Abstract
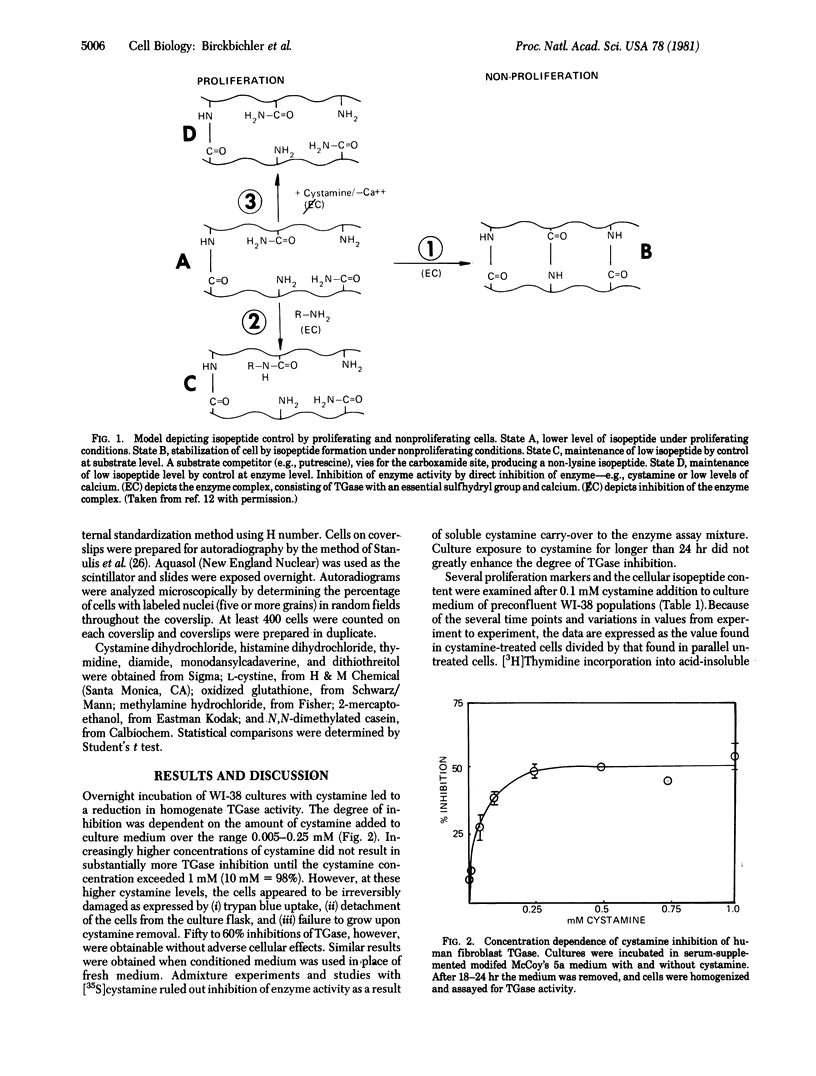
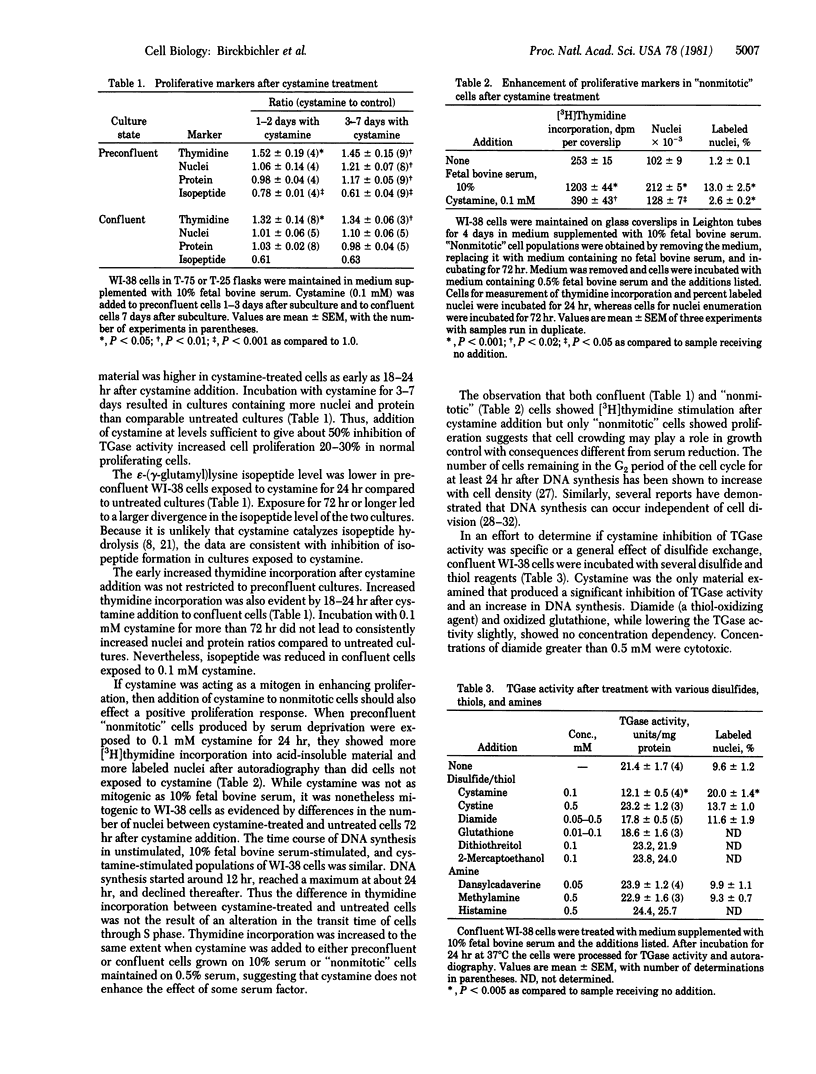
Cystamine inhibited transglutaminase activity (R-glutaminyl-peptide:amine gamma-glutamyltransferase, EC 2.3.2.13) of proliferating WI-38 cells in a dose-dependent manner over the concentration range 0.005-0.25 mM when added to the culture medium. The epsilon-(gamma-glutamyl)lysine content in the cells was decreased and several proliferation markers were enhanced. "Non-mitotic" cells were stimulated by cystamine (about 25% of that observed with 10% fetal bovine serum) to undergo DNA synthesis with subsequent increases in nuclei number. Numerous other disulfides, thiols, and amines were ineffective when added to culture medium. The findings are supportive of the concept that growth control involves a relationship between isopeptide crosslinks and proliferation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abernethy J. L., Hill R. L., Goldsmith L. A. epsilon-(gamma-Glutamyl)lysine cross-links in human stratum corneum. J Biol Chem. 1977 Mar 25;252(6):1837–1839. [PubMed] [Google Scholar]
- Birckbichler P. J., Carter H. A., Orr G. R., Conway E., Patterson M. K., Jr epsilon-(gamma-Glutamyl)lysine isopeptide bonds in normal and virus transformed human fibroblasts. Biochem Biophys Res Commun. 1978 Sep 14;84(1):232–237. doi: 10.1016/0006-291x(78)90287-5. [DOI] [PubMed] [Google Scholar]
- Birckbichler P. J., Dowben R. M., Matacic S., Loewy A. G. Isopeptide bonds in membrane proteins from eukaryotic cells. Biochim Biophys Acta. 1973 Jan 2;291(1):149–155. doi: 10.1016/0005-2736(73)90070-9. [DOI] [PubMed] [Google Scholar]
- Birckbichler P. J., Orr G. R., Conway E., Patterson M. K., Jr Transglutaminase activity in normal and transformed cells. Cancer Res. 1977 May;37(5):1340–1344. [PubMed] [Google Scholar]
- Birckbichler P. J., Patterson M. K., Jr Cellular transglutaminase, growth, and transformation. Ann N Y Acad Sci. 1978 Jun 20;312:354–365. doi: 10.1111/j.1749-6632.1978.tb16813.x. [DOI] [PubMed] [Google Scholar]
- Birckbichler P. J., Patterson M. K., Jr Transglutaminase and epsilon-(gamma-glutamyl) lysine isopeptide bonds in eukaryotic cells. Prog Clin Biol Res. 1980;41:845–855. [PubMed] [Google Scholar]
- Broome J. D., Jeng M. W. Promotion of replication in lymphoid cells by specific thiols and disulfides in vitro. Effects on mouse lymphoma cells in comparison with splenic lymphocytes. J Exp Med. 1973 Sep 1;138(3):574–592. doi: 10.1084/jem.138.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. J., Davies D. R., Levitzki A., Maxfield F. R., Milhaud P., Willingham M. C., Pastan I. H. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980 Jan 10;283(5743):162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- Fink M. L., Chung S. I., Folk J. E. gamma-Glutamylamine cyclotransferase: specificity toward epsilon-(L-gamma-glutamyl)-L-lysine and related compounds. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4564–4568. doi: 10.1073/pnas.77.8.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Park M. H., Chung S. I., Schrode J., Lester E. P., Cooper H. L. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980 Apr 25;255(8):3695–3700. [PubMed] [Google Scholar]
- Griffith O. W., Larsson A., Meister A. Inhibition of gamma-glutamylcysteine synthetase by cystamine: an approach to a therapy of 5-oxoprolinuria (pyroglutamic aciduria). Biochem Biophys Res Commun. 1977 Dec 7;79(3):919–925. doi: 10.1016/0006-291x(77)91198-6. [DOI] [PubMed] [Google Scholar]
- Harding H. W., Rogers G. E. Epsilon-(gamma-glutamyl)lysine cross-linkage in citrulline-containing protein fractions from hair. Biochemistry. 1971 Feb 16;10(4):624–630. doi: 10.1021/bi00780a013. [DOI] [PubMed] [Google Scholar]
- Hatzfeld J., Buttin G. Temperature-sensitive cell cycle mutants: a chinese hamster cell line with a reversible block in cytokinesis. Cell. 1975 Jun;5(2):123–129. doi: 10.1016/0092-8674(75)90020-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lebo R. V., Kredich N. M. Inactivation of human gamma-glutamylcysteine synthetase by cystamine. Demonstration and quantification of enzyme-ligand complexes. J Biol Chem. 1978 Apr 25;253(8):2615–2623. [PubMed] [Google Scholar]
- Levitzki A., Willingham M., Pastan I. Evidence for participation of transglutaminase in receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1980 May;77(5):2706–2710. doi: 10.1073/pnas.77.5.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy A. G., Matacic S. S. Modulation of the epsilon-(gamma-glutamic)lysine cross-link in cellular proteins. I. In vivo and in vitro studies. Biochim Biophys Acta. 1981 Mar 27;668(1):167–176. doi: 10.1016/0005-2795(81)90160-4. [DOI] [PubMed] [Google Scholar]
- Loewy A. G., Matacic S. S., Rice P., Stern J. Modulation of the epsilon-(gamma-glutamic)lysine cross-link in cellular proteins. II. Fractionation studies. Biochim Biophys Acta. 1981 Mar 27;668(1):177–185. doi: 10.1016/0005-2795(81)90161-6. [DOI] [PubMed] [Google Scholar]
- Lorand L., Rule N. G., Ong H. H., Furlanetto R., Jacobsen A., Downey J., Oner N., Bruner-Lorand J. Amine specificity in transpeptidation. Inhibition of fibrin cross-linking. Biochemistry. 1968 Mar;7(3):1214–1223. doi: 10.1021/bi00843a043. [DOI] [PubMed] [Google Scholar]
- Lorand L., Weissmann L. B., Epel D. L., Bruner-Lorand J. Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4479–4481. doi: 10.1073/pnas.73.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matacic S. S., Loewy A. G. Presence of the epsilon-(gamma-glutamic)lysine crosslink in cellular proteins. Biochim Biophys Acta. 1979 Feb 26;576(2):263–268. doi: 10.1016/0005-2795(79)90401-x. [DOI] [PubMed] [Google Scholar]
- Matacić S., Loewy A. G. The identification of isopeptide crosslinks in insoluble fibrin. Biochem Biophys Res Commun. 1968 Feb 26;30(4):356–362. doi: 10.1016/0006-291x(68)90750-x. [DOI] [PubMed] [Google Scholar]
- Namboodiri M. A., Favilla J. T., Klein D. C. Activation of pineal and brain acetyl-COA hydrolase by cystamine: an apparent case of disulfide exchange. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1166–1173. doi: 10.1016/0006-291x(79)92002-3. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Quittner S., Rubin A. L., Stenzel K. H. Transglutaminase activity in human lymphocytes: early activation by phytomitogens. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1157–1161. doi: 10.1073/pnas.75.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano J. J., Finlayson J. S., Peyton M. P. [Cross-link in fibrin polymerized by factor 13: epsilon-(gamma-glutamyl)lysine]. Science. 1968 May 24;160(3830):892–893. doi: 10.1126/science.160.3830.892. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977 Jun;11(2):417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Siefring G. E., Jr, Apostol A. B., Velasco P. T., Lorand L. Enzymatic basis for the Ca2+-induced cross-linking of membrane proteins in intact human erythrocytes. Biochemistry. 1978 Jun 27;17(13):2598–2604. doi: 10.1021/bi00606a022. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Wigglesworth N. M. Cell line which is temperature-sensitive for cytokinesis. J Cell Physiol. 1972 Oct;80(2):253–259. doi: 10.1002/jcp.1040800212. [DOI] [PubMed] [Google Scholar]
- Stanulis B. M., Sheldon S., Grove G. L., Cristofalo V. J. Scintillation fluid shortens exposure times in autoradiography. J Histochem Cytochem. 1979 Oct;27(10):1303–1307. doi: 10.1177/27.10.512316. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Lindl P. A. A CHO-cell mutant with a defect in cytokinesis. Somatic Cell Genet. 1976 Sep;2(5):387–400. doi: 10.1007/BF01542720. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Notides A. C., Pabalan S. S., Lorand L. Transamidase reactions involved in the enzymic coagulation of semen: isolation of -glutamyl- -lysine dipeptide from clotted secretion protein of guinea pig seminal vesicle. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2322–2325. doi: 10.1073/pnas.69.8.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissinger W., Wang R. J. Studies on cell division in mammalian cells. IV. A temperature-sensitive cell line defective in post-metaphase chromosome movement. Exp Cell Res. 1978 Mar 1;112(1):89–94. doi: 10.1016/0014-4827(78)90528-1. [DOI] [PubMed] [Google Scholar]
- van Wijk R., Zoutewelle G., van de Poll K. W. Regulation of initiation of DNA synthesis in relation to mitosis in cultured hepatoma cells. Cell Biol Int Rep. 1979 Aug;3(5):447–452. doi: 10.1016/0309-1651(79)90006-7. [DOI] [PubMed] [Google Scholar]


