Abstract
Patients with poor-risk leukemia have a high relapse rate despite allogeneic transplant. We report on the phase II trial of an intensified allogeneic transplant regimen whose aim was tolerable toxicity and durable remission. Study patients (n=30) had unfavorable first remission cytogenetics, progression from myelodysplasia or active disease due to induction failure or relapse. Conditioning was intravenous busulfan, targeted to a first-dose plasma area under the curve (AUC) of 700–900 µM·min, VP-16 at 30 mg/kg of adjusted ideal body weight and fractionated total body irradiation (FTBI) at 1200 cGy in ten fractions. Graft-versus-host disease (GVHD) prophylaxis was cyclosporine A and mycophenolate mofetil. Regimen-related toxicities (Bearman) included grade 3 mucositis in 29 patients (97%) and grade 4 in one, grade 2–3 sinusoidal obstructive syndrome in 2 patients (7%), and grade 2–3 skin toxicity in 8 patients (27%). The 30- and 100-day transplant-related mortalities were 0% and 7% respectively. The median follow-up was 83.7 months (60.7–96.4) for surviving patients. The 5-yr overall and disease-free survival was 40% for all patients. Cumulative 5-yr relapse incidence was 23% and transplant-related mortality was 37%. We have shown promising overall survival and relapse incidence in these poor-risk patients, who typically have few curative options.
Introduction
Relapse is the major cause of treatment failure in allogeneic hematopoietic cell transplant (HCT) patients with poor-risk leukemia, especially those with active disease due to induction failure or relapse. Relapse rates ranging from 28–69% have been demonstrated depending on the particular study regimen and patient population1–5. A key element in obtaining stable remission in patients with active disease is the intensity of the preparative regimen. High intensity regimens, while improving relapse rates, tend to offset this gain with an increased transplant-related mortality (TRM) due to regimen-related toxicity, tissue damage and increased incidence and/or severity of graft-versus-host disease (GVHD).
In an effort to improve relapse incidence while reducing TRM we have developed a busulfan (BU), fractionated total body irradiation (FTBI), etoposide (VP-16) regimen, tailored to reduce extramedullary toxicity. The rationale for choosing this combination of agents was as follows: 1) the drugs do not exhibit cross-resistance, 2) all three demonstrate dose-response curves, 3) VP16, a topoisomerase II inhibitor synergizes in vitro with an alkylating agent (like BU) to kill HL-60 promyelocytic leukemia cells6, and 4) these three agents in various combination regimens (which may also include cyclophosphamide) show some clinical efficacy for allogeneic and autologous HCT in relapsed leukemia.
The BU/FTBI/VP-16 combination was first tested by our group in a Phase I/II trial using oral busulfan prior to the availability of IV BU7. Escalating doses of oral BU were added to a preparative regimen of FTBI (12 Gy in 10 fractions) and VP-16 (60 mg). The maximum tolerated dose of BU was 12 mg/kg (oral) and the median plasma area under the curve (AUC) for the patients treated with 11 mg/kg (MTD-1) was 892 µM·min (460-1627). BU doses greater than 7 mg/kg were associated with improved disease-free survival (DFS).
In the current study, IV busulfan was used for its more consistent bioavailability and lower incidence of sinusoidal obstructive syndrome (SOS)8 and was targeted to a first-dose plasma AUC of 700–900 µM·min, based on data from the previous phase I trial of oral BU. The VP-16 dose was lowered to 30 mg/kg based on a data from a trial by Kroger et al. showing significant decreases in hepatic toxicity, SOS and acute GVHD with increased overall survival for the 30mg/kg VP-16 dose versus the 45mg/kg dose in Bu/Cy/VP-16 regimens9. Based on the literature and our own preliminary data, we designed and performed this prospective phase II trial of IV busulfan, targeted to an AUC of 700–900 µM·min, VP-16 and fractionated TBI conditioning, prior to allogeneic transplant in poor-risk leukemia patients at City of Hope.
Patients and methods
Inclusion Criteria
The Internal Review Board (IRB) at City of Hope approved this study and all patients were consented according to the Declaration of Helsinki. Study enrollment occurred between February 2000 and October 2004 at City of Hope, Duarte and also one patient at Good Samaritan, Phoenix. Enrollment for this protocol included patients of age ≥16 to ≤50, with advanced or poor-risk acute leukemia based on the following criteria: acute lymphoblastic leukemia (ALL) or acute myelogenous leukemia (AML) after induction failure or in relapse, and AML in first remission (CR1) with unfavorable cytogenetics or evolved from myelodysplasia. Eligibility also required the availability of an HLA-identical sibling donor. Organ function requirements were as follows: cardiac ejection fraction ≥50%, serum creatinine ≤1.2 or creatinine clearance >80 ml/min, bilirubin ≤SGOT and SGPT <5 times the upper limit of normal, FEV1 and DLCO >50% predicted normal value. Additional criteria were absence of active infection and time from last chemotherapy ≥28 days.
Patients
Patient characteristics and pre-transplant status are shown in Table 1. Six patients in CR1 were placed on this protocol due to unfavorable cytogenetics, based on SWOG criteria10, 11, or progression from MDS. Three patients with AML in CR1 with unfavorable cytogenetics had t(9:22), 11q 23 abnormality and a complex karyotype. Twenty-two patients had active AML or ALL due to induction failure or relapse. Two patients had active, untreated MDS (RAEB-t), which would now be classified as AML based on the current WHO classification 12. Of the 24 patients with induction failure or relapse, median WBC was 3.35 × 109/L (0.9–29.2), blasts in bone marrow were 16.5 % (0–95) and blasts in peripheral blood were 3.5% (0–92). Two patients with induction failures had fewer than 5% blasts in the marrow at the time of transplant; this reflects the fact that following pre-treatment prior to transplant conditioning, the ANC and platelet counts had not recovered sufficiently to fulfill the definition of complete remission. Patient comorbitity at the time of transplant was calculated using the Pretransplant Assessment of Mortality (PAM)13 score calculator available on the internet at http://cdsweb.fhcrc.org/pam/. The median PAM score was 26 with a range of 22 to 34.
Table 1.
Patient characteristics pre-transplant
| Characteristic | N (%) or median (range) |
|---|---|
| Patient Age | 37 (19–50) |
| Patient Sex | |
| Female | 15 (50%) |
| Male | 15 (50%) |
| Disease Status at Transplant | |
| AML (including 2 MDS) | 24 (80%) |
| CR1 | 6 (20%) |
| R1 | 6 (20%) |
| IF | 10 (33%) |
| untreated RAEB-t | 2 (6%) |
| ALL | 6 (20%) |
| R1 | 1 (3%) |
| R2 | 1 (3%) |
| IF | 4 (13%) |
| Cytogenetics | |
| Indeterminate* | 1 (3%) |
| Intermediate | 14 (47%) |
| Unfavorable | 15 (50%) |
| Stem Cell source | |
| Bone Marrow | 1 (3%) |
| Peripheral Blood | 29 (97%) |
| For patients with active disease | |
| WBC pre-conditioning | 3.4 (0.9–29.2) |
| % blasts in bone marrow | 16.5 (0–95) |
| % blasts in peripheral blood | 3.5 (0–92) |
Cytogenetic risk is classified as indeterminate by Pullarkat et al. 2008 [9]. CR1 = 1st complete remission, R1 = 1st relapse, R2 = 2nd relapse, IF = induction failure, WBC = white blood cell count
Treatment Regimen
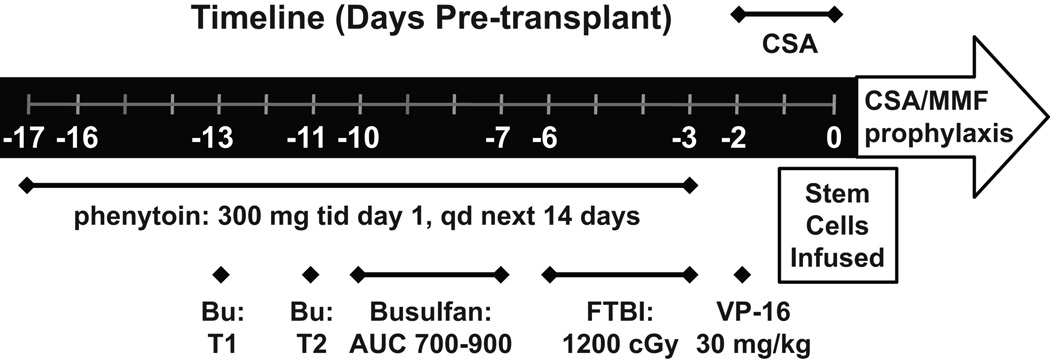
The treatment regimen is diagrammed in Figure 1. Prior to start of the preparative regimen, on day -17 (with day 0 = transplant day), phenytoin was administered 300 mg orally three times for one day, then 300 mg/day orally or IV for 14 days to prevent seizures. On day -13, a test dose of busulfan was administered at 22 mg/m2 body surface area. After measuring plasma concentrations at day -12, the subsequent doses were adjusted to target an AUC between 700–900 µM·min, based on the patient body surface area (BSA). The maximum possible dose was set to 27.25 mg/m2. On day -11 calculated BU dose was administered and blood levels retested. Further dose adjustments were made for AUCs > 1000 µM·min. The calculated target dose was administered in 14 doses over 4 days. On days -6 through -3, a total of 1200 cGy FTBI was given in 10 fractions. On day -2, VP-16 was dosed at 30 mg/kg of adjusted ideal bodyweight. Peripheral blood stem cells were transfused on day 0. GVHD prophylaxis was cyclosporine (CSA) and mycophenolate mofetil (MMF), starting at day -1 for all patients. After patients were able to eat and drink, IV administration of GVHD prophylaxis was switched to the oral route. MMF taper was begun at day 100 and CSA taper began at 6 months if there was no evidence of GVHD.
Figure 1. Treatment schema.
Treatments are listed under timeline with dosages. Bu = bulsulfan, T1 = test dose 1, T2 = test dose 2, FTB I= fractionated total body irradiation, CSA = cylclosporine, MMF = mycophenolate mofetil, qd = once daily, tid =t hrice daily
Busulfan AUC Calculation
Serial heparinized blood samples (4 ml each) were collected around the busulfan test dose at the following times; pre-dose, immediately prior to the end of the busulfan infusion, and then at 15, 30, 60, 180 and 240 minutes after the end of the infusion. The same sampling schedule was repeated around the 2nd dose in most patients. Busulfan concentrations in plasma were measured according to the previously published gas-liquid chromatographic method of Chen14 et al.
Busulfan AUC was estimated using a one-compartment pharmacokinetic model with first-order elimination in ADAPT II software (USC Biomedical Simulations Resource, Los Angeles, CA). The goodness-of-fit of the model-derived AUC was further confirmed by non-compartmental methods using the rule of linear trapezoids.
Statistics
Overall survival estimates were calculated based on the product-limit method, and 95% confidence intervals were calculated using the logit transformation and the Greenwood variance estimate15. Cumulative relapse incidence (RI) was calculated controlling for non-relapse related death as a competing risk and conversely, TRM was calculated controlling for relapse as a competing risk16. Differences between survival curves were assessed by log-rank or Gray’s test17 as appropriate. The significance of demographic and treatment features collected from HCT recipients was assessed using Cox proportional-hazards regression analysis18 and its competing-risks analogue19. The following parameters were included in the analysis: patient age, sex, disease group (AML/CR1, AML/R1, AML/IF, ALL), 2nd dose busulfan AUC (per unit increase and also per 100-unit increase), pre-conditioning bone marrow blasts, pre-conditioning peripheral cell blasts, cytogenetics (intermediate vs unfavorable), white blood cell count at diagnosis (continuous), white blood cell count pre-conditioning (continuous). Statistical significance was defined at the P value less than or equal to 0.05 level.
Results
Engraftment
Engraftment was defined as the first day of absolute neutrophil count greater than 500 per microliter of blood, when counts were maintained above 500 for three consecutive days. All patients engrafted successfully. The median time, post-transplant, to reach an absolute neutrophil count (ANC) of ≥ 500/µl was 11 days (range 8–23), and a platelet count ≥ 20/µl was 18 days (range 14–65). Bone marrow at day 30 was negative for leukemia for all patients excepting one with persistent disease.
Regimen-related toxicity
Regimen related toxicity (RRT) was rated for severity according to the Bearman scoring system20. 29/30 patients developed grade 3 mucositis, requiring opioid infusions and total parenteral nutrition, and one patient required intubation for airway protection. SOS of the liver grades 2 and 3 occurred in 2 patients (7%). Skin toxicity grades 2–3 occurred in 8 patients (27%) and manifested as an erythematous rash involving axilla and groin areas as previously described by Linker21.
Graft-versus-host disease
The total incidence of acute GVHD, as defined by Glucksberg et al22. was 63% for grades II-IV and 23% for grades III-IV. Chronic GVHD, by the classical limited/extensive classification23, 24, occurred at a rate of 82% overall, 5/28 patients (18%) having limited disease and 18/28 (64%) with extensive. Cummulative incidence of aGVHD calculated with death as a competing risk was 0.47 (95% CI: 0.30, 0.65) at 30 days and 0.63 (95% CI: 0.45, 0.79) at 100 days. Cummulative incidence of cGVHD with death as a competing risk was 0.40 (95% CI: 0.24, 0.59) at 1 year and 0.53 (95% CI: 0.35, 0.71) at 5 years. Two patients died before day 100, never entering the risk-set for chronic GVHD. The median time of follow-up was 83.7 months (60.7–96.4) for surviving patients. At analysis date eight patients had completely discontinued immunosuppression, two used only local therapy, one used cyclosporin 50 mg twice daily on alternating days with 10 mg prednisone, and one took 10 mg prednisone daily.
Busulfan Dose Adjustment
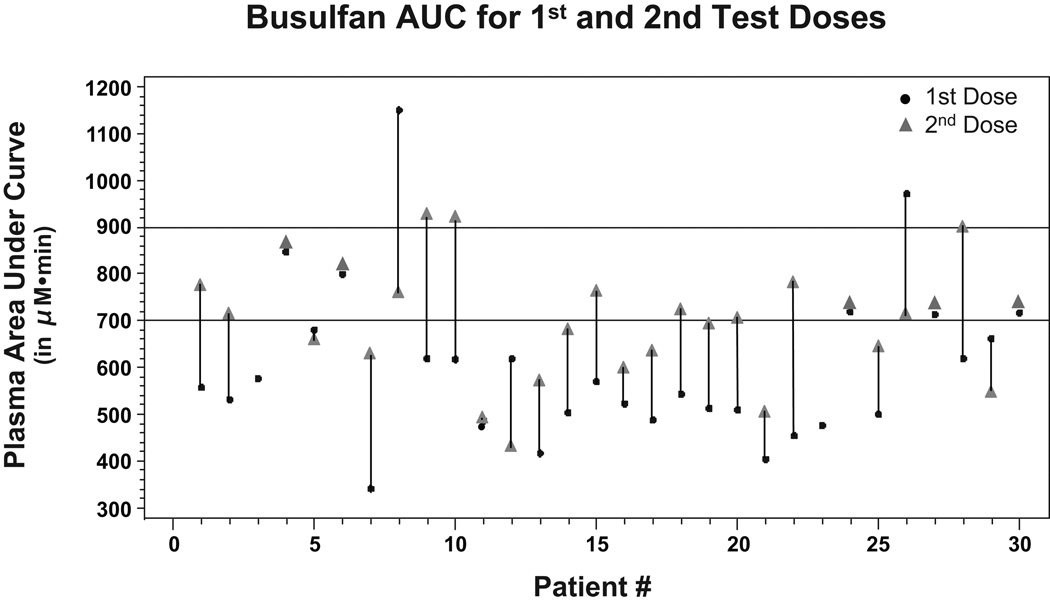
Figure 2 plots the first- and second-dose busulfan AUCs. Busulfan test-dose AUCs are available for 30 patients and 2nd-dose AUCs are available for 28 patients. Of the 30 patients with test-dose AUCs, 23 had AUCs below the target range, 5 were within the range and 2 were above the range. Of the 23 patients with a test dose below the range, for 2nd-dose AUCs, the repeat dose came within the range for 8 patients, went above the range for 3 patients and remained below the range for 12 patients. Both patients whose test dose was above the target range had repeat-dose AUCs within the range after dose adjustments. The median first-dose AUC was 518 µM·min, while the median second dose AUC was 684 µM·min. No patient had a busulfan AUC greater than 1000 µM·min at second dose, so further adjustments were unnecessary.
Figure 2. Busulfan AUC.
For each patient, area under the curve in µM·min is designated for both the 1st dose (22mg/m2) and 2nd dose (adjusted based on 1st). The target range AUC of 700–900 is indicated by horizontal lines.
Outcomes
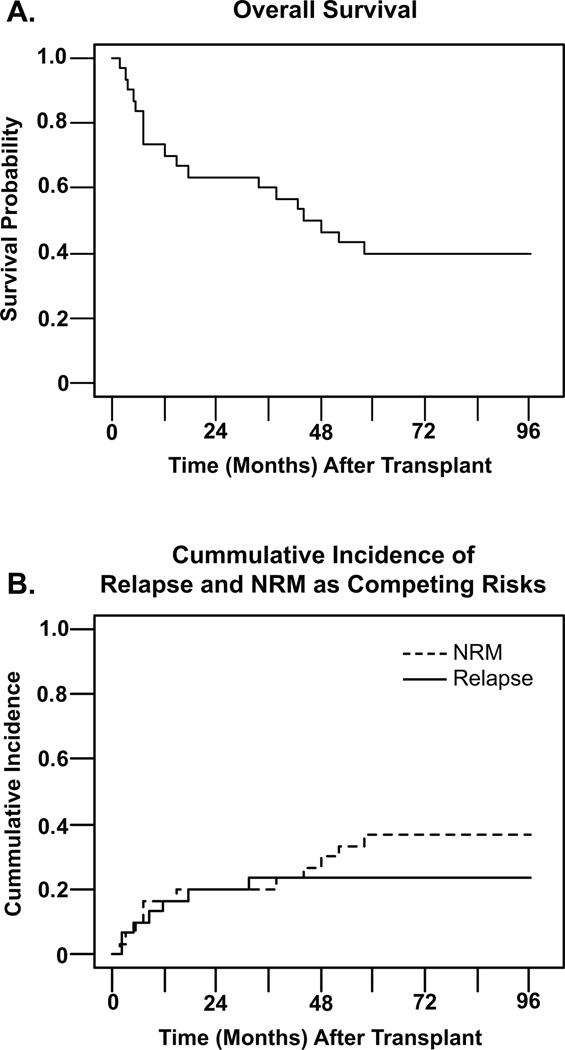
The median time of follow-up was 46.3 months for all patients (range 2.0–96.4), and 83.7 months (60.7–96.4) for surviving patients. Eighteen of 30 patients (60%) had died as of the date of analysis, and the cause of death is reported in Table 2. The 5-yr overall survival rate (OFS) was 40% for all patients, with a confidence interval (CI) of 23% to 57%, with the survival curve shown in figure 3A. Disease-free survival at 5 years was also 40%. Figure 3B depicts 5-yr cumulative relapse incidence (RI) (23%; CI 11–42%) and transplant-related mortality (TRM) (37%; CI 21–55%) for the total study group, calculated as competing risks. The 30-day and 100-day transplant-related mortalities were 0% and 7% respectively. Four distinct subgroups were compared for 5-yr OS and RI, AML/CR1 (n=6), AML/R1 (n=6) AML/IF (n=10), and active ALL (n=6). Five-year OS for the groups was 67% (CI 20%-90%, AML/CR1), 50% (CI 11%-80%, AML/R1), 30% (7%-38%, AML/IF) and 33% (CI 5%-68%, ALL); no significant differences were seen between groups. The 5-yr cumulative incidence of relapse controlling for TRM in the groups was 0% (AML/CR1), 0% (AML/R1), 30% (CI 9%-65%, AML/IF) and 50% (CI 13%-87%, ALL); no significant differences were seen between groups. A proportional-hazards model regression analysis was performed to look at 2nd-dose busulfan AUC as a continuous-variable predictor of relapse with non-relapse death as a competing risk; AUC was borderline significant at p=0.06. The risk ratio was 1.00 with 95% CI (1.00, 1.01) per unit increase of AUC. When calculated per 100 unit increase of AUC the risk ratio was 1.62 with 95% CI (0.98, 2.68). Busulfan AUC as a continuous variable was also analyzed as a risk factor for TRM (with relapse death as a competing risk) and GVHD incidence. AUC was borderline significant as a risk factor for TRM (p=0.06), but showed no significant impact on either aGVHD or cGVHD. Twelve of thirty patients were living at the analysis date of this report. At last followup, surviving patients had the following Karnofsky Performance Status (KPS) scores: eight were 90%, one was 80–90%, two at 80% and one at 70–80%.
Table 2.
Causes of death
| Cause of Death | Total n=18 |
|---|---|
| disease progression | 7 |
| cGVHD-related (organ failure & infection) | 7 |
| Leukoencephalopathy | 1 |
| Encephalitis | 1 |
| Interstitial Pneumonitis | 1 |
| Bacterial ARDS | 1 |
ARDS = acute respiratory distress syndrome
Figure 3. Outcomes.
Panel A. shows the overall survival in months for all 30 patients on the study. Panel B. shows both cummulative incidence of relapse (solid line) and non-relapse mortality (dashed line) in months, calculated as competing risks. NRM = non-relapse mortality.
Discussion
The goal of this study was to intensify the conditioning regimen for high-risk leukemia transplant patients to decrease the incidence of relapse while minimizing associated morbidity and mortality. This targeted BU/FTBI/VP-16 pre-transplant conditioning regimen was very effective in achieving disease control in patients with high-risk myeloid malignancy, both those in remission and with active disease at the time of HCT. Among the patients in first remission (with unfavorable cytogenetics or evolved from MDS, n=6) and those with relapsed AML (n=6) there were no relapses post-transplant. The Five-year OS and DFS of 40 % for the total population compare favorably with the literature where survival percentages for advanced patients are 17–36 % 1, 2, 4, 5. Modulating the preparative regimen to decrease toxicity resulted in a very low 100-day TRM of 7%, with an overall 5-yr TRM of 37% primarily due to complications of cGVHD. Based on our median PAM score13 of 26 (range 22–34), the expected 2-year mortality is 56% (53–60), which corresponds to an overall survival at 2 years of 44%. Our 2-yr OS (see Figure 3A) was 63% (95% confidence of 44–78%), so survival is at, or greater than, predicted oucomes based on the PAM score.
This IV busulfan protocol achieved the target AUC of 700–900 µM·min in 54% of patients at second dose. The data presented in Figure 2 illustrates the difficulty of targeting busulfan exposures within a narrow therapeutic range. Even though IV busulfan levels are more stable than oral dosing levels, the use of PK measurements is valuable, as dose adjustment was required frequently. The chosen range of 700–900 µM·min, with a true target of 800 µM·min, in the setting of normal intra-patient pharmacokinetic variability, is probably impractical. The majority of the AUCs outside the range were below the lower limit of 700 µM·min. In several cases, this was due to the self-imposed limitation on the maximum allowable dose increase (0.8 mg/kg = 27.25 mg/m2). Interestingly, if we had chosen a true target of 800 µM·min and increased the range to ± 25% (i.e. 600–1000 µM·min), 22 of the 28 patients with repeat AUCs would have been in the target range.
Despite the increased intensity of this regimen, mortality due to regimen-related toxicity (RRT) was improved compared to other high-intensity regimens in the literature and is reflected in the 7% 100-day TRM2–4, 9. The primary toxicity was mucositis, with 29/30 patients experiencing grade 3 and 1/30 experiencing grade 4. However, other complications, including SOS, were rare and there were no deaths attributable to RRT. Bu/Cy/TBI, Bu/Cy/VP-16 and Cy/TBI/VP16 high-intensity regimens have resulted in multiple lethal RRT events ranging from 5–16% of patients, typically lung toxicity and SOS2–4, 9. Regulation of the busulfan dose using AUC and lowering of the etoposide dose to 30 mg/kg, likely both contributed to prevention of lethality from RRT9, 25.
In this study the incidence of aGVHD II-IV was 63% and cGVHD was 82%. In comparison, the CSA/MMF prophylactic regimen used by Dean et al., for myeloablative matched-sibling bone-marrow transplants shows rates of 58% acute and 42% chronic GVHD.26 Cutler et al., for peripheral blood myeloablative transplants using sirolimus/tacrolimus prophylaxis, saw GVHD rates of 20.5% acute and 59.1% chronic GVHD27. The relatively high rates of GVHD in our study are possibly due to the use of peripheral blood stem cells, the advanced disease stage of the patients and the significant mucosal injury related to this conditioning regimen. To address this issue, in our follow-up study, we have replaced the GVHD prophylaxis regimen with tacrolimus/sirolimus. Recent studies using tacrolimus/sirolimus for GVHD prophylaxis at this institution show lower rates of GVHD than CSA/MMF regimens following peripheral blood transplants28, 29. Despite the rate of GVHD, survival in such a poor-prognosis group of patients is relatively high and the twelve surviving patients are not severely disabled, as indicated by KPS scores (avg. 86%, median 90%). Eight of these twelve patients had completely discontinued immunosuppressive therapy at analysis date, while the remaining four were on minimal immunosuppression.
Disease-free survival of 40% at 5-yrs and relapse incidence of 23% is particularly encouraging for patients with advanced leukemias (ALL and AML). The prognosis for AML patients in active relapse or induction failure is so poor that they are not considered eligible for transplant at many institutions. Overall survival rates of 50% for AML patients in R1 and 30% for patients in IF support transplant using this conditioning regimen as a reasonable option. If TRM could be further improved, survival possibilities for these high-risk patients could become even more favorable. The use of targeted busulfan has decreased the hepatic and pulmonary toxicity previously associated with this drug, however mucositis remains a challenge with this regimen. Since this trial was initiated, advances in the use of palifermin30 and targeted irradiation techniques such as radioimmunotherapy or total marrow irradiation (TMI), have the potential to decrease the incidence and severity of mucositis.
Based on the data from this phase II trial, City of Hope is currently accruing on a phase I trial of targeted IV BU, VP-16 and targeted total marrow irradiation (TMI) using CT image-guided intensity modulated radiotherapy delivered by a Tomotherapy Hi-Art System (Tomotherapy, Inc. Madison, WI)31, with tacrolimus/sirolimus GVHD prophylaxis in poor-risk AML patients. We anticipate further refinement of these favorable results in the future via the decreased extramedullary side effects of TMI, a more effective GVHD regimen and the selection of AML patients most likely to benefit from this protocol.
Acknowledgements
This study was supported in part by grants from the National Institute of Health, CA30206 and CA33572. We would like to acknowledge the dedicated nurses of the City of Hope Bone Marrow Unit for excellent care of our patients and support of medical research.
Footnotes
Authorship
Contributions: A.S. designed the original study, performed the research, analyzed the data and wrote the paper; T.S. analyzed the data and wrote the paper; M. O’D critically reviewed the paper; A.D. and A.T. analyzed the data; A.N., P.P, V.P, D.S, R S, J. W. and J.A treated patients and critically reviewed the paper; S.T. analyzed the data and wrote the paper; and S.F. designed the original study and critically reviewed the paper.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Blume KG, Kopecky KJ, Henslee-Downey JP, Forman SJ, Stiff PJ, LeMaistre CF, et al. A prospective randomized comparison of total body irradiation-etoposide versus busulfan-cyclophosphamide as preparatory regimens for bone marrow transplantation in patients with leukemia who were not in first remission: a Southwest Oncology Group study. Blood. 1993;81(8):2187–2193. [PubMed] [Google Scholar]
- 2.Giralt SA, LeMaistre CF, Vriesendorp HM, Andersson BS, Dimopoulos M, Gajewski J, et al. Etoposide, cyclophosphamide, total-body irradiation, and allogeneic bone marrow transplantation for hematologic malignancies. J Clin Oncol. 1994;12(9):1923–1930. doi: 10.1200/JCO.1994.12.9.1923. [DOI] [PubMed] [Google Scholar]
- 3.Hirabayashi N, Goto S, Ishii M, Yuge M, Mitsuma A, Noda N. Busulfan, cyclophosphamide and total body irradiation as conditioning for allogeneic bone marrow transplantation for acute and chronic myeloid leukemia. Bone Marrow Transplant. 1998;21(11):1079–1083. doi: 10.1038/sj.bmt.1701244. [DOI] [PubMed] [Google Scholar]
- 4.Lynch MH, Petersen FB, Appelbaum FR, Bensinger WI, Clift RA, Storb R, et al. Phase II study of busulfan, cyclophosphamide and fractionated total body irradiation as a preparatory regimen for allogeneic bone marrow transplantation in patients with advanced myeloid malignancies. Bone Marrow Transplant. 1995;15(1):59–64. [PubMed] [Google Scholar]
- 5.Mengarelli A, Iori A, Guglielmi C, Romano A, Cerretti R, Torromeo C, et al. Standard versus alternative myeloablative conditioning regimens in allogeneic hematopoietic stem cell transplantation for high-risk acute leukemia. Haematologica. 2002;87(1):52–58. [PubMed] [Google Scholar]
- 6.Chang TT, Gulati SC, Chou TC, Vega R, Gandola L, Ibrahim SM, et al. Synergistic effect of 4-hydroperoxycyclophosphamide and etoposide on a human promyelocytic leukemia cell line (HL-60) demonstrated by computer analysis. Cancer Res. 1985;45(6):2434–2439. [PubMed] [Google Scholar]
- 7.Stein A, O'Donnell MR, Parker P, Snyder DS, Nademanee A, Krishnan A, et al. Phase I-II study of escalating doses of busufan (BU) in combination with fractioned total body irradiation (FTBI) and etoposide (VP-16) as a preparative regimen for allogeneic bone marrow transplant (BMT) for patients with advanced leukemias. Blood. 1998;92(10-Sup 1) Abstract # 517. [Google Scholar]
- 8.Nguyen L, Leger F, Lennon S, Puozzo C. Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: a population pharmacokinetic study. Cancer Chemother Pharmacol. 2006;57(2):191–198. doi: 10.1007/s00280-005-0029-0. [DOI] [PubMed] [Google Scholar]
- 9.Kroger N, Zabelina T, Sonnenberg S, Kruger W, Renges H, Stute N, et al. Dose-dependent effect of etoposide in combination with busulfan plus cyclophosphamide as conditioning for stem cell transplantation in patients with acute myeloid leukemia. Bone Marrow Transplant. 2000;26(7):711–716. doi: 10.1038/sj.bmt.1702598. [DOI] [PubMed] [Google Scholar]
- 10.Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89(9):3323–3329. [PubMed] [Google Scholar]
- 11.Pullarkat V, Slovak ML, Kopecky KJ, Forman SJ, Appelbaum FR. Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of Southwest Oncology Group 9400 study. Blood. 2008;111(5):2563–2572. doi: 10.1182/blood-2007-10-116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 13.Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med. 2006;144(6):407–414. doi: 10.7326/0003-4819-144-6-200603210-00007. [DOI] [PubMed] [Google Scholar]
- 14.Chen TL, Grochow LB, Hurowitz LA, Brundrett RB. Determination of busulfan in human plasma by gas chromatography with electron-capture detection. J Chromatogr. 1988;425(2):303–309. doi: 10.1016/0378-4347(88)80034-3. [DOI] [PubMed] [Google Scholar]
- 15.Breslow NE, Day NE. Statistical methods in cancer research: volume II, the design and analysis of cohort studies. IARC Sci Publ. 1987;82:1–406. [PubMed] [Google Scholar]
- 16.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–554. [PubMed] [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1140–1154. [Google Scholar]
- 18.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society. 1972;B34:187–220. [Google Scholar]
- 19.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 20.Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6(10):1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 21.Linker CA, Ries CA, Damon LE, Rugo HS, Wolf JL. Autologous bone marrow transplantation for acute myeloid leukemia using busulfan plus etoposide as a preparative regimen. Blood. 1993;81(2):311–318. [PubMed] [Google Scholar]
- 22.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57(2):267–276. [PubMed] [Google Scholar]
- 25.Copelan EA, Bechtel TP, Avalos BR, Elder PJ, Ezzone SA, Scholl MD, et al. Busulfan levels are influenced by prior treatment and are associated with hepatic veno-occlusive disease and early mortality but not with delayed complications following marrow transplantation. Bone Marrow Transplant. 2001;27(11):1121–1124. doi: 10.1038/sj.bmt.1703047. [DOI] [PubMed] [Google Scholar]
- 26.Dean R, Rybicki LA, Sobecks RM, Copelan E, Kalaycio M, Andresen S, et al. Comparison of cyclosporine and methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Blood. 2008;112(11) Ab2240. [Google Scholar]
- 27.Cutler C, Li S, Ho VT, Koreth J, Alyea E, Soiffer RJ, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura R, Palmer J, Parker P, Stein A, Stiller T, Pullarkat V, et al. Improved outcome after reduced intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome usingtacroliums/siroliums-based GVHD prophylaxis. Blood (ASH Annual Meeting Abstracts) 2009;114 Abstract 2771. [Google Scholar]
- 29.Snyder DS, Palmer J, Gaal K, Stein AS, Pullarkat V, Sahebi F, et al. Improved Outcomes Using Tacrolimus/Sirolimus for Graft Versus Host Disease prophylaxis with a Reduced Intensity Conditioning Regimen for Allogeneic Hematopoietic Cell Transplant as treatment of Myelofibrosis. Biol Blood Marrow Transplant. 2009 doi: 10.1016/j.bbmt.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351(25):2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 31.Wong JY, Rosenthal J, Liu A, Schultheiss T, Forman S, Somlo G. Image-guided total-marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2009;73(1):273–279. doi: 10.1016/j.ijrobp.2008.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]