Abstract
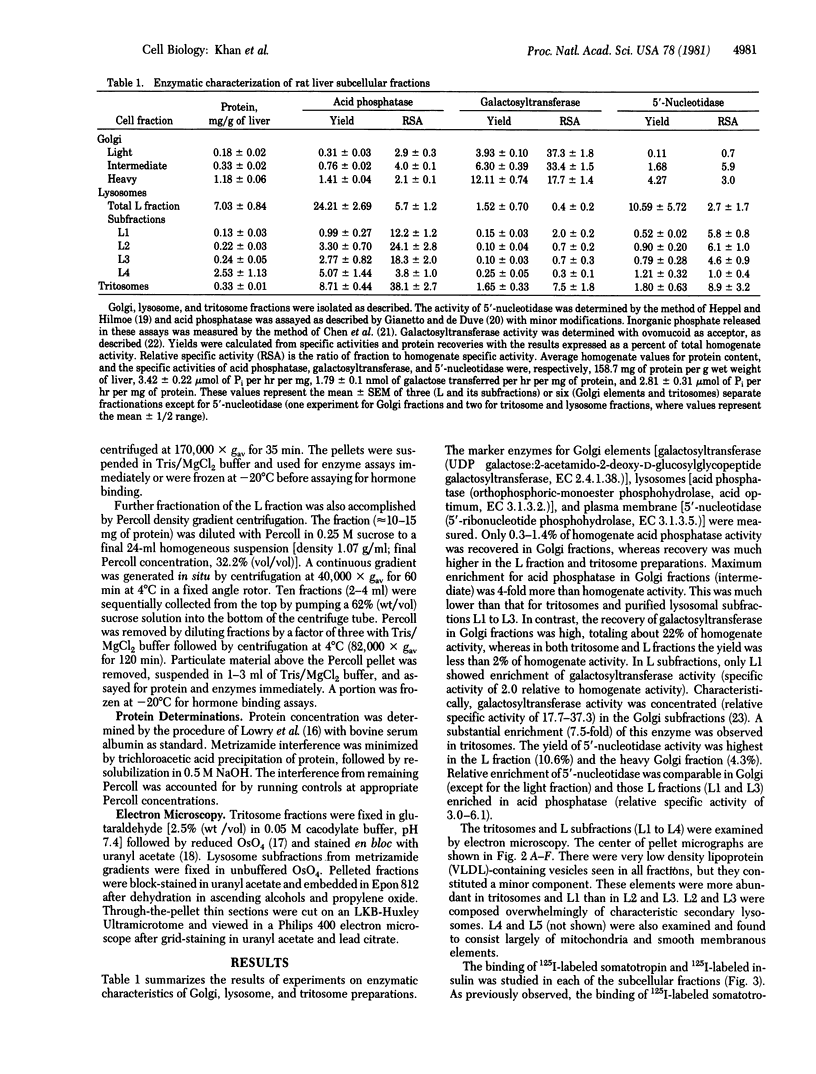
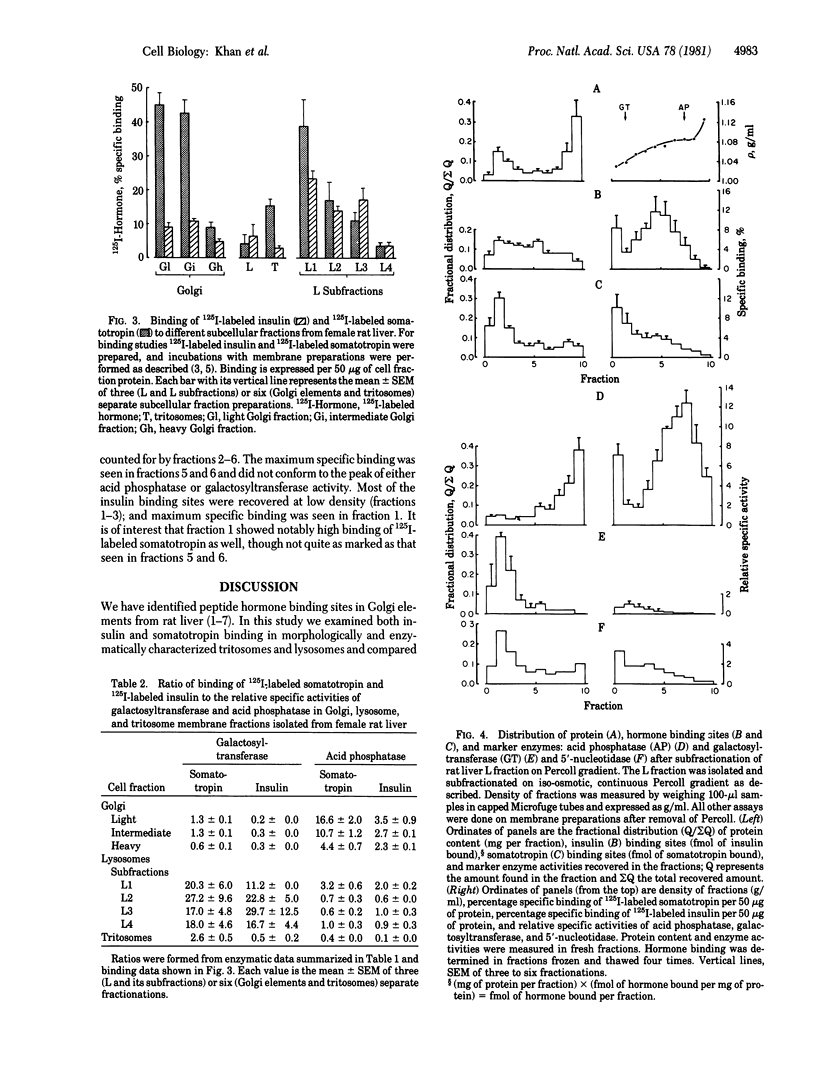
Previous studies have established the presence of polypeptide hormone receptors in Golgi fractions from rodent liver. In this study we attempted to identify peptide hormone receptors in other intracellular elements, particularly lysosomes. Tritosomes were prepared by a standard procedure, and highly purified secondary lysosomes were prepared by fractionating the L fraction of rat liver in a discontinuous metrizamide gradient into subfractions L1 to L4. Binding of 125I-labeled insulin and 125I-labeled somatotropin was studied with membranes prepared from osmotically shocked fractions. The L2 and L3 fractions, virtually devoid of galactosyltransferase (UDP galactose:2-acetamido-2-deoxy-D-glucosylglycopeptide galactosyltransferase, EC 2.4.1.38) but highly enriched in acid phosphatase [orthophosphoric-monoester phosphohydrolase (acid optimum), EC 3.1.3.2], appeared as classical secondary lysosomes by electron microscopy. When compared with Golgi fractions, the level of specific binding per 50 micrograms of protein of 125I-labeled somatotropin in L2 and L3 was 1/3, whereas that of 125I-labeled insulin was comparable. L1, which was reduced in acid phosphatase and increased in galactosyltransferase activities, showed higher hormone binding than did L2 and L3. This was not attributable to Golgi fraction contamination, as evident by specific binding/galactosyltransferase ratios. Binding to tritosome membranes could be largely accounted for by variable contamination with Golgi fractions as judged by specific binding/galactosyltransferase ratios. To clarify the distribution of receptor sites in lysosomal preparations, we fractionated the entire L fraction on a continuous Percoll gradient. Acid phosphatase and galactosyltransferase activities were segregated to the high and low density ranges of the gradient, respectively; however, the fractions enriched in hormone binding were of intermediate density, distinct from Golgi and lysosomal biochemical markers. We conclude that intracellular receptors are found not only in galactosyltransferase-containing very low density lipoprotein-marked Golgi vesicles but also in a unique vesicle of intermediate density between classical Golgi and lysosomal structures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergeron J. J., Evans W. H., Geschwind I. I. Insulin binding to rat liver Golgi fractions. J Cell Biol. 1973 Dec;59(3):771–776. doi: 10.1083/jcb.59.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Posner B. I., Josefsberg Z., Sikstrom R. Intracellular polypeptide hormone receptors. The demonstration of specific binding sites for insulin and human growth hormone in Golgi fractions isolated from the liver of female rats. J Biol Chem. 1978 Jun 10;253(11):4058–4066. [PubMed] [Google Scholar]
- Bergeron J. J., Sikstrom R., Hand A. R., Posner B. I. Binding and uptake of 125I-insulin into rat liver hepatocytes and endothelium. An in vivo radioautographic study. J Cell Biol. 1979 Feb;80(2):427–443. doi: 10.1083/jcb.80.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz R., Stäubli W. Detergent influence on rat-liver galactosyltransferase activities towards different acceptors. Eur J Biochem. 1977 Jul 1;77(1):181–192. doi: 10.1111/j.1432-1033.1977.tb11656.x. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Barazzone P., Freychet P., Le Cam A., Orci L. Intracellular localization of 125I-labeled insulin in hepatocytes from intact rat liver. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2803–2807. doi: 10.1073/pnas.76.6.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. Cell junctions in amphibian skin. J Cell Biol. 1965 Jul;26(1):263–291. doi: 10.1083/jcb.26.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Freychet P. O., Orci L. Internalization of polypeptide hormones: mechanism, intracellular localization and significance. Diabetologia. 1980 Apr;18(4):263–274. doi: 10.1007/BF00251003. [DOI] [PubMed] [Google Scholar]
- HEPPEL L. A., HILMORE R. J. Purification and properties of 5-nucleotidase. J Biol Chem. 1951 Feb;188(2):665–676. [PubMed] [Google Scholar]
- Josefsberg Z., Posner B. I., Patel B., Bergeron J. J. The uptake of prolactin into female rat liver. Concentration of intact hormone in the Golgi apparatus. J Biol Chem. 1979 Jan 10;254(1):209–214. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertoft H., Wärmegård B., Hök M. Heterogeneity of lysosomes originating from rat liver parenchymal cells. Metabolic relationship of subpopulations separated by density-gradient centrifugation. Biochem J. 1978 Jul 15;174(1):309–317. doi: 10.1042/bj1740309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner B. I., Bergeron J. J., Josefsberg Z., Khan M. N., Khan R. J., Patel B. A., Sikstrom R. A., Verma A. K. Polypeptide hormones: intracellular receptors and internalization. Recent Prog Horm Res. 1981;37:539–582. doi: 10.1016/b978-0-12-571137-1.50016-5. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Gonzalez R. M., Guyda H. J. Intracellular (Golgi) receptors for insulinlike peptides in rat liver. Can J Biochem. 1980 Oct;58(10):1075–1081. doi: 10.1139/o80-145. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Josefsberg Z., Bergeron J. J. Intracellular polypeptide hormone receptors. Characterization of insulin binding sites in Golgi fractions from the liver of female rats. J Biol Chem. 1978 Jun 10;253(11):4067–4073. [PubMed] [Google Scholar]
- Posner B. I., Josefsberg Z., Bergerson J. J. Intracellular polypeptide hormone receptors. Characterization and induction of lactogen receptors in the Golgi apparatus of rat liver. J Biol Chem. 1979 Dec 25;254(24):12494–12499. [PubMed] [Google Scholar]
- Posner B. I., Patel B., Verma A. K., Bergeron J. J. Uptake of insulin by plasmalemma and Golgi subcellular fractions of rat liver. J Biol Chem. 1980 Jan 25;255(2):735–741. [PubMed] [Google Scholar]
- Posner B. I., Raquidan D., Josefsberg Z., Bergeron J. J. Different regulation of insulin receptors in intracellular (Golgi) and plasma membranes from livers of obese and lean mice. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3302–3306. doi: 10.1073/pnas.75.7.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattiaux R., Wattiaux-De Coninck S., Ronveaux-dupal M. F., Dubois F. Isolation of rat liver lysosomes by isopycnic centrifugation in a metrizamide gradient. J Cell Biol. 1978 Aug;78(2):349–368. doi: 10.1083/jcb.78.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]




