyard, & van Schayck, 2010; McClure et al., 2009; Quanjer & Enright, 2010). Since measuring lung function by simple spirometry is underutilized in primary care (Ferguson et al., 2000; Kaminsky, Marcy, Bachand, & Irvin, 2005), we conducted a pilot study to determine the impact of using the lung age concept on quit attempts by current smokers presenting, instead, to the hospital pulmonary function testing (PFT) laboratory.
Methods
Eligible participants were current smokers referred to the PFT laboratory by their physician. The PFT technologist invited them to participate in the trial, which was explained as a study of the smoking habits of patients having PFTs. The true nature of the study, to determine the effects of the intervention on quit attempt rate, was not revealed at that time. The University of Vermont Institutional Review Board approved the study, and all patients agreeing to participate provided written informed consent.
All subjects completed a brief questionnaire to obtain demographic data and information about symptoms, daily use of cigarettes, nicotine addiction based on the time-to-first-cigarette scoring component of the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), and motivation to quit smoking (Solomon, Scharoun, Flynn, Secker-Walker, & Sepinwall, 2000; Solomon et al., 2005). Participants were then randomized to the control or intervention group. If assigned to the control group, the PFT technologist completed the lung function testing according to American Thoracic Society/European Respiratory Society quality criteria (Miller, Hankinson, & Brusasco, 2005) and then gave the participant an information sheet on smoking cessation resources in the community as recommended by current guidelines (Fiore, Bailey, & Cohen, 2000). This process lasted less than 1 min. If assigned to the intervention group, the technologist completed the lung function testing and then helped the participant find his/her lung age on a graph drawn according to Fletcher and Peto (1977). Based on whether lung age was normal (forced expiratory volume in 1 s, FEV1 ≥ 80% predicted; Hankinson, Odencrantz, & Fedan, 1999; which defined a normal lung age) or abnormal (FEV1 < 80% predicted, which defined a high lung age), the PFT technologist then followed a standardized script to share lung function results with participants in order to enhance their motivation to quit. This process involved motivational interviewing (Lai, Cahill, Qin, & Tang, 2010) using lung age to educate the patient about the dangers of smoking, elicit feedback about motivation to quit and barriers to quitting, and provide information on strategies and resources to help quit smoking. The PFT technologist also gave participants in the intervention group the same information sheet that they gave subjects in the control group. The entire intervention took approximately 15 min. Following discharge from the PFT laboratory, we also sent participants in the intervention group a letter signed by the physician investigator reminding them of their lung function results, emphasizing the link between smoking and disease, and providing them with local toll-free Quit Line telephone numbers.
All participants were called 1 month after their initial PFT laboratory visit. At this time, a trained interviewer who had no prior contact with the participants and was unaware of their group assignment asked the same questions initially asked during the day of the PFT laboratory visit. The question, “Since your breathing test 1 month ago, have you made any attempt to quit smoking that lasted 24 hours or longer?” provided data for the primary outcome of the study, quit attempt rate at 1 month (Carpenter & Hughes, 2005). If any quit attempt was made, we also asked for the number of such attempts. The true nature of the study was then disclosed at the end of the interview.
Data Analysis
The primary outcome measure was the incidence of one or more quit attempts during the 1 month after the PFT test and was determined on all participants randomized (intention-to-treat), with participants unable to be contacted for follow-up assumed to be continued smokers. Secondary outcomes included daily use of cigarettes, abstinence rates, motivation to quit, and nicotine dependence. We stratified data based on lung function (high vs. normal lung age). We used mixed model analysis of variance or chi-square analysis and Fisher’s exact test to compare outcomes before and after the intervention and between groups. We used multivariate logistic regression to determine independent risk factors associated with quit attempts. p values < .05 were considered statistically significant.
Results
A total of 67 participants were enrolled, and 51 completed the study. Among those not completing the study, 13 were unable to be contacted by telephone despite repeated attempts, 2 refused further participation, and 1 died. Most patients were referred to the PFT laboratory for shortness of breath (n = 32, 48%), an abnormal chest x-ray (n = 10, 15%), cough (n = 8, 12%), or preoperative evaluation (n = 4, 6%). The demographic characteristics and baseline features regarding smoking habits, nicotine dependence, and motivation to quit were not different between the 51 participants who completed the study and the 67 who initially enrolled (data not shown).
Participants were evenly distributed into the control (n = 33) and intervention (n = 34) groups with respect to age, sex, education, nicotine addiction, and motivation to quit (Table 1). In general, participants were highly addicted to nicotine but wanted to quit smoking. The incidence of one or more quit attempts at 1 month was n = 8 (24%) control versus n = 11 (32%) intervention, p = .59, Figure 1, with patients who made a quit attempt averaging 2.3 such attempts during that period. When broken down by whether participants had normal or high lung age (Figure 1), those with a high lung age had a higher incidence of quit attempts if assigned to the intervention group (n = 9, 39%) than if assigned to the control group (n = 3, 17%). Those participants with a normal lung age had a higher incidence of quit attempts if assigned to the control group (n = 5, 33%) than if assigned to the intervention group (n = 2, 18%). None of these differences were statistically significant (p = .38), but there was a trend toward significant association between quit attempts and the interaction of group assignment and lung age (odds ratio [OR] = 11.2, p = .089). The incidence of abstinence at 1 month was small and did not differ between groups (n = 0, 0% control vs. n = 3, 8.8% intervention, p = .24). The only factor that was associated with making a quit attempt was less nicotine dependence (OR = 5.4, 95% CI = 1–29, p = .049). There were significant differences in baseline cigarette consumption between the control-normal lung age and control-high lung age groups (p < .01) and the control-normal lung age and intervention-high lung age groups (p = .02). Compared with baseline levels, cigarette consumption following the intervention was significantly lower in the control-high lung age group (p < .01) and nearly significantly lower in the intervention-high lung age group (p = .08). There were no statistically significant differences between groups at baseline or postintervention or within groups pre- and postintervention in nicotine dependence or motivation to quit.
Table 1.
Demographic Characteristics and Lung Age of Study Participants
| Characteristic | Control-normal lung age | Control-high lung age | Intervention-normal lung age | Intervention- high lung age | p value |
| N | 15 | 18 | 11 | 23 | |
| Age (years) | 49.8 | 57.5 | 49.0 | 51.3 | .15 |
| Sex (% male) | 40 | 11 | 45 | 30 | .18 |
| Cigarettes per day | |||||
| Preintervention | 13.9 | 26.2 | 18.9 | 22.9 | <.01 |
| Postintervention | 14.0 | 17.5a | 16.8 | 15.4a | .77 |
| Pack-years | 23.9 | 54.6 | 32.4 | 42.3 | <.01 |
| Age started smoking (years) | 17.5 | 16.4 | 15.7 | 14.2 | .08 |
| FEV1b (L, % predicted) | 3.0 (94) | 1.5 (60) | 2.6 (88) | 1.5 (52) | <.01 |
| Lung age (years) | 45.2 | 87.7 | 61.9 | 90.3 | <.01 |
| COPDc (% present) | 53 | 72 | 64 | 87 | .14 |
| Time to first cigarette (% <30 min) | 67 | 72 | 91 | 87 | .32 |
| How much want to stop (1 = none to 4 = a lot) | 3.7 | 4.0 | 3.5 | 3.5 | .32 |
| How much want to continue (1 = none to 4 = a lot) | 2.1 | 2.6 | 2.4 | 2.4 | .80 |
| Intend to quit in next 30 days? (1 = no to 5 = definitely) | 3.5 | 2.9 | 2.9 | 3.0 | .51 |
| Number of smokers in household besides participant | 0.9 | 0.8 | 0.7 | 0.8 | .94 |
| Education (% less than high school) | 27 | 28 | 63 | 48 | .16 |
Note. aSignificantly different than value preintervention.
FEV1 = forced expiratory volume in 1 s.
COPD = chronic obstructive pulmonary disease.
Figure 1.
Quit attempt rate per group at 1 month, shown for control versus intervention (open bars) as well as breakdown by lung age (normal or high, shaded bars). p value at top is for difference between control and intervention groups and p value at bottom is for difference across lung age categories among the control and intervention groups.
Discussion
The main finding from this study is that using the lung age concept through a motivational interviewing approach applied in the PFT laboratory may increase quit attempts among current smokers with high lung age but may reduce quit attempts rate among smokers with normal lung function.
Measuring lung function by spirometry to motivate smoking cessation has been a controversial topic because of conflicting data (Bize et al., 2009; Bohadana et al., 2005; Boushey et al., 2005; Enright & Crapo, 2000; Ferguson et al., 2000; Kotz et al., 2009; Mannino, 2006; McClure et al., 2009; Wilt et al., 2005; Young et al., 2010). Low FEV1 is an independent risk factor for death from COPD and all causes as well as for lung cancer, cardiovascular disease, and stroke (Mannino et al., 2000; Young et al., 2007). Thus, telling a smoker that he/she has low lung function might be thought to motivate smoking cessation (Young et al., 2010) since quitting smoking can improve outcomes (Anthonisen, Skeans, & Wise, 2005). However, a recent Cochrane review (Bize et al., 2009) found little evidence to support this approach other than a recently reported study of using lung age derived from spirometry. In that study, Parkes et al. (2008) demonstrated that revealing a smoker’s lung age obtained during screening spirometry in primary care settings led to increased smoking cessation.
Our study did not find a statistically significant effect of communicating lung age; however, our study differed from that by Parkes et al. because we tested smokers referred to a hospital PFT laboratory, not general smokers in the community. Indeed, we had more smokers with a higher mean burden of cigarette use (21 vs. 17 cigarettes/day) and lower mean lung function (74% vs. 90% predicted). Interestingly, these factors might have influenced motivation to quit in opposite ways, the former associated with a higher degree of nicotine dependence and hence less likelihood of quitting and the latter associated with a greater likelihood of quitting (Gorecka et al., 2003). Our data show that patients with higher cigarette consumption but lower lung function were able to significantly reduce their consumption after the visit to the PFT laboratory, suggesting that smokers with impaired lung function may respond better to smoking cessation interventions (Bednarek et al., 2006; Gorecka et al., 2003; McClure et al., 2009). Our study also differed from that of Parkes et al. because we examined quit attempts at 1 month, not abstinence rate at 1 year. Despite these differences, our data suggest an important interaction between lung age and the intervention received: The nearly significant interaction between group and lung age on the quit attempt rate (p = .089) supports the finding of Parkes et al. that knowledge of poor lung function (high lung age) motivates smokers to quit (Parkes et al., 2008).
However, our data also suggest that smokers with normal lung age were less likely to make a quit attempt if they were informed of their normal lung function. This has been a concern with using spirometry to motivate smoking cessation (Kotz et al., 2010; McClure et al., 2009; Quanjer & Enright, 2010). Recently, McClure et al. (2009) documented such an effect, although it appeared to be short lived. Parkes et al. (2008) did not show this effect. We speculate that since the participants in our study were all patients referred for PFTs due to some concern about their breathing, the finding of normal lung function results may have led to relief of anxiety and less motivation to quit. This effect may not have occurred in smokers invited to participate in the study by Parkes et al. because, by virtue of their accepting the invitation, they might have been more open to health promotion (Kotz et al., 2010; Parkes et al., 2008).
The current study provides support for using brief motivational interviewing (Britt, Hudson, & Blampied, 2004; Lai et al., 2010; Miller & Rollnick, 1991) in the setting of the PFT laboratory, which provides a unique opportunity to focus smoking cessation strategies on current smokers with potential lung problems. Coupling physiological feedback with motivational interviewing enhances the technique because a key component of this approach is to provide personalized feedback of risk to motivate subjects to change behavior (Borrelli et al., 2002). Motivational interviewing also allows a brief intervention that is suitable to the busy practice of a PFT laboratory (Colella & Laver, 2005; Lai et al., 2010). In addition, the process takes advantage of a “teachable moment” (McBride, Emmons, & Lipkus, 2003) and utilizes the PFT technologist as a credible (Hovland & Weiss, 1952) nonphysician (Fiore et al., 2008) source of information.
Our study suggests that we need to develop a better approach to motivate smokers with normal lung age to quit. For these subjects, we tried to emphasize the good fortune of having normal lung function that now was the time to quit before lung damage developed and that smoking affects many other organs besides the lungs. However, it appears that this approach was not adequate. Perhaps the message needs to be stronger (e.g., enhanced by visual images), delivered over a longer period of time, or repeated at regular intervals.
There are several limitations to our study. First, as a pilot project only, this study had a low sample size and limited power. However, the doubling of the quit attempt rate among smokers with high lung age in the intervention group (39%) compared with those in the control group (17%) suggests that with a greater sample size, this substantial difference may have become statistically significant. Measuring quit attempts at only 1 month provided a limited timeframe within which to assess the impact of the intervention, but it is likely that most of any motivational effect from revealing lung age would have occurred soon thereafter.
Second, our results apply only to the unique population of patients referred to our PFT laboratory. Subtle differences in the interaction between the PFT technologists and the smokers involved in the study may have influenced the results. In addition, we did not measure or control for physician tobacco use interventions that might have occurred at subsequent physician visits that followed testing.
Third, although we were interested in focusing on the effect of lung age per se on quit attempt rate, the intervention group received a more intensive intervention and a follow-up letter. While the participants were blinded as to the true nature of the intervention, the PFT technologists were not. We tried to maintain fidelity and reduce any cross contamination of the intervention during the study through periodic practice meetings with the PFT technologists, but we did not monitor the actual patient–technologist interactions.
Conclusions
We conclude that using the lung age concept through a motivational interviewing approach can be performed in a busy hospital PFT laboratory and may motivate smokers with a high lung age to make a quit attempt but may result in less motivation to quit among those with normal lung age. It is possible that the lung age concept should only be used in smokers with high lung age. Further work is needed to refine the approach to smokers with normal lung age in whom evidence of harm is not apparent.
Funding
This work was supported by National Institutes of Health, National Cancer Institute (R03 CA126417).
Declaration of Interests
None declared.
Acknowledgments
The authors would like to thank the PFT technologists who participated in this study: Amy Carpenter, Deborah Hunton, Karlinda King, Teresa LaRose, and Lisa Philips. We also thank Joan Skelly of the Biostatistics Unit of University of Vermont for help with planning this study, Greg Dana and Barbara Branch of the Office of Health Promotions Research at University of Vermont for help with conducting this study, and Maura Pierson for administrative support.
References
- Anthonisen N, Skeans M, Wise R, Manfeda J, Kanner R, Connett J, et al. The effects of smoking cessation intervention on 14.5-year mortality. Annals of Internal Medicine. 2005;142:233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. doi:10.1016/j.accreview.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Bednarek M, Gorecka D, Wielgomas J, Czajkowska-Malinowska M, Regula J, Mieszko-Filipczk G, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax. 2006;61:869–873. doi: 10.1136/thx.2006.059071. doi:10.1136/thx.2006.059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bize R, Burnand B, Mueller Y, Rege W, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database of Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD004705.pub3. CD004705.pub2. doi:10.1002/14651858.CD004705.pub3. [DOI] [PubMed] [Google Scholar]
- Bohadana A, Nilsson F, Martinet Y. Detecting airflow obstruction in smoking cessation trials. A rationale for routine spirometry. Chest. 2005;128:1252–1257. doi: 10.1378/chest.128.3.1252. doi:10.1378/chest.128.3.1252. [DOI] [PubMed] [Google Scholar]
- Borrelli B, McQuaid E, Becker B, Hammond K, Papandonatos G, Fritz G, et al. Motivating parents of kids with asthma to quit smoking: The PAQS project. Health Education Research. 2002;17:659–669. doi: 10.1093/her/17.5.659. doi:10.1093/her/17.5.659. [DOI] [PubMed] [Google Scholar]
- Boushey H, Enright P, Samet J. Spirometry for chronic obstructive pulmonary disease case finding in primary care? American Journal of Respiratory and Critical Care Medicine. 2005;172:1481–1482. doi: 10.1164/rccm.2509009. doi:10.1164/rccm.2509009. [DOI] [PubMed] [Google Scholar]
- Britt E, Hudson S, Blampied N. Motivational interviewing in health settings: A review. Patient Education and Counseling. 2004;53:147–155. doi: 10.1016/S0738-3991(03)00141-1. doi:10.1016/S0738-3991(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Hughes J. Defining quit attempts: What difference does a day make? Addiction. 2005;100:257–259. doi: 10.1111/j.1360-0443.2004.00952.x. doi:0.1111/j.1360-0443.2004.00952.x. [DOI] [PubMed] [Google Scholar]
- Colella C, Laver J. Setting the stage for changing health behavior. Nurse Practitioner. 2005;30:68–70. doi: 10.1097/00006205-200510000-00021. doi:10.1097/00006205-200510000-0021. [DOI] [PubMed] [Google Scholar]
- Enright P, Crapo R. Controversies in the use of spirometry for early recognition and diagnosis of chronic obstructive pulmonary disease in cigarette smokers. Clinics in Chest Medicine. 2000;21:645–652. doi: 10.1016/s0272-5231(05)70174-x. doi:10.1016/50272-5231(05)70174-X. [DOI] [PubMed] [Google Scholar]
- Enright P, Kaminsky D. Strategies for screening for chronic obstructive pulmonary disease. Respiratory Care. 2003;48:1194–1201. Retrieved from http://www.rcjournal.com. [PubMed] [Google Scholar]
- Ferguson G, Enright P, Buist A, Higgins M. Office spirometry for lung health assessment in adults. A consensus statement from the National Lung Health Education Program. Chest. 2000;117:1146–1161. doi: 10.1378/chest.117.4.1146. doi:10.1378/chest.117.4.1146. [DOI] [PubMed] [Google Scholar]
- Fiore M, Bailey W, Cohen S, Dorfman S, Goldstein M, Gritz E, et al. Treating tobacco use and dependence: Clinical practice guideline. Rockville, MD: Department of Health and Human Services, Public Health Service; 2000. [Google Scholar]
- Fiore M, Jaen C, Baker T, Bailey W, Benowitz N, Curry S. Treating tobacco use and dependence: 2008 update. 2008 Retrieved from www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hsahcpr&part=A28163. [Google Scholar]
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. British Medical Journal. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. doi:10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorecka D, Bednarek M, Nowinski A, Puscinska E, Goljan-Geremek A, Zielinski J. Diagnosis of airflow limitation combined with smoking cessation advice increases stop-smoking rate. Chest. 2003;123:1916–1923. doi: 10.1378/chest.123.6.1916. doi:10.1378/chest.123.6.1916. [DOI] [PubMed] [Google Scholar]
- Hankinson J, Odencrantz J, Fedan K. Spirometric reference values from a sample of the general US population. American Journal of Respiratory and Critical Care Medicine. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. Retrieved from http://www.atsjournals.org. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerstrom K. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi:10.1111/j.1360-0443.1991.tb01879.X. [DOI] [PubMed] [Google Scholar]
- Hovland C, Weiss W. The influence of source credibility on communication effectiveness. Public Opinion Quarterly. 1952;15:635–650. doi:10.1086/266350. [Google Scholar]
- Kaminsky D, Marcy T, Bachand M, Irvin C. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respiratory Care. 2005;50:1639–1648. doi:10.1016/j.rmedu.2006.03.016. [PubMed] [Google Scholar]
- Kotz D, Huibers M, West R, Wesseling G, van Schayck O. What mediates the effect of confrontational counselling on smoking cessation in smokers with COPD? Patient Education and Counseling. 2009;76:16–24. doi: 10.1016/j.pec.2008.11.017. doi:0.1016/j.pec.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Kotz D, Wesseling G, Aveyard P, van Schayck O. Smoking cessation and development of respiratory health in smokers screened with normal spirometry. Respiratory Medicine. 2010;105:243–249. doi: 10.1016/j.rmed.2010.07.010. doi:10.1016/j.rmed.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Lai D, Cahill K, Qin Y, Tang J. Motivational interviewing for smoking cessation. Cochrane Database of Systematic Reviews. 2010;1 doi: 10.1002/14651858.CD006936.pub2. CD006936. doi:10.1002/14651858.CD006936.pub2. [DOI] [PubMed] [Google Scholar]
- Mannino D. Spirometric screening: Does it work? Thorax. 2006;61:834–835. doi: 10.1136/thx.2006.061317. doi:10.1136/thx.2006.059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino D, Aguayo S, Petty T, Redd S. Low lung function and incident lung cancer in the Unites States. Data from the First National Health and Nutrition Examination Survey Follow-up. Archives of Internal Medicine. 2003;163:1475–1480. doi: 10.1001/archinte.163.12.1475. doi:10.1001/archinte.163.12.1475. [DOI] [PubMed] [Google Scholar]
- Mannino D, Gagnon R, Petty T, Lydick E. Obstructive lung disease and low lung function in adults in the Unites States. Archives of Internal Medicine. 2000;160:1683–1689. doi: 10.1001/archinte.160.11.1683. doi:10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- McBride C, Emmons K, Lipkus I. Understanding the potential of teachable moments: The case of smoking cessation. Health Education Research. 2003;18:156–170. doi: 10.1093/her/18.2.156. doi:10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- McClure J, Ludman E, Grothaus L, Pabiniak C, Richards J. Impact of spirometry feedback and brief motivational counseling on long-term smoking outcomes: A comparison of smokers with and without lung impairment. Patient Education and Counseling. 2010;80:280–283. doi: 10.1016/j.pec.2009.11.002. doi:10.1016/j.pec.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Hankinson J, Brusasco J, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. European Respiratory Journal. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. doi:10.1183109031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Miller W, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York, NY: Guilford Press; 1991. [Google Scholar]
- Morris J, Temple W. Spirometric “lung age” estimation for motivating smoking cessation. Preventive Medicine. 1985;14:655–662. doi: 10.1016/0091-7435(85)90085-4. doi:10.1016/0091-7435(85)90085-4. [DOI] [PubMed] [Google Scholar]
- Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: The step2quit randomized controlled trial. British Medical Journal. 2008;336:598–600. doi: 10.1136/bmj.39503.582396.25. doi:10.1136/bmj.39503.582396.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty T, Enright P. Simple office spirometry for primary care practitioners. Tarrytown, NY: Alpha Medica; 2003. [Google Scholar]
- Quanjer P, Enright P. Should we use ‘lung age’? Primary Care Respiratory Journal. 2010;19:197–199. doi: 10.4104/pcrj.2010.00045. doi:10.4104/pcrj.2010.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon L, Marcy T, Howe K, Skelly J, Reinier K, Flynn B. Does extended proactive telephone support increase smoking cessation among low-income women using nicotine patches? Preventive Medicine. 2005;40:306–313. doi: 10.1016/j.ypmed.2004.06.005. doi:10.1016/j.ypmed.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Solomon L, Scharoun G, Flynn B, Secker-Walker R, Sepinwall B. Free nicotine patches plus proactive telephone peer support to help low-income women stop smoking. Preventive Medicine. 2000;31:68–74. doi: 10.1006/pmed.2000.0683. doi:10.1006/pmed.2000.0683. [DOI] [PubMed] [Google Scholar]
- Wasswa-Kintu S, Gan W, Man S, Pare P, Sin D. Relationship between reduced forced expiratory volume in one second and risk of lung cancer: A systematic review and meta-analysis. Thorax. 2005;60:570–575. doi: 10.1136/thx.2004.037135. doi:10.1136/thx.2004.03713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilt T, Niewoehner D, Kim C-B., Kane R, Linabery A, Tacklind J, et al. Use of spirometry for case finding, diagnosis, and management of chronic obstructive pulmonary disease (COPD) 2005 doi: 10.1037/e439492005-001. Evidence Report/Technology Assessment No. 121. Agency for Healthcare Research and Quality, Publication No. 05-E017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Hopkins R, Eaton T. Forced expiratory volume in one second: Not just a lung function test but a marker of premature death from all causes. European Respiratory Journal. 2007;30:616–622. doi: 10.1183/09031936.00021707. doi:10.1183/09031936.0002170. [DOI] [PubMed] [Google Scholar]
- Young R, Hopkins R, Smith M, Hogarth D. Smoking cessation: The potential role of risk assessment tools as motivational triggers. Postgraduate Medicine. 2010;86:26–33. doi: 10.1136/pgmj.2009.084947. doi:10.1136/pgmj.2009.084947. [DOI] [PubMed] [Google Scholar]