Abstract
Multivalency is a common principle in the recognition of cellular receptors, and multivalent agonists and antagonists have played a major role in understanding mammalian cell receptor biology. The study of bacterial cell receptors using similar approaches, however, has lagged behind. Herein we describe our efforts toward the development of a dendrimer-based multivalent probe for studying AI-2 quorum sensing receptors. From these studies, we have discovered a chemical probe specific for Lsr-type AI-2 quorum sensing receptors with the potential for enabling the identification of new bacterial species that utilize AI-2 as a quorum sensing signaling molecule.
Multivalency is a powerful underlying logic in biological recognition.1–3 In chemical biology, multivalent ligands have been widely used to antagonize cellular receptors.1–3 To employ such techniques, researchers often rely on polymeric scaffolds that are able to display multiple copies of a ligand or even multiple ligands.1–4 In particular, the use of dendrimers, a class of monodisperse polymers, in drug-, antibody- and peptide-delivery systems has rapidly expanded within recent years due to the favorable properties that dendrimers possess (biocompatible, nonimmunogenic, nonmutagenic, nontoxic, water-soluble).5–9 Additionally, decoration of the termini of dendrimers with targeting, solubilizing and imaging groups has also been reported.7–10 With respect to imaging, while many dendritic probes have been used to study mammalian receptor biology using fluorescence microscopy,11–15 no analogous experiments have been reported for bacteria.16
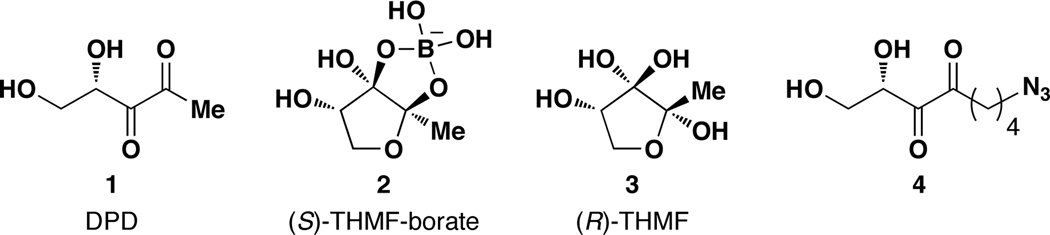
In bacterial quorum sensing, only one quorum sensing signaling molecule has been discovered that facilitates both Gram-negative and Gram-positive bacterial communication, AI-2, a class of furanone-based quorum-sensing signaling molecules derived from 4,5-dihydroxy-2,3-pentanedione (DPD, 1, Figure 1).17,18 Although the DPD synthase, luxS, has been identified in over 55 bacterial species, the exact chemical structure of the AI-2 signaling molecule(s) along with their respective receptor(s) have been confirmed in only two species, Vibrio harveyi and Salmonella typhimurium.18 Interestingly, the chemical form of this autoinducer is dependent on species: V. harveyi utilizes a boron-containing AI-2 molecule, (2S, 4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran borate ((S)-THMF-borate, 2, Figure 1),19 while S. typhimurium uses (R)-THMF (3, Figure 1).20 Moreover, these Gram-negative bacterial species also use functionally different AI-2 receptors, which have low sequence homology (11%) and electrostatically and sterically diverse binding sites.21,22 LuxP is a periplasmic binding protein that together with LuxQ, a membrane sensor histidine kinase, controls quorum sensing in V. harveyi upon the binding of 2 in its positively charged binding pocket.21 LsrB, on the other hand, is part of an ATP binding cassette transporter, and binds 3 in its net negatively charged binding pocket to faciltate AI-2 internalization and quorum sensing in S. typhimurium.22 Intriguingly, LuxPQ receptor systems have only been identified in Vibrionales, while the Lsr receptor complex has been identified in many pathogenic bacterial species.23–25 However, these studies solely relied on genetic and structural predictions,23–25 and no probe has been reported to rapidly analyze bacteria for AI-2 binding receptors. Herein, we report the design and synthesis of a multivalent chemical probe that is capable of specifically recognizing Lsr-type AI-2 receptors.
Figure 1.
Structures of DPD, AI-2 quorum sensing signaling molecules and an azido-DPD analogue.
Our group has reported the synthesis of a series of alkyl-DPD analogues that demonstrated a molecular basis for highly discriminatory recognition in AI-2 quorum sensing.26 Additionally, from these studies, an azidobutyl-containing DPD analogue (4, Figure 1) was found to be a quorum-sensing antagonist in both V. harveyi and S. typhimurium.26 As this analogue is amenable to modification using click chemistry, we were interested in exploiting the azido motif of 4 for the synthesis of DPD-based dendritic probes using CuI-catalyzed azide-alkyne cycloaddition chemistry.27–29 It is important to note here that while reports of the use of functionalized dendrimers in cancer therapeutics are numerous,5–10 only limited studies exploring their use as antibacterials have been reported,9,30–33 none of which target quorum sensing specifically.34
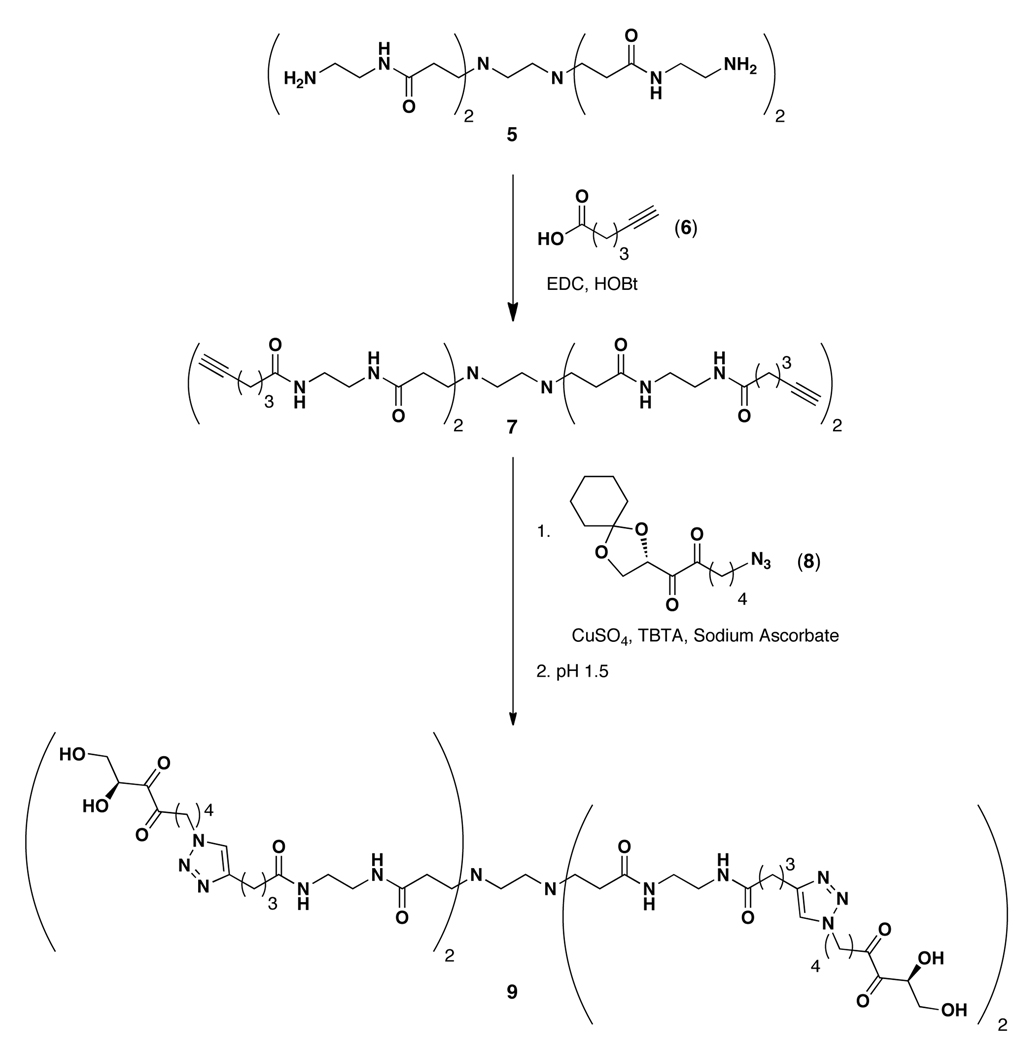
The synthesis of our DPD-based dendritic probe is shown in Scheme 1, and began with commercially available PAMAM dendrimer 5 (generation 0.0). The N-terminal amines of 5 were first converted to alkynyl-amides 7 by coupling with 5-hexynoic acid (6) using standard coupling conditions. The alkynyl moieties were then utilized to install protected azido-DPD analogues 8 using CuI-catalyzed azide-alkyne cycloaddition chemistry. Click chemistry has been widely used for both dendrimer assembly using azide- or alkyne-modified dendrons or for terminal conjugation of various small molecules.35–37 Following azide-alkyne cycloaddition, the purified dendrimers were dissolved in pH 1.5 buffer to afford deprotected alkyl-DPD-functionalized dendrimer 9.26
Scheme 1.
Synthesis of DPD dendrimer 9.
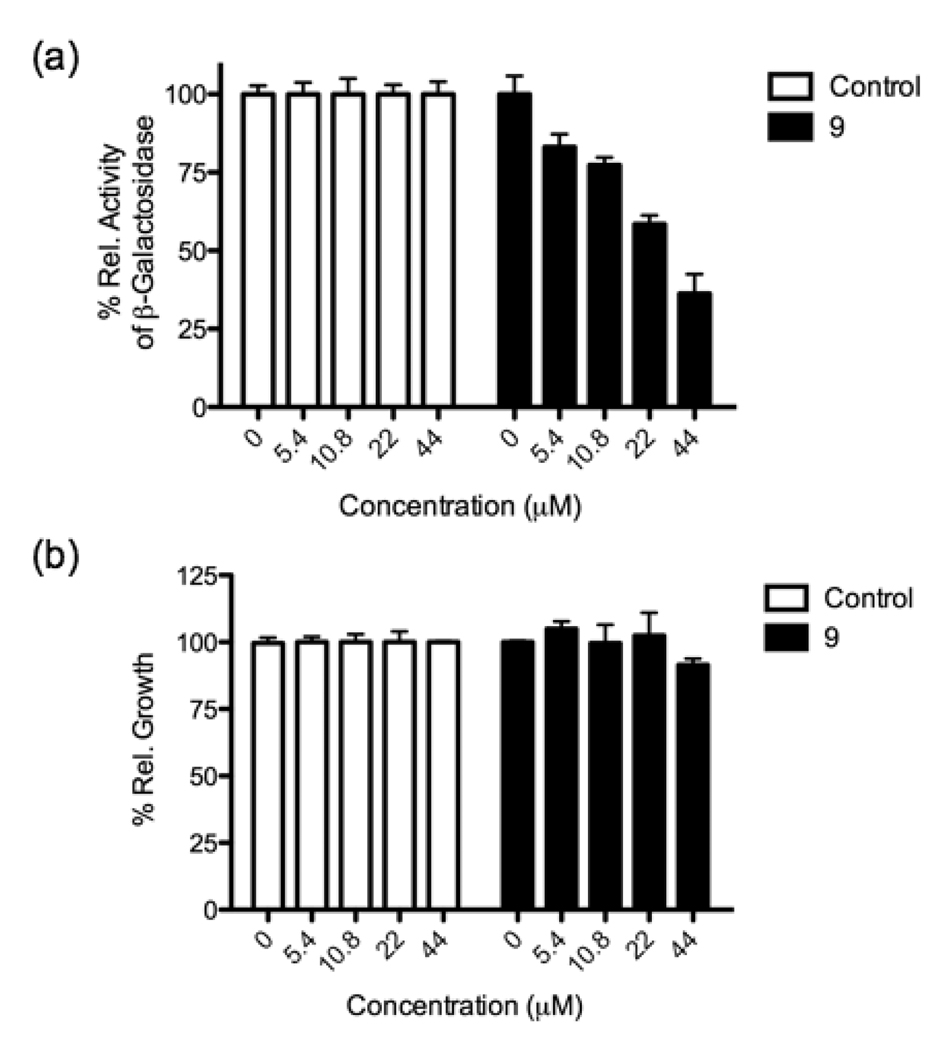
To determine the effect of conjugating azidobutyl-DPD to the dendrimer, we first tested 9 for its activity as a quorum-sensing antagonist in S. typhimurium. Assays were conducted using S. typhimurium strain Met844, a ΔluxS strain with a lacZ-lsr fusion, as previously reported.26 The lacZ fusion, which encodes for the biosynthesis of β-galactosidase under lsr promoter control, allows for the monitoring of AI-2-dependent lsr activation.38 As Figure 2a shows, DPD dendrimer 9 exhibited dose dependent inhibition of quorum sensing in S. typhimurium with an IC50 value of 29.5 µM, similar to that of 4 (20.3 µM).26 Importantly, no effect on growth was noted at any of the concentrations examined (Figure 2b). We note that although the inhibitory activity of DPD dendrimer 9 was not enhanced as has previously been reported for other multivalent probes,1–3 this may be readily interpreted in that 9 must be internalized to bind the periplasmic LsrB receptor and antagonize quorum sensing. Thus, only one DPD molecule may be required for binding LsrB; however, multiple ligand display was sought for probe versatility and future de novo receptor discovery, vide infra.
Figure 2.
Quorum sensing modulation (a) and growth impact (b) by DPD dendrimer 9. The antagonist assay was performed in the presence of 50 µM 1.
To examine the feasibility of larger dendrimers, generations 1.0 and 2.0 with 8 and 16 DPD molecules, respectively, were also examined; however, each carried a serious liability, they were toxic to the bacteria with a likely basis being outer membrane disruption. Of additional note, a DPD dimer was also examined; however, no quorum sensing antagonism was observed. To confirm our inhibition data, we also examined the downregulation of quorum sensing relevant genes using RT-PCR, and a noted decrease in LsrB, LsrF and LsrK expression was observed (see Supporting Information). Thus, DPD dendrimer 9 selectively inhibits quorum sensing in S. typhimurium. Based on these successful results in S. typhimurium, we were also interested in examining quorum sensing antagonist activity of 9 in V. harveyi; however, no activity was noted in this species.
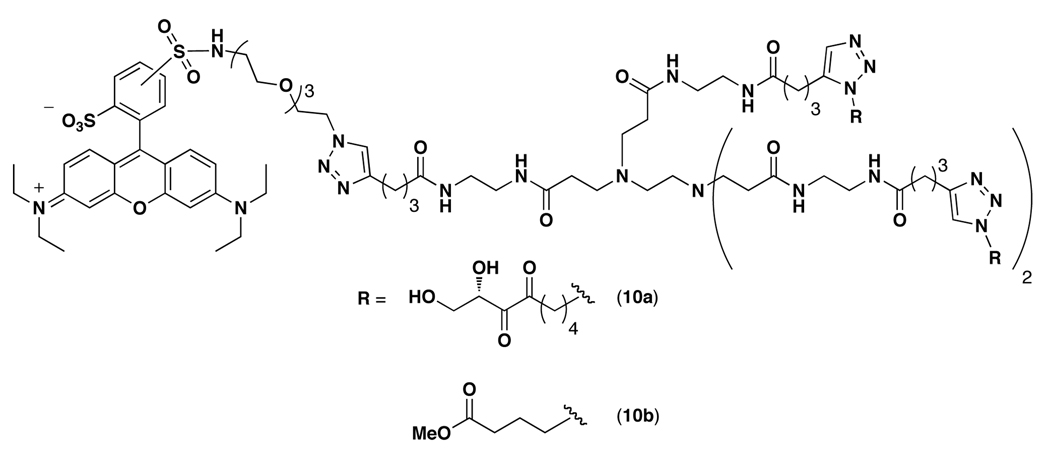
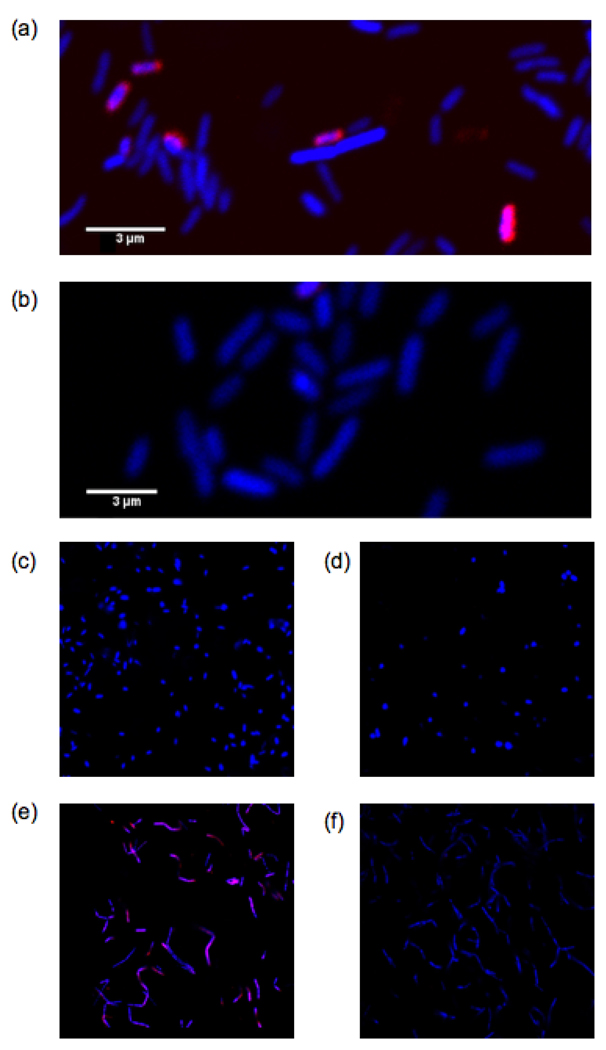
Based on our activity data and the fact that S. typhimurium and V. harveyi use functionally different quorum sensing receptors, LsrB, part of an ABC transporter,22 and LuxP, a periplasmic binding protein,21 respectively, we decided to establish whether DPD dendrimer 9-based constructs may be useful as specific probes for Lsr-type AI-2 quorum sensing receptors. This hypothesis was also driven by the seminal studies of Chmielewski who demonstrated the capacity of multivalent ligands to act as potent inhibitors of ABC transporter drug efflux systems.39–41 To engage this logic, we synthesized DPD-rhodamine dendrimer 10a and control dendrimer 10b (Figure 3; see Supporting Information for synthetic details) for fluorescence imaging studies. Importantly, dendrimer 10a retained antagonistic activity in S. typhimurium supporting its use as a probe (see Supporting Information). Bacteria were stained with either dendrimer 10a or 10b (50 µM each) and the cells were analyzed using confocal microscopy for rhodamine fluorescence. As Figures 4a and 4b show, dendrimer 10a successfully stained S. typhimurium. Moreover, no fluorescence was observed after staining with dendrimer 10b indicating that the interaction between DPD-rhodamine dendrimer 10a and LsrB is specific. As an additional control for Lsr specificity, we also performed imaging experiments in V. harveyi. As Figures 4c and 4d show, no fluorescence was observed with either dendrimer 10a or 10b providing additional evidence that dendrimer 10a is specific for Lsr-type quorum sensing receptors.
Figure 3.
Structure of rhodamine-containing DPD and control ligand dendrimers.
Figure 4.
Fluorescence imaging with dendrimers 10a and 10b (50 µM each). Images were obtained using a Zeiss LSM 710 laser scanning confocal microscope. Rhodamine fluorescence is shown in pink. Hoescht 33342 (blue), a DNA stain, was used as a control. (a), (b) Fluorescence imaging in S. typhimurium with 10a and 10b, respectively. (c), (d) Fluorescence imagining in V. harveyi with 10a and 10b, respectively. (e), (f) Fluorescence imaging in B. cereus with 10a and 10b, respectively.
Finally, we sought to examine the versatility of our probe in another bacterial species known to contain an Lsr-type AI-2 receptor. Recent biochemical evidence has shown that Bacillus cereus, a Gram-positive bacterial species, possesses functional AI-2 receptors with 63% homology to LsrB.25 B. cereus was stained with either dendrimer 10a or 10b as described above. As Figures 4e and 4f show, DPD dendrimer probe 10a was also capable of recognizing the B. cereus AI-2 receptor. Thus, dendrimer probe 10a is able to specifically bind Lsr-type AI-2 receptors in both Gram-negative and Gram-positive bacterial species. This finding could be broadly useful as Lsr system orthologs have been genetically identified in bacterial species belonging to the Enterobacteriaceae, Rhizobiaceae, Bacillaceae, Pasteurellaceae and Rhodobacteriaceae families, while LuxPQ systems have only been identified in Vibrionales indicating the potential importance of Lsr systems for AI-2 quorum sensing.23–25
In conclusion, we have developed a multivalent chemical probe for Lsr-type AI-2 receptors. More specifically, using this multivalent scaffold approach, an AI-2 quorum sensing antagonist in S. typhimurium and an imaging agent for bacterial species utilizing Lsr-type AI-2 receptors have been identified. Although antagonism was achieved with our probe (9), efficacy could be readily improved upon, possibly by examining other dendrimer scaffolds or altering the linker length. These considerations notwithstanding, this multivalent scaffold was key in obtaining an effective imaging agent as similar imaging experiments failed with a rhodamine-conjugated DPD molecule. Thus, an understanding of these multivalent receptor-ligand interactions should provide valuable tools for discovering new bacterial species that utilize Lsr-type AI-2 receptor. Furthermore, since the dendrimer ligands are modular, both fluorescence and affinity tags can be employed to facilitate such studies. Most importantly, however, since our probe is capable of binding Lsr-type receptors in both Gram-negative and Gram-positive bacteria, it may facilitate our understanding of interspecies bacterial communication.
Supplementary Material
ACKNOWLEDGMENT
We thank Professor Bonnie Bassler (Princeton University) for kindly providing S. typhimurium strain Met844. We gratefully acknowledge financial support from the NIH (Grant AI077644 to K.D.J. and Grants AI080715 and AI085324 to G.F.K.) and the Skaggs Institute for Chemical Biology.
Footnotes
ASSOCIATED CONTENT
Synthetic protocols for and characterization of dendrimers 9, 10a and 10b and assay and imaging protocols. Complete reference 31. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Kiessling LL, Gestwicki JE, Strong LE. Curr. Opin. Chem. Biol. 2000;4:696. doi: 10.1016/s1367-5931(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 2.Kiessling LL, Gestwicki JE, Strong LE. Angew. Chem. Int. Ed. 2006;45:2348. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mammen M, Choi S, Whitesides GM. Angew. Chem. Int. Ed. 1998;37:2754. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Rolland O, Turrin C, Caminade A, Majoral J. New J. Chem. 2009;33:1809. [Google Scholar]
- 5.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. Polymer J. 1985;17:117. [Google Scholar]
- 6.Grayson SM, Frechet JMJ. Chem. Rev. 2001;101:3819. doi: 10.1021/cr990116h. [DOI] [PubMed] [Google Scholar]
- 7.Gillies ER, Frechet JMJ. Drug Disc. Today. 2005;10:35. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 8.Patri AK, Majoros IJ, Baker JR., Jr Curr. Opin. Chem. Biol. 2002;6:466. doi: 10.1016/s1367-5931(02)00347-2. [DOI] [PubMed] [Google Scholar]
- 9.Boas U, Heegaard PMH. Chem. Soc. Rev. 2004;33:43. doi: 10.1039/b309043b. [DOI] [PubMed] [Google Scholar]
- 10.Svenson S, Chauharr AS. Nanomedicine. 2008;3:679. doi: 10.2217/17435889.3.5.679. [DOI] [PubMed] [Google Scholar]
- 11.Quintana A, Raczka E, Piehler L, Lee I, Myc A, Majoros IJ, Patri AK, Thomas T, Mule J, Baker JR., Jr Pharm. Res. 2002;19:1310. doi: 10.1023/a:1020398624602. [DOI] [PubMed] [Google Scholar]
- 12.Choi Y, Thomas T, Kotlyar A, Islam MT, Baker JR., Jr Chem. Biol. 2005;12:35. doi: 10.1016/j.chembiol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Regino CAS, Koyama Y, Hama Y, Gunn AJ, Bernardo M, Kobayashi H, Choyke PL, Brechbiel MW. Bioconj. Chem. 2007;18:1474. doi: 10.1021/bc0701085. [DOI] [PubMed] [Google Scholar]
- 14.Shi X, Wang SH, Shen M, Antwerp ME, Chen X, Li C, Petersen EJ, Huang Q, Weber WJ, Jr, Baker JR., Jr Biomacromol. 2009;10:1744. doi: 10.1021/bm9001624. [DOI] [PubMed] [Google Scholar]
- 15.Thomas T, Shukla R, Kotlyar A, Kukowska-Latallo J, Baker JR., Jr Bioorg. Med. Chem. Lett. 2010;20:700. doi: 10.1016/j.bmcl.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Live cell labeling of an acylhomoserine lactone quorum sensing receptor has been reported, see Rayo J, Amara N, Krief P, Meijler MM. J. Am. Chem. Soc. 2011;133:7469. doi: 10.1021/ja200455d.
- 17.Schauder S, Shokat K, Surette MG, Bassler BL. Mol. Microbiol. 2001;41:463. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 18.Waters CM, Bassler BL. Annu. Rev. Cell Dev. Biol. 2005;21:319. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Nature. 2002;415:545. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 20.Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM. Mol. Cell. 2004;15:677. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Neiditch MB, Federle MJ, Miller ST, Bassler BL, Hughson FM. Mol. Cell. 2005;18:507. doi: 10.1016/j.molcel.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Taga ME, Semmelhack JL, Bassler BL. Mol. Microbiol. 2001;42:777. doi: 10.1046/j.1365-2958.2001.02669.x. [DOI] [PubMed] [Google Scholar]
- 23.Sun J, Daniel R, Wagner-Dobler I, Zeng A. BMC Evol. Biol. 2004;4:36. doi: 10.1186/1471-2148-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezzonico F, Duffy B. BMC Microbiol. 2008;8:154. doi: 10.1186/1471-2180-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira CS, de Regt AK, Brito PH, Miller ST, Xavier KB. J. Bacteriol. 2009;191:6975. doi: 10.1128/JB.00976-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowery CA, Park J, Kaufmann GF, Janda KD. J. Am. Chem. Soc. 2008;130:9200. doi: 10.1021/ja802353j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rostovtsov VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Tornoe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 29.Meldal M, Tornoe CW. Chem. Rev. 2008;108:2952. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 30.Johansson EMV, Kolomiets E, Rosenau F, Jaeger K-E, Darbre T, Reymond J-L. New J. Chem. 2007;31:1291. [Google Scholar]
- 31.Johansson EMV, et al. Chem. Biol. 2008;15:1249. doi: 10.1016/j.chembiol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Kolomiets E, Swiderska MA, Kadam RU, Johansson EMV, Jaeger K-E, Darbre T, Reymond J-L. ChemMedChem. 2009;4:562. doi: 10.1002/cmdc.200800380. [DOI] [PubMed] [Google Scholar]
- 33.Hou S, Zhou C, Liu Z, Young AW, Shi Z, Ren D, Kallenbach NR. Bioorg. Med. Chem. Lett. 2009;19:5478. doi: 10.1016/j.bmcl.2009.07.077. [DOI] [PubMed] [Google Scholar]
- 34.Quorum sensing control using other polymers has been reported, see: Kato N, Kobayashi A, Motohashi H, Ozonoe Y, Morohoshi T, Ikeda T. Progr. Colloid Polym. Sci. 2009;136:155. Piletska EV, Stavroulakis G, Karim K, Whitcombe MJ, Chianella I, Sharma A, Eboigbodin KE, Robinson GK, Piletsky SA. Biomacromol. 2010;11:975. doi: 10.1021/bm901451j. Piletska EV, Stavroulakis G, Larcombe LD, Whitcombe MJ, Sharma A, Primrose S, Robinson GK, Piletsky SA. Biomacromol. 2011;12:1067. doi: 10.1021/bm101410q.
- 35.Wu P, Malkoch M, Hunt JN, Vestberg R, Kaltgrad E, Finn MG, Fokin VV, Sharpless KB, Hawker CJ. Chem. Commun. 2005:5775. doi: 10.1039/b512021g. [DOI] [PubMed] [Google Scholar]
- 36.Malkoch M, Schleicher K, Drockenmuller E, Hawker CJ, Russell TP, Wu P, Fokin VV. Macromolecules. 2005;38:3663. [Google Scholar]
- 37.Franc G, Kakkar A. Chem. Commun. 2008:5267. doi: 10.1039/b809870k. [DOI] [PubMed] [Google Scholar]
- 38.Taga ME, Miller ST, Bassler BL. Mol. Microbiol. 2003;50:1411. doi: 10.1046/j.1365-2958.2003.03781.x. [DOI] [PubMed] [Google Scholar]
- 39.Pires MM, Hrycyna CA, Chmielewski J. Biochemistry. 2006;45:11695. doi: 10.1021/bi0608109. [DOI] [PubMed] [Google Scholar]
- 40.Pires MM, Emmert D, Hrycyna CA, Chmielewski J. Mol. Pharm. 2009;75:92. doi: 10.1124/mol.108.050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Namanja HA, Emmert D, Pires MM, Hrycyna CA, Chmielewski J. Biochem. Biophys. Res. Commun. 2009;388:672. doi: 10.1016/j.bbrc.2009.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.