Abstract
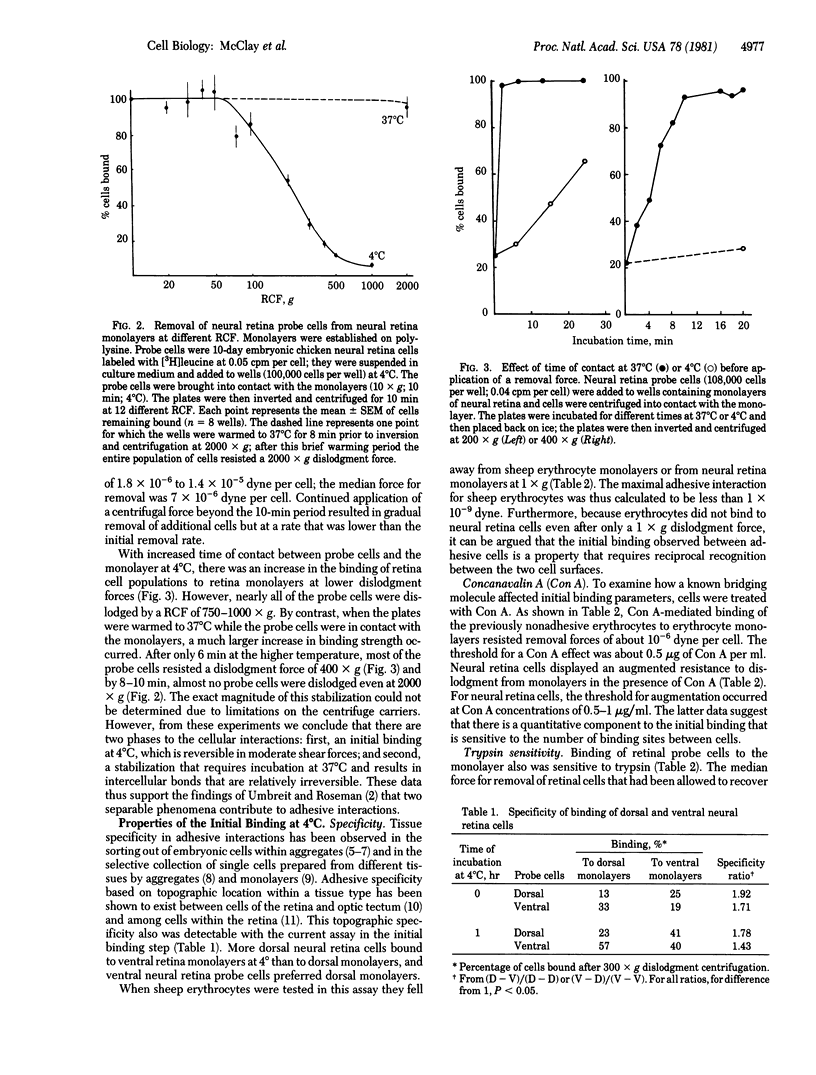
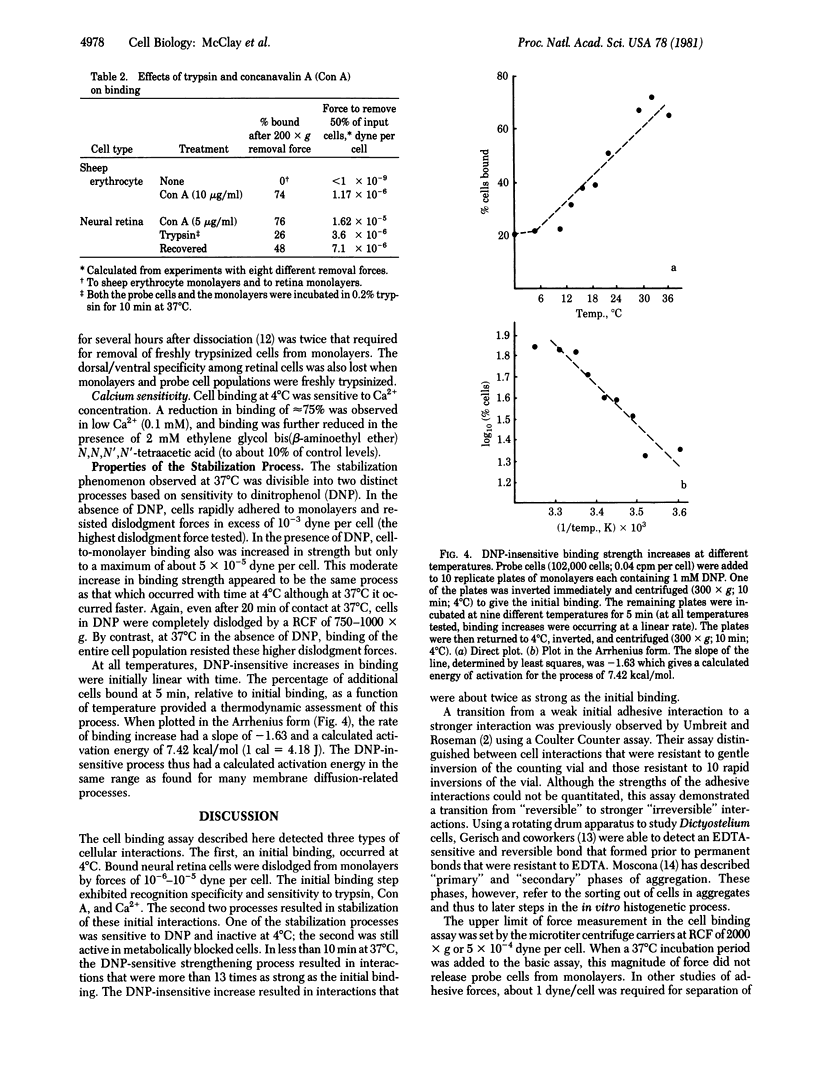
The hypothesis that intercellular adhesion can be subdivided into two separable phenomena--an initial recognition event and a subsequent stabilization--is supported by the use of a cell binding assay that provides a quantitative measure of intercellular binding strengths. Radioactive single cells are brought into contact with cell monolayers at 4 degrees C in sealed compartments. The compartments are inverted and a centrifugal force is then applied to dislodge the probe cells from the monolayers. By varying the speed of centrifugation, the force maintaining associations between embryonic chicken neural retina cells was determined to be on the order of 10(-5) dyne. Topographic specificities of single neural retina cells for retinal monolayers from various regions of the retina were detected with this assay and corresponded to those observed in more traditional assays at 37 degrees C. Also observed were two time- and temperature-dependent stabilization processes in which the force required for dislodgment increased. One of the stabilization processes was sensitive to dinitrophenol and was inactive at 4 degrees C; the second was still active in metabolically blocked cells. The metabolic-dependent process resulted in interactions at least 13 times as strong as the initial binding. The metabolic-independent process resulted in about a 2-fold increase in binding strength and had a temperature dependence similar to that of membrane diffusional phenomena.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbera A. J., Marchase R. B., Roth S. Adhesive recognition and retinotectal specificity. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2482–2486. doi: 10.1073/pnas.70.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Beug H., Gerisch G., Kempff S., Riedel V., Cremer G. Specific inhibition of cell contact formation in Dictyostelium by univalent antibodies. Exp Cell Res. 1970 Nov;63(1):147–158. doi: 10.1016/0014-4827(70)90343-5. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. I., Rock K., Glaser L. A gradient of adhesive specificity in developing avian retina. Proc Natl Acad Sci U S A. 1976 Feb;73(2):410–414. doi: 10.1073/pnas.73.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laris P. C., Pershadsingh H. A., Johnstone R. M. Monitoring membrane potentials in Ehrlich ascites tumor cells by means of a fluorescent dye. Biochim Biophys Acta. 1976 Jun 17;436(2):475–488. doi: 10.1016/0005-2736(76)90209-1. [DOI] [PubMed] [Google Scholar]
- MOSCONA A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961 Jan;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- Marchase R. B., Vosbeck K., Roth S. Intercellular adhesive specificity. Biochim Biophys Acta. 1976 Dec 14;457(3-4):385–416. doi: 10.1016/0304-4157(76)90005-8. [DOI] [PubMed] [Google Scholar]
- McCLAY D. R., Gooding L. R., Fransen M. E. A requirement for trypsin-sensitive cell-surface components for cell-cell interactions of embryonic neural retina cells. J Cell Biol. 1977 Oct;75(1):56–66. doi: 10.1083/jcb.75.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. THE DEVELOPMENT IN VITRO OF CHIMERIC AGGREGATES OF DISSOCIATED EMBRYONIC CHICK AND MOUSE CELLS. Proc Natl Acad Sci U S A. 1957 Jan 15;43(1):184–194. doi: 10.1073/pnas.43.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S. Studies on intercellular adhesive selectivity. Dev Biol. 1968 Dec;18(6):602–631. doi: 10.1016/0012-1606(68)90029-8. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. S. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool. 1970 Apr;173(4):395–433. doi: 10.1002/jez.1401730406. [DOI] [PubMed] [Google Scholar]
- Umbreit J., Roseman S. A requirement for reversible binding between aggregating embryonic cells before stable adhesion. J Biol Chem. 1975 Dec 25;250(24):9360–9368. [PubMed] [Google Scholar]
- WEISS L. The measurement of cell adhesion. Exp Cell Res. 1961;Suppl 8:141–153. doi: 10.1016/0014-4827(61)90345-7. [DOI] [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]


