Summary
Axonemes are microtubule-based organelles of crucial importance in the structure and function of eukaryotic cilia and flagella. Despite great progress in understanding how axonemes are assembled, the signals that initiate axoneme outgrowth remain unknown. Here, we identified phosphatidylinositol phosphates (phosphoinositides) as key regulators of early stages of axoneme outgrowth in Drosophila melanogaster spermatogenesis. In a study of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] function in developing Drosophila male germ cells, we depleted PtdIns(4,5)P2 by expression of a potent phosphoinositide phosphatase. Phosphatase expression dramatically inhibited sperm tail formation and perturbed microtubule organization in a manner reversible by co-expression of a PtdIns 4-phosphate 5-kinase. Depletion of PtdIns(4,5)P2 caused increased levels of basal body γ-tubulin and altered the distribution of proteins known to be required for axoneme assembly. Examination of PtdIns(4,5)P2-depleted spermatids by transmission electron microscopy revealed defects in basal body docking to the nuclear envelope, and in axoneme architecture and integrity of the developing flagellar axoneme and axial sheath. Our results provide the first evidence that phosphoinositides act at several steps during flagellar biogenesis, coordinately regulating microtubule and membrane organization. They further suggest that phosphoinositides play evolutionarily conserved roles in flagella and cilia, across phyla and in structurally diverse cell types.
Keywords: Axoneme, Basal body, Flagella, Cilia, Microtubules, Phosphoinositides, Phosphatidylinositol, SigD, Sktl, Drosophila, Spermatogenesis
Introduction
Eukaryotic cilia and flagella project from the cell surface, allowing cells to interact with their environment. Motile cilia and flagella contain axonemes, composed of nine outer doublet microtubules (MTs) and a central pair of MTs (9 + 2), and play fundamental roles in a variety of physiological processes, including cell signaling, mucus clearance and sperm motility (Davenport and Yoder, 2005). Defects in assembly of motile axonemes lead to human diseases and developmental disorders, including respiratory dysfunction, defects in left-right asymmetry, hydrocephaly and infertility (reviewed in Badano et al., 2006; Bisgrove and Yost, 2006).
Axonemes are assembled on basal bodies, which are modified centrioles containing nine triplet MTs. Centrioles are required for axoneme assembly, because flies mutant for core centriolar proteins, Sas-4 and Sas-6, lack axonemes (Basto et al., 2006; Rodrigues-Martins et al., 2007). Prior to flagellar axoneme assembly, the basal body attaches to the nuclear envelope. This process (henceforth termed basal body docking) has been described in diverse cells, including insect and mammalian sperm (Tates, 1971; Manandhar et al., 1999; Li et al., 2004) and the unicellular alga Chlamydomonas reinhardtii (Salisbury, 1995). In Drosophila, axoneme assembly is independent of basal body docking, because mutant flies lacking dynein light chain or γ-tubulin ring complex (γ-TuRC) proteins produce normal, stable axonemes, yet their basal bodies fail to dock (Li et al., 2004; Vogt et al., 2006). Organization of axonemal MTs into a classic 9 + 2 arrangement depends on incorporation of testis-specific tubulin isoforms (Hoyle and Raff, 1990; Hutchens et al., 1997), and also on the fragile X-related protein FXR (FMRP), the whirligig gene and the basal-body-associated proteins Centrosomin (Cnn) and Uncoordinated (Unc) (Green et al., 1990; Li et al., 1998; Baker et al., 2004; Zhang et al., 2004). Despite decades of research, little is known about the signals that promote basal body docking, ensure the proper transition from basal body to axonemal MT arrays or preserve the integrity of the developing flagellar axoneme.
Drosophila spermatogenesis offers an excellent model for studying axoneme assembly, because the stages are well-characterized and easily identified by light and transmission electron microscopy (TEM), and the spermatids grow to an impressive 1.8 mm in length (Tates, 1971; Fuller, 1993). Male germ cells develop in syncytial cysts, with individual cells remaining connected by cytoplasmic bridges. Cysts of 16 primary spermatocytes undergo meiotic cytokinesis, generating cysts of 64 haploid spermatids that will develop into mature sperm. In early spermatids, the basal body becomes localized to a perinuclear region. During docking, the basal body becomes firmly embedded in the nuclear envelope. Axoneme assembly initiates at the protein body stage, when an electron-dense, phase-dark structure appears in the nucleus. Two giant mitochondrial derivatives elongate along the growing axoneme, which becomes covered by a membranous axial sheath.
The membrane lipid phosphatidylinositol (PtdIns) and its phosphorylated derivates (phosphoinositides) (Fig. 1A) control vital cellular functions. For example, PtdIns 4-phosphate [PtdIns(4)P] is synthesized from PtdIns by PtdIns 4-kinases at the Golgi, where it recruits proteins involved in post-Golgi transport (reviewed in De Matteis and Godi, 2004; Roth, 2004). PtdIns(4)P 5-kinases phosphorylate PtdIns(4)P to yield PtdIns(4,5)P2, an important plasma membrane regulator of membrane trafficking and actin assembly (reviewed in Yin and Janmey, 2003; Di Paolo and De Camilli, 2006; Janetopoulos and Devreotes, 2006). Hydrolysis of PtdIns(4,5)P2 by phospholipase C (PLC) leads to production of the second messengers diacyglycerol (DAG) and inositol trisphosphate (InsP3), which in turn promotes Ca2+ release from the ER (Fig. 1A). Despite the wealth of information from studies on single cells, surprisingly little is known about the functions of phosphoinositides in animal development. We have developed a Drosophila model in which to study phosphoinositides in spermatogenesis.
Fig. 1.
SigD expression depletes plasma membrane PtdIns(4,5)P2. (A) Simplified PtdIns pathway. PtdIns (PI) can be phosphorylated by PtdIns 4-kinases (PI4K), such as Drosophila Fwd, to yield PtdIns(4)P [PI4P]. PtdIns(4)P is phosphorylated by PtdIns(4)P 5-kinases (PIP5K), such as Drosophila Sktl, to produce PtdIns(4,5)P2 (PIP2). PtdIns(4,5)P2 is hydrolyzed by phospholipase C (PLC), generating the second messengers inositol trisphosphate (InsP3, IP3) and diacylglycerol (DAG). InsP3 can lead to release of Ca2+ from intracellular stores (via the InsP3 receptor) and to synthesis of other inositol polyphosphates, such as inositol hexakisphosphate (InsP6, IP6). Alternatively, PtdIns(4,5)P2 can be sequentially dephosphorylated by 5-phosphatases (5-P’ase), including Salmonella SigD, to regenerate PtdIns(4)P, which in turn can be dephosphorylated by 4-phosphatases (4-P’ase), such as Drosophila Sac1, to produce PtdIns. (B-E′) Phase-contrast (B–E) and corresponding fluorescence (B′-E′) images of male germ cells expressing PLCδ-PH-GFP (B,C,E) or PLCδ-PH-RFP (D), which bind PtdIns(4,5)P2. (B′) PtdIns(4,5)P2 is found in the plasma membrane (arrow) of wild-type male germ cells. (C′) SigD expression diminishes PtdIns(4,5)P2 at the plasma membrane and causes accumulation of PLCδ-PH-GFP puncta in the cytoplasm (arrow). (D′) Co-expression of Sktl partially restores plasma membrane PtdIns(4,5)P2 (arrow), although some cells exhibit non-specific PLCδ-PH-RFP localization in the cytoplasm. (E′) Expression of SigDdead has no effect on PtdIns(4,5)P2 accumulation. (F–G′) Phase-contrast (F,G) and corresponding fluorescence (F′,G′) images of male germ cells expressing RFP-PH-FAPP to detect PtdIns(4)P. (F′) PtdIns(4)P localizes to the Golgi in spermatocytes (arrowhead) and to the acroblast in spermatids (arrow). (G′) SigD expression increases PtdIns(4)P levels in both spermatocytes (arrowhead) and spermatids (arrow). Note that, although the exposure times for F′ and G′ were identical, the contrast in F′ was increased by 50% to make the PtdIns(4)P localization more obvious. Scale bars: 20 μm.
Drosophila phosphoinositides have been implicated in regulating cell proliferation, differentiation, apical-basal polarity, actin organization and cytokinesis (Leevers et al., 1996; Brill et al., 2000; Bateman and McNeill, 2004; Wong et al., 2005; Pilot et al., 2006; Pinal et al., 2006). However, because enzymes that synthesize and dephosphorylate phosphoinositides are often essential, it has been difficult to study phosphoinositide function in differentiating cells. For example, mutations affecting the Drosophila PtdIns(4)P 5-kinase Skittles (Sktl) are lethal and clones of sktl mutant cells are largely inviable, making it difficult to assess PtdIns(4,5)P2 function in vivo (Hassan et al., 1998). Similarly, the PtdIns 4-phosphatase Sac1 is essential, limiting analysis of the consequences of PtdIns(4)P overproduction (Wei et al., 2003). In the course of studies examining PtdIns(4,5)P2 function in Drosophila male-germ-cell cytokinesis, we depleted plasma membrane PtdIns(4,5)P2 by targeted expression of a lipid phosphatase (Wong et al., 2005). Here, we show that PtdIns(4,5)P2 depletion blocks sperm tail formation and causes defects in microtubule organization. In particular, PtdIns(4,5)P2 depletion reversibly affects basal body docking to the nuclear envelope, as well as axoneme architecture and integrity of the developing flagellar axoneme and axial sheath. Our results provide new evidence that phosphoinositides act at several steps during flagellar biogenesis, coordinately regulating microtubule and membrane organization. Because phosphoinositides and products of PtdIns(4,5)P2 hydrolysis are implicated in ciliary morphogenesis in vertebrate epithelial cells and embryos (Baranova et al., 1996; Sarmah et al., 2005; Vieira et al., 2006; Nachury et al., 2007), our findings suggest that phosphoinositides play evolutionarily conserved roles in flagella and cilia, across phyla and in structurally diverse cell types.
Results
Expression of SigD depletes PtdIns(4,5)P2 and causes accumulation of PtdIns(4)P
To identify roles for PtdIns(4,5)P2 in Drosophila spermatogenesis, we expressed the Salmonella phosphoinositide phosphatase SigD (Marcus et al., 2001) under control of the spermatocyte-specific β2-tubulin promoter (Hoyle and Raff, 1990; Wong et al., 2005). SigD dephosphorylates several phosphoinositides on the D-3, D-4 or D-5 positions of the inositol ring in vitro and depletes PtdIns(4,5)P2 in tissue-culture cells (Norris et al., 1998; Marcus et al., 2001; Terebiznik et al., 2002). To confirm that SigD expression depleted plasma membrane PtdIns(4,5)P2, we examined green fluorescent protein (GFP) fused to the pleckstrin homology (PH) domain of PLCδ (PLCδ-PH-GFP), a fluorescent marker that specifically binds PtdIns(4,5)P2 (Lemmon et al., 1995; Varnai and Balla, 1998). PLCδ-PH-GFP was primarily associated with the plasma membrane of control cells (Fig. 1B′) (Wong et al., 2005). By contrast, spermatocytes (not shown) and spermatids from SigD testes generally lacked detectible plasma membrane PtdIns(4,5)P2, although occasional bright spots of PLCδ-PH-GFP fluorescence were observed in the cytoplasm (Fig. 1C′).
To determine whether SigD acts as a 5-phosphatase in vivo (Fig. 1A), we used red fluorescent protein (RFP) fused to the PH domain of FAPP (RFP-PH-FAPP), which binds Golgi-associated PtdIns(4)P (Dowler et al., 2000; Godi et al., 2004). In control cells, PtdIns(4)P localized to the Golgi in spermatocytes (Fig. 1F′, arrowhead) and to the developing acroblast in spermatids (Fig. 1F′, arrow). Golgi and acroblast localization were confirmed by colocalization of RFP-PH-FAPP with a GFP fusion to the Drosophila Cog5 homolog Fws (Farkas et al., 2003) (supplementary material Fig. S1). In cells expressing SigD, PtdIns(4)P levels were increased, as evidenced by stronger Golgi localization of RFP-PH-FAPP in spermatocytes (Fig. 1G′, arrowhead) and enlarged structures containing PtdIns(4)P in spermatids (Fig. 1G′, arrow). The intensity ratio of the RFP-PH-FAPP signal to background in line scans of spermatocytes and spermatids was significantly different in SigD-expressing cells versus wild-type controls (P<0.0001; see Materials and Methods). Consistent with the apparent accumulation of PtdIns(4)P in SigD-expressing cells, co-expression of Sktl PtdIns(4)P 5-kinase, which employs PtdIns(4)P as a substrate, partially restored plasma membrane PtdIns(4,5)P2 (Fig. 1D′, arrow). SigD phosphatase activity was required for its effect on phosphoinositide levels, because expression of catalytically dead SigD (SigDdead) (Marcus et al., 2001) had no effect on either PtdIns(4,5)P2 (Fig. 1E′) or PtdIns(4)P (not shown). These results establish that targeted expression of SigD is a useful tool for examining PtdIns(4,5)P2 function during male-germ-cell development.
Phosphoinositides are required for sperm tail formation
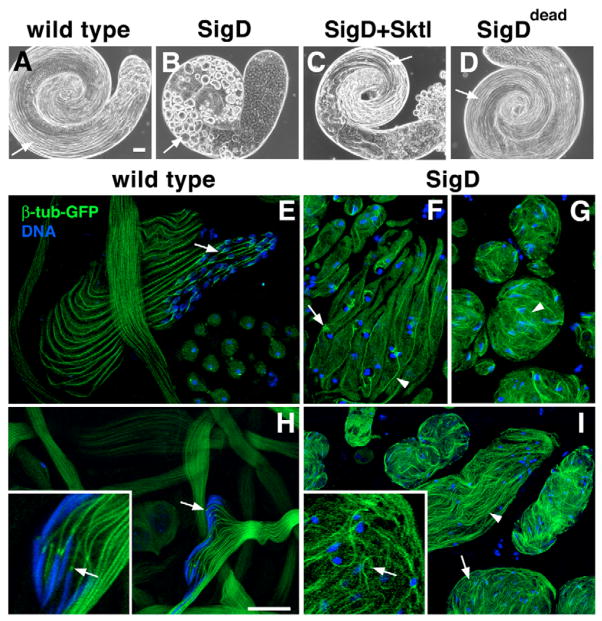
Transgenic lines depleted of PtdIns(4,5)P2 were male-sterile, indicating that SigD expression disrupted sperm development. To identify the stages of spermatogenesis affected by PtdIns(4,5)P2 depletion, we dissected and analyzed live-testis preparations by phase-contrast microscopy. PtdIns(4,5)P2 depletion caused defects in meiotic cytokinesis (Wong et al., 2005) and a dramatic block in sperm tail formation. In contrast to wild-type testes, which contained many long rope-like cysts of elongating or individualizing spermatids (Fig. 2A), SigD lines lacked elongating spermatids (Fig. 2B). Cysts from young SigD-expressing males were predominantly ovoid or circular in shape (Fig. 2B, arrow), whereas cysts from 3-day-old males were more elongated, appearing as tangled tubular structures (not shown). SigD catalytic activity was required for this effect on sperm tail formation, because testes from males expressing SigDdead were morphologically normal (Fig. 2D) and the males were fertile.
Fig. 2.
PtdIns(4,5)P2 depletion inhibits sperm tail formation and causes defects in microtubule organization. (A–D) Phase-contrast micrographs of whole Drosophila testes. (A) A wild-type testis has many rope-like cysts of elongating spermatids (arrow). (B) Expression of SigD causes the accumulation of ovoid spermatid cysts (arrow). (C) Co-expression of Sktl with SigD restores spermatid elongation (arrow). (D) Expression of SigDdead has no effect on sperm tail formation (arrow). (E–I) Confocal fluorescence micrographs of spermatids expressing β-tubulin-GFP (β-tub-GFP, green) and stained for DNA (blue). (E,H) Wild-type spermatids. (E) β-tub-GFP is incorporated into elongating axonemes and perinuclear forked microtubule (MT) arrays (arrow). (H) After individualization, β-tub-GFP is found in prominent perinuclear puncta (arrows) and axonemes (inset, magnified cyst). (F,G,I) SigD-expressing spermatids. (F) Early elongating cyst showing β-tub-GFP in axonemes (arrowhead) and large diffuse perinuclear MT arrays (arrow). (G) Cysts with tangled tails of β-tub-GFP (arrowhead). (I) Later-stage cysts show poorly aligned MTs (arrowhead) and puncta dissociated from nuclei (arrows) (inset, magnified cyst). Scale bars: 50 μm (A–D), 20 μm (E–I).
To determine whether the observed defect in sperm tail formation was a consequence of altered levels of PtdIns(4,5)P2 or PtdIns(4)P, we used two strategies to manipulate PtdIns(4)P or PtdIns(4,5)P2 levels in flies expressing SigD. First, we showed that mutations in fwd, which encodes a PtdIns 4-kinase required in spermatocytes for the accumulation of PtdIns(4)P (Fig. 1A) (H.-C.W., G.P. and J.A.B., unpublished) (Brill et al., 2000), failed to suppress the effects of SigD expression, suggesting that the block in sperm tail growth was not due to increased levels of PtdIns(4)P (not shown). Second, we co-expressed the PtdIns(4)P 5-kinase Sktl, which strongly suppressed the spermatid elongation defect caused by SigD (Fig. 2C), suggesting that PtdIns(4,5)P2 is required for spermatid elongation.
Phosphoinositides affect microtubule and basal body organization
Differentiating spermatids contain a variety of MT arrays, including MTs involved in nuclear shaping, cytoplasmic MTs that run parallel to the axoneme, and MTs of the axoneme itself (Tates, 1971; Tokuyasu, 1974). To determine whether PtdIns(4,5)P2 depletion caused defects in MT organization, we examined MTs in live testis preparations using β-tubulin-GFP (β-tub-GFP) (Inoue et al., 2004). In elongating spermatids, β-tub-GFP was incorporated into distinct MT arrays associated with developing sperm tails and haploid nuclei (Fig. 2E,H). In early stages of elongation, MTs formed a branched structure around each nucleus (Fig. 2E, arrow), and sperm tails were parallel and smooth. In late stages of sperm development, β-tub-GFP remained associated with the axoneme (H.-C.W. and J.A.B., unpublished) and was highly concentrated in a spot adjacent to the needle-shaped nucleus (Fig. 2H, arrows).
PtdIns(4,5)P2 depletion had a profound effect on MT organization (Fig. 2F,G,I). In cysts of elongating spermatids, branched structures still formed but were diffuse and loosely associated with the nuclei (Fig. 2F, arrow). Moreover, the apparent increase in background GFP suggested cytoplasmic accumulation of unassembled or aggregated tubulin. MTs were generally disorganized and tangled, even in the more elongated cysts (Fig. 2G,I, arrowheads). In addition, perinuclear foci of β-tub-GFP were not evident (Fig. 2I, arrows) and nuclei, which were scattered throughout the cyst, remained minimally elongated.
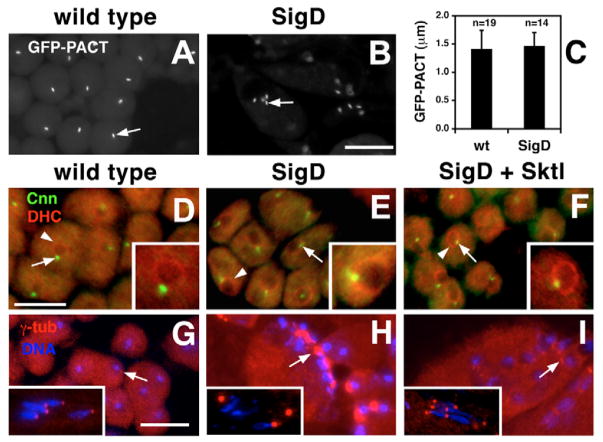
To determine whether the aberrant MT organization in PtdIns(4,5)P2-depleted spermatids was due to defects in basal body architecture, we examined the distribution of core and peripheral basal-body-associated proteins. The core basal body proteins PACT and Sas-4 formed short perinuclear rods in all spermatids examined (GFP-PACT, Fig. 3A,B; Sas-4-GFP, not shown) (Basto et al., 2006), suggesting that the core structure of the basal body was unaffected by expression of SigD. Indeed, there was no significant difference in the length of the GFP-PACT signal in wild-type versus PtdIns(4,5)P2-depleted spermatids (Fig. 3C).
Fig. 3.
PtdIns(4,5)P2 depletion affects peripheral basal body proteins. (A–C) Analysis of the core basal body protein GFP-PACT. (A,B) Fluorescence micrographs of (A) wild-type and (B) SigD-expressing spermatids, showing the morphology of GFP-PACT-containing basal bodies (arrows). Note that some SigD-expressing cells are multinucleate and contain four basal bodies (B, arrow). (C) Analysis of the length of the GFP-PACT signal in wild-type (wt) versus PtdIns(4,5)P2-depleted (SigD) spermatids indicates no significant difference in the length of GFP-PACT-containing basal bodies (P=0.0957). (D–F) Fluorescence micrographs of spermatids stained for the basal-body-associated protein Centrosomin (Cnn, green, arrows) and dynein heavy chain (DHC, red, arrowheads). (D) In wild-type spermatids, Cnn localizes to puncta that are tightly associated with DHC in the nuclear envelope (arrow and arrowhead). (E) In SigD-expressing spermatids, Cnn localizes to diffuse comet-shaped structures that are not always tightly associated with the nuclear envelope (arrow). DHC localization appears normal (arrowhead). (F) Spermatids co-expressing SigD and Sktl exhibit normal DHC and Cnn localization. (G–I) Spermatids stained for γ-tubulin (γ-tub, red) and DNA (blue). (G) Early and late (inset) wild-type spermatids. Note that early spermatids have barely discernible γ-tub foci (arrow), whereas late spermatids exhibit prominent γ-tub puncta that closely associate with nuclei (one per nucleus). (H) Early and late (inset) SigD-expressing spermatids show aberrant γ-tub accumulation (arrow) and loose association of γ-tub and nuclei (inset). (I) Early and late (inset) spermatids expressing SigD and Sktl show restored γ-tub localization (arrow). Scale bars, 10 μm (A,B,D–F), 20 μm (G–I).
In contrast to the core proteins, the more peripheral basal-body-associated proteins Cnn and γ-tubulin (Li et al., 1998; Sampaio et al., 2001) were mislocalized, as indicated by indirect immunofluorescence experiments. In wild-type spermatids, Cnn localized to a spot tightly associated with dynein heavy chain (DHC), which marks the nuclear envelope (Fig. 3D). Similarly, γ-tubulin was present in a small focus adjacent to the nuclear DNA (Fig. 3G). In PtdIns(4,5)P2-depleted spermatids, Cnn localized to diffuse, comet-shaped structures (Fig. 3E, inset) and its association with DHC in the nuclear envelope was occasionally disrupted. Large accumulations of γ-tubulin were found near the nuclei in PtdIns(4,5)P2-depleted spermatids (Fig. 3H) and, in older cysts, basal bodies were not always found near nuclear DNA (Fig. 3G,H, compare insets).
SigD expression specifically affected the distribution of peripheral basal body proteins in post-meiotic spermatids, because depletion of PtdIns(4,5)P2 had no obvious effect on localization of Cnn and γ-tubulin at centrosomes in primary spermatocytes (not shown). Furthermore, co-expression of Sktl largely rescued the defects in basal body Cnn and γ-tubulin localization caused by expression of SigD (Fig. 3F,I), confirming that these effects were due to altered levels of phosphoinositides.
Phosphoinositides are required for axoneme outgrowth and architecture
To determine whether the defects we observed in MT distribution corresponded to defects in axoneme assembly, we examined the distribution of Unc, a protein required for proper axonemal architecture (Baker et al., 2004), and found that depletion of PtdIns(4,5)P2 dramatically influenced the distribution of Unc-GFP. Unc-GFP was previously reported to localize only to the basal body (Fig. 4A, arrows) (Baker et al., 2004). However, we found that it was also concentrated at the distal end of each elongating spermatid (Fig. 4A, arrowhead). The morphology of the two ends was distinct, with basal body Unc-GFP forming an elongated cylinder (Fig. 4B′, arrow) and distal Unc-GFP appearing as a prominent spot (Fig. 4D′, arrowhead in inset). Strikingly, the distance between the two strong Unc-GFP signals correlated with spermatid length, consistent with its role in axoneme assembly (Fig. 4D′, inset, and Fig. 4F′).
Fig. 4.
PtdIns(4,5)P2 depletion affects axoneme outgrowth. (A) Diagrams showing Unc-GFP (green) localization in developing spermatids. (i) Unc-GFP is found at the basal body in early round spermatids (arrow). Nucleus (white circle) and mitochondrial derivatives (black circle), which are wrapped together at this stage, are shown. (ii–iv) Elongating spermatids. (ii) Protein body stage. (iii) Later stage of elongation. (iv) Late stage of elongation showing nuclear shaping. During elongation, Unc-GFP is found at the basal body (iv, arrow) and at the elongating end (iv, arrowhead). Not to scale. (B–H′) Phase-contrast (B–H) and corresponding fluorescence (B′–H′) micrographs of spermatids expressing Unc-GFP (green) and stained for DNA (blue). (B,D,F) Wild-type spermatids. (B′) Unc-GFP (arrow) is present at the basal body of early round spermatids. (D′) As spermatids elongate, Unc-GFP signals split into two parts, a cylindrical structure at the basal body (arrows) and a prominent dot associated with the growing end (arrowheads). In addition, a weak Unc-GFP signal can be seen along the entire length of the elongating sperm tail (inset, between arrow and arrowhead). (F′) In later stages, Unc-GFP signals are clearly observed at opposite ends of the elongating cyst [arrow, nuclei (mostly on a different focal plane); arrowhead, growing end]. Note that the contrast of this image was increased by 30% to make the localization of Unc-GFP more obvious. (C,E,G,H) Spermatids expressing SigD. (C′) Unc-GFP localization appears comet-shaped (arrow) in SigD-expressing spermatids. Association of Unc-GFP with nuclear DNA is also disrupted. (E′) In a rare elongated SigD-expressing cyst, Unc-GFP splits into two parts (arrow, arrowhead), and a weak signal along the sperm tail can be seen. (G′) SigD cyst at the protein body stage. Note the failure of Unc-GFP to split into two signals. (H′) Later-stage cyst with Unc-GFP signals scattered in the cytoplasm. Scale bars: 10 μm (B,C,G), 20 μm (D,E), 50 μm (F,H). Inset in D is approximately the same scale as G.
Although, Unc-GFP was present at the basal body in early PtdIns(4,5)P2-depleted spermatids, its distribution was abnormally elongated and appeared comet-shaped rather than cylindrical (Fig. 4C′, arrow). In addition, association of Unc-GFP with the nucleus was often disrupted (Fig. 4C′,G′). In most cysts, distal spots of Unc-GFP were not identifiable at the protein body stage (Fig. 4Aii), when axoneme assembly normally would have begun (compare Fig. 4D′, inset, with Fig. 4G′). In less-affected cysts, spermatid elongation proceeded further and Unc-GFP was present at both the basal body and the distal end (Fig. 4E′). However, even in these mildly affected cysts, association of Unc-GFP with the nucleus appeared defective. The aberrant Unc-GFP localization suggested a defect in axoneme outgrowth in spermatids with altered phosphoinositide levels.
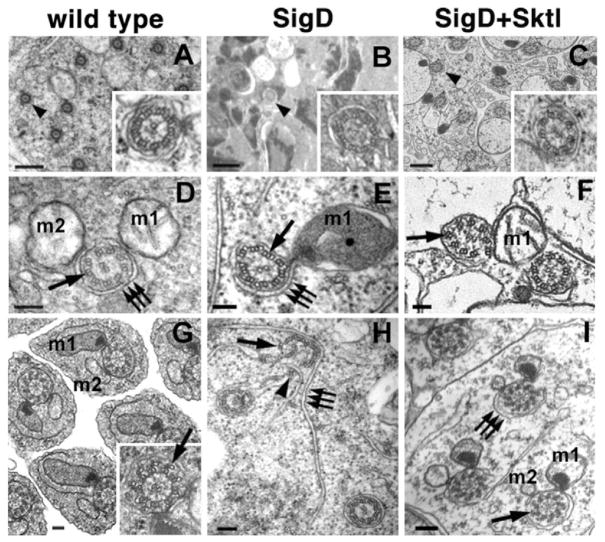
To examine axonemes at an ultrastructural level, we used TEM. Sections through the central portion of wild-type testes revealed axonemes organized in parallel (Fig. 5A). Each 9 + 2 axoneme contained nine outer doublet MTs and a central pair, surrounded by an axial sheath (Fig. 5A, inset and Fig. 5D) and accompanied by two mitochondrial derivatives (Fig. 5D,G). More-mature axonemes contained accessory MTs rotationally and centripetally displaced from the doublet MTs (Fig. 5G, arrow).
Fig. 5.
PtdIns(4,5)P2 depletion affects axoneme architecture. (A–C) Transmission electron micrographs (TEMs) showing the distribution of axonemes (arrowheads) in spermatid cysts. (A) Wild-type cysts have many axonemes with a regular 9 + 2 arrangement (inset, magnified axoneme). (B) Spermatid cysts expressing SigD have few axonemes, and these axonemes have aberrant MT organization (inset). (C) Co-expression of Sktl with SigD restores axonemes of normal architecture (inset). (D–I) TEMs showing axonemal architecture and axial membranes. Major (m1) and minor (m2) mitochondrial derivatives are indicated. (D,G) Wild-type axonemes. (D) Wild-type spermatids have doublet MTs (arrow) surrounded by axial membranes (triple arrows) adjacent to the two mitochondrial derivatives. (G) Axonemes from later-stage spermatids develop accessory MTs that are centripetally displaced from the outer doublet MTs (inset, arrow). (E,H) Axonemes from SigD-expressing spermatids. (E) Note the presence of triplet MTs (arrow). (H) Axial membranes (triple arrows) fail to ensheath axonemes that are falling apart (arrowhead). No SigD axonemes with accessory MTs were observed. (F,I) Co-expression of Sktl with SigD restored axonemal doublets and accessory MTs (arrow, I); however, occasional defects in doublet orientation were observed (arrow, F). Scale bars: 500 nm (A–C), 100 nm (D–I).
PtdIns(4,5)P2-depleted flies exhibited striking defects in the number and organization of axonemes. Sections through testes expressing SigD revealed few axonemes (Fig. 5B), and these were often oriented in different directions (not shown). The few axonemes observed contained triplet as well as doublet MTs (Fig. 5E,H, arrows), and more-mature axonemes with accessory tubules were not observed. In addition, ensheathment of the axoneme by the axial membrane was often compromised, and the sheath was typically dissociated from the mitochondrial derivatives. In cases in which the morphology of the sheath was most aberrant (Fig. 5H, triplet arrows), the axonemes were splayed and mislocalized MT doublets were occasionally found associated with extensions of the axial membranes (Fig. 5H, arrowheads).
Co-expression of Sktl with SigD rescued axoneme assembly and restored the stability of the ensheathed structure (Fig. 5C,F,I). Sktl co-expression restored morphologically normal accessory tubules and axial sheaths (Fig. 5I), although the orientation of the doublet MTs was occasionally disrupted (Fig. 5F, arrow). Thus, formation of outer doublet MTs, axoneme ensheathment by the axial membrane and axoneme outgrowth require proper levels of PtdIns(4,5)P2.
Phosphoinositides are required for basal body docking to the nuclear envelope
In addition to axoneme architecture, our TEM studies of PtdIns(4,5)P2-depleted spermatids also identified basal body docking as a phosphoinositide-dependent step. In wild-type early spermatids (Tates, 1971), the basal body penetrated through the outer nuclear membrane and attached perpendicular to the surface of the nuclear envelope (Fig. 6A). In older spermatids, an electron-dense centriolar adjunct formed a collar around the basal body (Fig. 6B,C). In PtdIns(4,5)P2-depleted spermatids, the overall morphology of the basal body and centriolar adjunct appeared fairly normal (Fig. 6E–G). Strikingly, however, basal bodies were typically mislocalized, often lying tangential to the nuclear envelope (Fig. 6E,F). Axonemes were not observed in spermatids at the protein body stage (Fig. 6F), suggesting a delay in axoneme assembly. In the few late spermatids in which axonemes were observed, axonemal architecture appeared superficially normal, in that a regular pattern of axonemal dynein arms was present along the length of the axoneme. However, in contrast to wild type (Fig. 6D), the basal body was not embedded in the nuclear envelope (Fig. 6H). Co-expression of Sktl with SigD largely restored orientation of the basal body with respect to the nuclear envelope as well as basal body docking, although nuclear morphology remained somewhat aberrant (Fig. 6I,J), and not all basal bodies were associated with a nucleus (Fig. 6K). Thus, the levels of phosphoinositides are crucial for basal body docking at the nuclear envelope and for the normal timing of axoneme outgrowth.
Fig. 6.
PtdIns(4,5)P2 depletion interferes with basal body docking to the nuclear envelope. (A–K) TEMs showing developing basal bodies from spermatids at different stages of development. Nuclei (n) and basal bodies (arrows) are indicated. (A–D) Wild-type spermatids. (A) Longitudinal section through an early-stage spermatid showing attachment of the basal body to the nuclear envelope. (B) Longitudinal section through the nucleus, basal body and centriolar adjunct of an intermediate-stage spermatid. (C) Cross-section through an intermediate-stage spermatid showing the position of the centriole and centriolar adjunct with respect to the two mitochondrial derivatives (m1, m2). (D) Longitudinal section through the nucleus and basal body region of an advanced spermatid. (E–H) Spermatids from testes expressing SigD. (E,F) Longitudinal sections of early-stage SigD-expressing spermatids showing defects in orientation and attachment of the basal body and the centriolar adjunct to the nuclear envelope. (G) Cross-section through an intermediate-stage SigD-expressing spermatid showing dissociation of the basal body and centriolar adjunct from the nuclear envelope. (H) Basal body, enlarged centriolar adjunct and axoneme from a later-stage SigD-expressing spermatid. (I–K) Axonemes from testes co-expressing SigD and Sktl. (I) Longitudinal section through the nucleus and the centriolar adjunct of an intermediate-stage spermatid showing proper orientation of the basal body with respect to the nuclear envelope. (J) Longitudinal section through the nucleus and basal body region of an advanced spermatid showing the basal body embedded in the nuclear envelope. (K) Longitudinal section showing a basal body and corresponding axoneme not embedded in the nuclear membrane. Scale bars: 500 nm.
Discussion
Here, using a novel approach to deplete plasma membrane PtdIns(4,5)P2 in vivo, we demonstrate a requirement for PtdIns(4,5)P2 – or the correct balance of PtdIns(4,5)P2 and PtdIns(4)P – in flagellar biogenesis. Previous studies have implicated phosphoinositides in ciliary biogenesis. For example, PtdIns(4,5)P2 production is correlated with MT assembly on bovine retinal rod axonemes (Baranova et al., 1996) and with the formation of apical membranes, the site of cilia formation, in polarized MDCK cells (Martin-Belmonte et al., 2007). The PtdIns(4)P-binding protein Fapp2 was recently shown to establish a membrane domain required for ciliogenesis in MDCK cells (Vieira et al., 2006). Bbs5, a member of a protein complex (the BBS complex) involved in ciliary membrane formation, binds PtdIns(3)P in vitro (Nachury et al., 2007). Increased expression of a PtdIns 4-kinase and reduced levels of PtdIns(4,5)P2 are found in rodent models of polycystic kidney disease, a ciliary disease (Cuozzo et al., 2002). In addition, a recent report implicated Ipk1, the enzyme that synthesizes InsP6 [a metabolite downstream of PtdIns(4,5)P2 hydrolysis and InsP3] in ciliary function in zebrafish (Sarmah et al., 2005). Although these studies hint at roles for phosphoinositides in ciliary assembly or function, a requirement for PtdIns(4,5)P2 – or any other phosphoinositide – in ciliogenesis has never been established.
To study PtdIns(4,5)P2 function in vivo, we exploited the Salmonella lipid phosphatase SigD to deplete plasma membrane PtdIns(4,5)P2 in developing Drosophila male germ cells. Because the defects of the basal body and axoneme were rescued by co-expression of Sktl with SigD, alterations in PtdIns(4)P or PtdIns(4,5)P2 levels, or both, must account for the observed phenotypes. Mutations in Drosophila fwd cause defects in the accumulation of Golgi PtdIns(4)P and in cytokinesis (H.-C.W., unpublished) (Brill et al., 2000), yet fwd is not required for normal axoneme assembly (M.H., L.F. and J.A.B., unpublished). In addition, mutations in fwd failed to suppress the sperm tail formation defects caused by expression of SigD, suggesting that excess PtdIns(4)P is not responsible for the observed phenotypes.
Thus, PtdIns(4,5)P2 appears to be required in developing Drosophila sperm for basal body docking at the nuclear envelope, flagellar outgrowth and proper axoneme architecture. PtdIns(4,5)P2 itself could serve as an important signal in flagellar biogenesis. Although axoneme assembly is templated from the basal body, this process actually initiates prior to meiosis. In primary spermatocytes, centrioles migrate to the surface of the cell, where they protrude from the plasma membrane and develop short axonemes (Tates, 1971; Baker et al., 2004). These centrioles have a membrane cap that covers the distal (elongating) end of the axoneme. It is possible that PtdIns(4,5)P2 from the plasma membrane is incorporated into this cap, where it might provide a signal for the binding of proteins involved in basal body docking or initiation of flagellar axoneme outgrowth.
PtdIns(4,5)P2 is also upstream of the second messengers DAG, Ca2+ and the inositol polyphosphates (InsP3, InsP4, InsP5 and InsP6). By analogy to other systems, Ca2+ could bind to a centrin-like protein that links the basal body to the nuclear envelope; inositol polyphosphates might regulate flagellar assembly; and DAG, a lipid known to affect membrane curvature (Allan et al., 1978), might be required to promote ensheathment of the developing axoneme by the axial membrane.
The effect of PtdIns(4,5)P2 depletion on axonemal MTs is particularly intriguing, because the defects observed suggest a possible role for PtdIns(4,5)P2 in regulating the transition from basal body to axonemal MT arrays. PtdIns(4,5)P2 or a downstream second messenger could influence the stability or post-translational modification of particular tubulin isoforms, which in turn might affect the structure of the MT arrays incorporated into the axoneme. In support of this idea, SigD expression reduces the abundance of a slower-migrating form of acetylated α-tubulin (as determined by immunoblotting; H.-C.W., unpublished). Alternatively, because the triplet MTs found in PtdIns(4,5)P2-depleted spermatids resemble basal body MT triplets, PtdIns(4,5)P2 or a downstream metabolite might provide a signal that inhibits triplet MT formation in favor of assembly of doublet MTs and accessory tubules. Because formation of outer MT doublets is crucial for both ciliary and flagellar motility, understanding the mechanism of regulation of this transition will be an important area for future investigation.
Materials and Methods
Molecular biology
All transgenes were constructed using the testis vector tv3, a P-element transformation vector containing the spermatocyte-specific β2-tubulin (β2t) promoter and SV40 3′ sequences (Wong et al., 2005). RFP-PH-FAPP, a fusion of monomeric red fluorescent protein (mRFP1) (Campbell et al., 2002) to the N-terminus of the FAPP PH domain (Dowler et al., 2000), was made by fusion PCR using plasmids containing mRFP1 (from R. Tsien, University of California, San Diego, CA) and YFP-PH-FAPP (from D. Alessi, University of Dundee, Dundee, UK) as templates. PLCδ-PH-RFP was made by replacing GFP in PLCδ-PH-GFP (Varnai and Balla, 1998) with mRFP1. A catalytically inactive version of SigD, SigDdead, was obtained by mutagenizing SigD with the QuikChange XL site-directed mutagenesis kit (Stratagene) to introduce a previously characterized inactivating mutation (C460S). YFP-Sktl was made by fusing EYFP (Clontech) to the N-terminus of Sktl. All constructs were confirmed by DNA sequencing (The Centre for Applied Genomics, SickKids). Details of molecular cloning (primers, restriction sites, sequences) will be provided upon request.
Fly stocks
Flies were raised on standard cornmeal molasses agar at 25°C (Ashburner, 1990). Transgenes were introduced by injection of w1118 embryos as described (Wong et al., 2005). Lines expressing PLCδ-PH-GFP [which binds PtdIns(4,5)P2] and SigD were previously described (Wong et al., 2005). SigD lines were maintained by crossing transgenic (w+) females to w1118 males. Unc-GFP flies were from J. Baker and M. Kernan (Baker et al., 2004). β-tubulin-EGFP flies were from Y. Akiyama-Oda and H. Oda (JT Biohistory Research Hall, Takatsuki City, Japan) (Inoue et al., 2004). Fws-GFP flies were from M. Fuller (Farkas et al., 2003). PACT-GFP flies were from J. Raff (Basto et al., 2006). w1118 flies were used as wild-type controls.
Fluorescence microscopy and imaging
Immunofluorescence was performed essentially as described (Hime et al., 1996), except that testis isolation buffer (TIB) (Casal et al., 1990) was used instead of TB-1. Antibodies used were as follows: 1:250 rabbit anti-dynein-heavy-chain (a gift of T. Hays, University of Minnesota, Minneapolis, MN) (Hays et al., 1994; Li et al., 1998); 1:1000 anti-Centrosomin (a gift of T. Kaufman, Indiana University, Bloomington, IN) (Li et al., 1998). For γ-tubulin, samples were fixed in methanol-acetone (Bonaccorsi et al., 2000) and anti-γ-tubulin antibody GTU88 (Sigma) was used at a dilution of 1:1000. Rhodamine phalloidin (20 units/ml) was used as recommended by the manufacturer (Molecular Probes).
To image live squashed preparations of Drosophila male germ cells expressing fluorescent fusion proteins (PLCδ-PH-GFP, RFP-PH-FAPP, GFP-PACT, Unc-GFP), testes were dissected in TIB, transferred to a microscope slide and cut with tungsten needles in TIB containing 8.3 μg/ml Hoechst 33342 to stain DNA. Samples were squashed with a coverslip before viewing.
The difference in RFP-PH-FAPP localization to the Golgi in wild-type versus SigD-expressing cells was calculated from line scans using ImageJ (http://rsb.info.nih.gov/ij/). The minimum intensity along each line scan, corresponding to the background, was subtracted from the maximum intensity, corresponding to the Golgi localization. For each phenotype, 83 measurements were made on cells from different preparations. The values were imported in GraphPad Prism and the P value was calculated (P<0.0001).
To compare the lengths of centrioles in control and SigD-expressing spermatids, the lengths of the GFP-PACT signals were measured as previously described (Rodrigues-Martins et al., 2007), except that measurements were performed on spermatids rather than spermatocytes. To ensure our measurements reflected the entire length of the centriole, we used only centrioles that were in focus along their entire length.
Fluorescence images were acquired with an Axiocam CCD camera on an upright Zeiss Axioplan 2 microscope using Axiovision software. Specimens of testes expressing β-tubulin-EGFP were imaged with a Zeiss inverted LSM510 confocal microscope. Images of separate fluorochromes from multiply stained tissues were collected individually and combined using Adobe Photoshop. When necessary, images were adjusted only for brightness and contrast. Unless otherwise stated, in cases in which direct comparison of images was required, images were acquired using identical exposure times and adjusted in an identical manner.
Electron microscopy
Ultrastructural analysis of testes from 0- to 1-day-old males was carried out as previously described (Bazinet and Rollins, 2003). Sections were viewed with a JEOL JEM 1200EX transmission electron microscope (St John’s University), a JEOL JTE 141011 microscope (SickKids) or a Technai microscope (SickKids/Mt Sinai Hospital). Images were captured on film and scanned at high resolution (St John’s University), or obtained using digital acquisition software (SickKids/Mt Sinai Hospital). Images were manipulated (for brightness and contrast only) using Adobe Photoshop.
Acknowledgments
The authors wish to thank Sergio Grinstein, John Ashkenas and Monica Bettencourt-Dias for helpful discussions; John Ashkenas, Chi-Chung Hui and Helen McNeill for comments on the manuscript; Alla Buzina for Russian translation; Yew Meng Heng and Robert Temkin for assistance with TEM; Dario Alessi, Tamas Balla, Hugo Bellen, Brett Finlay, Sergio Grinstein, Sandra Marcus and Roger Tsien for plasmids; James Baker, Maurice Kernan, Hiroki Oda, Yasuko Akiyama-Oda, Margaret Fuller and Jordan Raff for flies; and Tom Hays and Thomas Kaufman for antibodies. The authors declare no competing interests. The authors are grateful for funding from SickKids Restracomp (postdoctoral fellowship to H.-C.W.), NSERC (postdoctoral fellowship to L.F.), NIH (#R15 HD043885-01A1 to C.B.), The Terry Fox Foundation (NCIC #016425 to J.A.B.) and CIHR (to J.A.B.).
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/121/7/1076/DC1
References
- Allan D, Thomas P, Michell RH. Rapid transbilayer diffusion of 1,2-diacylglycerol and its relevance to control of membrane curvature. Nature. 1978;276:289–290. doi: 10.1038/276289a0. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Drosophila: A Laboratory Handbook. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Baker JD, Adhikarakunnathu S, Kernan MJ. Mechanosensory-defective, male-sterile unc mutants identify a novel basal body protein required for ciliogenesis in Drosophila. Development. 2004;131:3411–3422. doi: 10.1242/dev.01229. [DOI] [PubMed] [Google Scholar]
- Baranova LA, Grits AN, Volotovskii ID. Phosphorylation of proteins and phosphoinositides in the axoneme of the bovine rod outer segments. The effect of structural factor. Biofizika. 1996;41:1258–1263. [PubMed] [Google Scholar]
- Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW. Flies without centrioles. Cell. 2006;125:1375–1386. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Bateman JM, McNeill H. Temporal control of differentiation by the insulin receptor/tor pathway in Drosophila. Cell. 2004;119:87–96. doi: 10.1016/j.cell.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Bazinet C, Rollins JE. Rickettsia-like mitochondrial motility in Drosophila spermiogenesis. Evol Dev. 2003;5:379–385. doi: 10.1046/j.1525-142x.2003.03045.x. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–4143. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi S, Giansanti MG, Cenci G, Gatti M. Cytological analysis of spermatocyte growth and male meiosis in Drosophila melanogaster. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 87–109. [Google Scholar]
- Brill JA, Hime GR, Scharer-Schuksz M, Fuller MT. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development. 2000;127:3855–3864. doi: 10.1242/dev.127.17.3855. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. A monomeric red fluorescent protein. Proc Natl Acad Sci USA. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J, Gonzalez C, Ripoll P. Spindles and centrosomes during male meiosis in Drosophila melanogaster. Eur J Cell Biol. 1990;51:38–44. [PubMed] [Google Scholar]
- Cuozzo FP, Mishra S, Jiang J, Aukema HM. Overexpression of kidney phosphatidylinositol 4-kinase β and phospholipase C γ1 proteins in two rodent models of polycystic kidney disease. Biochim Biophys Acta. 2002;1587:99–106. doi: 10.1016/s0925-4439(02)00072-8. [DOI] [PubMed] [Google Scholar]
- Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol. 2005;289:F1159–F1169. doi: 10.1152/ajprenal.00118.2005. [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Godi A. PI-loting membrane traffic. Nat Cell Biol. 2004;6:487–492. doi: 10.1038/ncb0604-487. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Dowler S, Currie RA, Campbell DG, Deak M, Kular G, Downes CP, Alessi DR. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem J. 2000;351:19–31. doi: 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas RM, Giansanti MG, Gatti M, Fuller MT. The Drosophila Cog5 homologue is required for cytokinesis, cell elongation, and assembly of specialized Golgi architecture during spermatogenesis. Mol Biol Cell. 2003;14:190–200. doi: 10.1091/mbc.E02-06-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MT. Spermatogenesis. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1993. pp. 71–147. [Google Scholar]
- Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol. 2004;6:393–404. doi: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- Green LL, Wolf N, McDonald KL, Fuller MT. Two types of genetic interaction implicate the whirligig gene of Drosophila melanogaster in microtubule organization in the flagellar axoneme. Genetics. 1990;126:961–973. doi: 10.1093/genetics/126.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan BA, Prokopenko SN, Breuer S, Zhang B, Paululat A, Bellen HJ. Skittles, a Drosophila phosphatidylinositol 4-phosphate 5-kinase, is required for cell viability, germline development and bristle morphology, but not for neurotransmitter release. Genetics. 1998;150:1527–1537. doi: 10.1093/genetics/150.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays TS, Porter ME, McGrail M, Grissom P, Gosch P, Fuller MT, McIntosh JR. A cytoplasmic dynein motor in Drosophila: identification and localization during embryogenesis. J Cell Sci. 1994;107:1557–1569. doi: 10.1242/jcs.107.6.1557. [DOI] [PubMed] [Google Scholar]
- Hime GR, Brill JA, Fuller MT. Assembly of ring canals in the male germ line from structural components of the contractile ring. J Cell Sci. 1996;109:2779–2788. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- Hoyle HD, Raff EC. Two Drosophila β tubulin isoforms are not functionally equivalent. J Cell Biol. 1990;111:1009–1026. doi: 10.1083/jcb.111.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens JA, Hoyle HD, Turner FR, Raff EC. Structurally similar Drosophila α-tubulins are functionally distinct in vivo. Mol Biol Cell. 1997;8:481–500. doi: 10.1091/mbc.8.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue YH, Savoian MS, Suzuki T, Mathe E, Yamamoto MT, Glover DM. Mutations in orbit/mast reveal that the central spindle is comprised of two microtubule populations, those that initiate cleavage and those that propagate furrow ingression. J Cell Biol. 2004;166:49–60. doi: 10.1083/jcb.200402052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetopoulos C, Devreotes P. Phosphoinositide signaling plays a key role in cytokinesis. J Cell Biol. 2006;174:485–490. doi: 10.1083/jcb.200603156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers SJ, Weinkove D, MacDougall LK, Hafen E, Waterfield MD. The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 1996;15:6584–6594. [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, O’Brien R, Sigler PB, Schlessinger J. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc Natl Acad Sci USA. 1995;92:10472–10476. doi: 10.1073/pnas.92.23.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Xu EY, Cecil JK, Turner FR, Megraw TL, Kaufman TC. Drosophila centrosomin protein is required for male meiosis and assembly of the flagellar axoneme. J Cell Biol. 1998;141:455–467. doi: 10.1083/jcb.141.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MG, Serr M, Newman EA, Hays TS. The Drosophila tctex-1 light chain is dispensable for essential cytoplasmic dynein functions but is required during spermatid differentiation. Mol Biol Cell. 2004;15:3005–3014. doi: 10.1091/mbc.E04-01-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manandhar G, Simerly C, Salisbury JL, Schatten G. Centriole and centrin degeneration during mouse spermiogenesis. Cell Motil Cytoskeleton. 1999;43:137–144. doi: 10.1002/(SICI)1097-0169(1999)43:2<137::AID-CM5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Marcus SL, Wenk MR, Steele-Mortimer O, Finlay BB. A synaptojanin-homologous region of Salmonella typhimurium SigD is essential for inositol phosphatase activity and Akt activation. FEBS Lett. 2001;494:201–207. doi: 10.1016/s0014-5793(01)02356-0. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Norris FA, Wilson MP, Wallis TS, Galyov EE, Majerus PW. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc Natl Acad Sci USA. 1998;95:14057–14059. doi: 10.1073/pnas.95.24.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot F, Philippe JM, Lemmers C, Lecuit T. Spatial control of actin organization at adherens junctions by a synaptotagmin-like protein Btsz. Nature. 2006;442:580–584. doi: 10.1038/nature04935. [DOI] [PubMed] [Google Scholar]
- Pinal N, Goberdhan DC, Collinson L, Fujita Y, Cox IM, Wilson C, Pichaud F. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr Biol. 2006;16:140–149. doi: 10.1016/j.cub.2005.11.068. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Martins A, Bettencourt-Dias M, Riparbelli M, Ferreira C, Ferreira I, Callaini G, Glover DM. DSAS-6 organizes a tube-like centriole precursor, and Its absence suggests modularity in centriole assembly. Curr Biol. 2007;17:1465–1472. doi: 10.1016/j.cub.2007.07.034. [DOI] [PubMed] [Google Scholar]
- Roth MG. Phosphoinositides in constitutive membrane traffic. Physiol Rev. 2004;84:699–730. doi: 10.1152/physrev.00033.2003. [DOI] [PubMed] [Google Scholar]
- Salisbury JL. Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol. 1995;7:39–45. doi: 10.1016/0955-0674(95)80043-3. [DOI] [PubMed] [Google Scholar]
- Sampaio P, Rebollo E, Varmark H, Sunkel CE, Gonzalez C. Organized microtubule arrays in γ-tubulin-depleted Drosophila spermatocytes. Curr Biol. 2001;11:1788–1793. doi: 10.1016/s0960-9822(01)00561-9. [DOI] [PubMed] [Google Scholar]
- Sarmah B, Latimer AJ, Appel B, Wente SR. Inositol polyphosphates regulate zebrafish left-right asymmetry. Dev Cell. 2005;9:133–145. doi: 10.1016/j.devcel.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Tates AD. Cytodifferentiation during spermatogenesis in Drosophila melanogaster: an electron microscope study. S-Gravenhage: Drukkerij J. H. Pasmans; 1971. [Google Scholar]
- Terebiznik MR, Vieira OV, Marcus SL, Slade A, Yip CM, Trimble WS, Meyer T, Finlay BB, Grinstein S. Elimination of host cell PtdIns(4,5)P2 by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol. 2002;4:766–773. doi: 10.1038/ncb854. [DOI] [PubMed] [Google Scholar]
- Tokuyasu KT. Dynamics of spermiogenesis in Drosophila melanogaster. IV Nuclear transformation. J Ultrastruct Res. 1974;48:284–303. doi: 10.1016/s0022-5320(74)80083-3. [DOI] [PubMed] [Google Scholar]
- Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium-and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt N, Koch I, Schwarz H, Schnorrer F, Nusslein-Volhard C. The γTuRC components Grip75 and Grip128 have an essential microtubule-anchoring function in the Drosophila germline. Development. 2006;133:3963–3972. doi: 10.1242/dev.02570. [DOI] [PubMed] [Google Scholar]
- Wei HC, Sanny J, Shu H, Baillie DL, Brill JA, Price JV, Harden N. The Sac1 lipid phosphatase regulates cell shape change and the JNK cascade during dorsal closure in Drosophila. Curr Biol. 2003;13:1882–1887. doi: 10.1016/j.cub.2003.09.056. [DOI] [PubMed] [Google Scholar]
- Wong R, Hadjiyanni I, Wei HC, Polevoy G, McBride R, Sem KP, Brill JA. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr Biol. 2005;15:1401–1406. doi: 10.1016/j.cub.2005.06.060. [DOI] [PubMed] [Google Scholar]
- Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Matthies HJ, Mancuso J, Andrews HK, Woodruff E, 3rd, Friedman D, Broadie K. The Drosophila fragile X-related gene regulates axoneme differentiation during spermatogenesis. Dev Biol. 2004;270:290–307. doi: 10.1016/j.ydbio.2004.02.010. [DOI] [PubMed] [Google Scholar]